Practice Guidelines for Monitoring Neuromuscular Blockade—Elements to Change to Increase the Quality of Anesthesiological Procedures and How to Improve the Acceleromyographic Method
Abstract
1. Introduction
2. Professional Perspectives
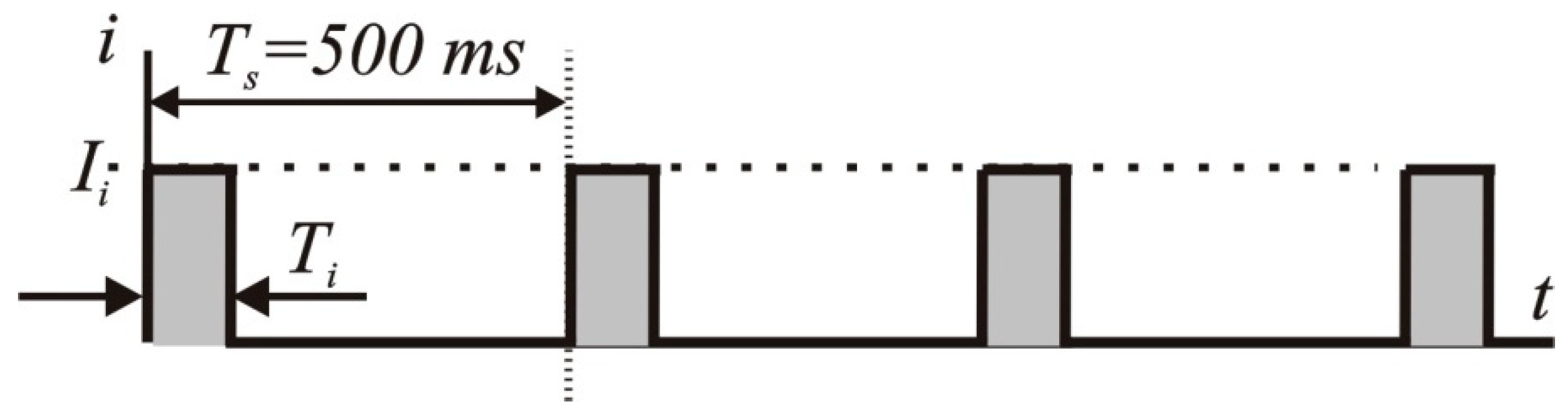
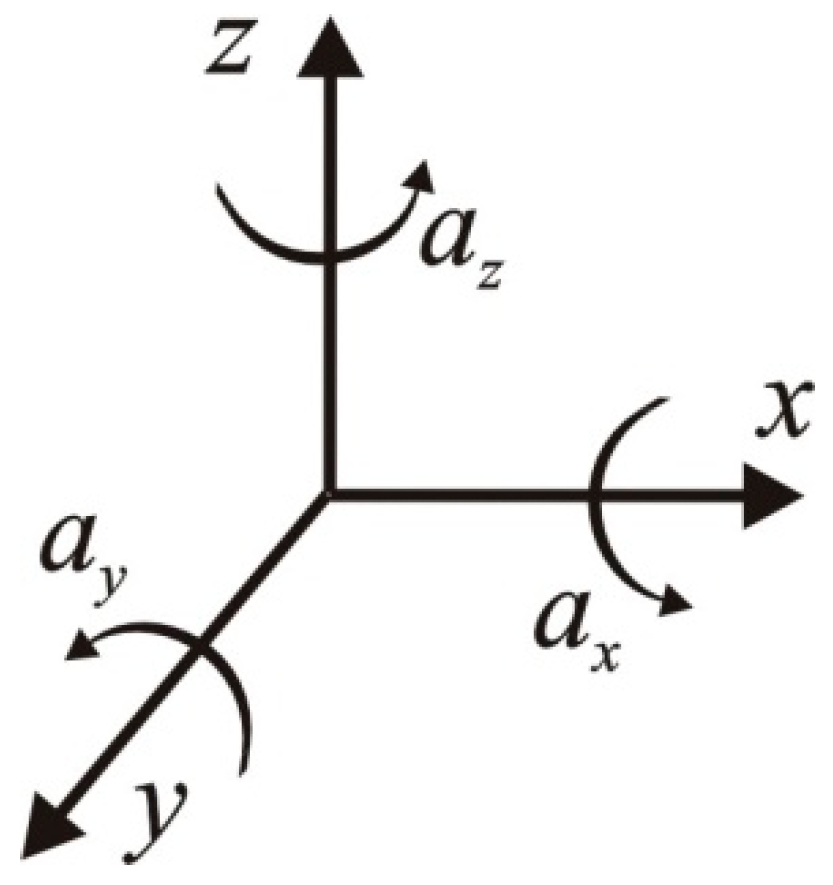
3. Technical Aspects
4. Physical Aspects
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thilen, S.R.; Weigel, W.A.; Todd, M.M.; Dutton, R.P.; Lien, C.A.; Grant, S.A.; Szokol, J.W.; Eriksson, L.I.; Yaster, M.; Grant, M.D.; et al. 2023 American Society of Anesthesiologists Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade: A report by American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology 2023, 138, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Buder, T.; Romero, C.S.; Lewald, H.; Lamperti, M.; Afshari, A.; Hristovska, A.M.; Schmartz, D.; Hinkelbein, J.; Longrois, D.; Popp, M.; et al. Peri-operative management of neuromuscular blockade. A guideline from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2023, 40, 82–94. [Google Scholar] [CrossRef]
- Klein, A.A.; Meek, T.; Allcock, E.; Cooki, T.M.; Mincher, N.; Morris, C.; Nimmo, A.F.; Pandit, J.J.; Pawa, A.; Rodney, G.; et al. Recommendation for standards of monitoring during anaesthesia and recovery 2021. Anaesthesia 2021, 76, 1212–1223. [Google Scholar] [CrossRef]
- Mulier, J.P.; Hunter JM de Boer, H.D. Seventy-five years since the birth of Liverpool anaesthetic technique. B J. Anaesth. 2021, 2, 343–347. [Google Scholar] [CrossRef]
- Brull, S.J.; Eriksson, L. The French Guidelines on muscle relaxants and reversal in anaesthesia: The chain is finally broken and soul is freed. Anaesth. Crit. Care Pain. Med. 2020, 39, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Dutu, M.; Ivascu, R.; Tudorache, O.; Morlova, D.; Stanca, A.; Negoita, S.; Conreci, D. Neuromuscular monitoring: An update. Rom. J. Anaesth. Intensive Care 2018, 25, 55–60. [Google Scholar] [PubMed]
- Koo, B.W.; Oh, A.Y.; Seo, K.S.; Han, H.S.; Yoon, Y.S. Randomized clinical trial of moderate versus deep neuromuscular block for low-pressure pneumoperitoneum during laparoscopic cholecystectomy. World J. Surg. 2016, 40, 2898–2903. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, Y.L.; Xu, C.M.; Lv, X.H.; Wan, Z.H. The effects of neuromuscular blockade on operating conditions during general anesthesia for spinal surgery. J. Neurosurg. Anesth. 2014, 26, 45–49. [Google Scholar] [CrossRef]
- Van Wijk, R.M.; Watts, R.W.; Ledowski, T.; Trochsler, M.; Moran, J.L.; Arenas, G.W. Deep neuromuscular block reduces intra-abdominal pressure requirements during laparoscopic cholecystectomy: A prospective observational study. Acta Anaesthesiol. Scand. 2015, 59, 434–440. [Google Scholar] [CrossRef]
- Bulka, C.M.; Terekhov, M.A.; Martin, B.J.; Dmochowski, R.R.; Hayes, R.M.; Ehrenfeld, J.M. Nondepolarizing neuromuscular blocking agents, reversal, and risk of postoperative pneumonia. Anesthesiology 2016, 125, 647–655. [Google Scholar] [CrossRef]
- Naguib, M.; Brull, S.J.; Johnson, K.B. Conceptual and technical insights into the basis of neuromuscular monitoring. Anaesthesia 2017, 72, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Errando-Oyonarte, C.L.; Moreno-Sanz, C.; Vila-Caral, P.; Ruiz de Adana-Belbel, J.C.; Vazquez- Alonso, E.; Ramirez- Rodriquez, J.M.; Veiga-Ruiz, G.; Guasch-Arevalo, E.; Lora-Tamayo, J.I. Recommendation on the use of deep neuromuscular blockade by anaesthesiologists and surgeons. AQUILES Consensus. Rev. Esp. Anestesiol. Reanim. 2017, 64, 95–104. [Google Scholar] [CrossRef]
- Blum, L.V.; Steeger, E.; Iken, S.; Lotz, G.; Zinn, S.; Piekarski, F.; Zacharowski, K.; Raimann, F.J. Effect of quantitative versus qualitative neuromuscular blockade monitoring on rocuronium consumption in patients undergoing abdominal and gynaecological surgery: A retrospective cohort study. J. Clin. Monit. Comp. 2023, 37, 509–516. [Google Scholar] [CrossRef]
- Lwin, N.S.; Leslie, K. Neuromuscular monitoring during general anaesthesia: A review of current national and international guidelines. BJA Open 2022, 3, 100028. [Google Scholar] [CrossRef]
- Dobson, G.; Chow, L.; Flexman, A.; Hurdle, H.; Kurrek, M.; Laflamme, C.; Perrault, M.A.; Sparrow, K.; Stacey, S.; Swart, P.; et al. Guidelines to the practice of anesthesia—Revised edition 2020. Can. J. Anesth. 2020, 67, 64–69. [Google Scholar] [CrossRef]
- Foldes, F.F. The impact of neuromuscular blocking agents on the development od anaesthesia and surgery. In Muscle Relaxants. Monographs in Anaesthesiology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 19, pp. 1–17. [Google Scholar]
- Checketts, M.R.; Alladi, R.; Ferguson, K.; Gemmel, L.; Handy, J.M.; Klein, A.A.; Love, N.J.; Misra, U.; Morris, C.; Nathanson, M.H.; et al. Association of Anaesthesiologists of Great Britain and Ireland. Recommendations for standards of monitoring during anaesthesia and recovery 2015. Anaesthesia 2016, 71, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Brull, S.J.; Kopman, A.F.; Hunter, J.M.; Fülesdi, B.; Arkes, H.R.; Elstein, A.; Todd, M.M.; Johnson, K.B. Consensus statement on perioperative use of neuromuscular monitoring. Anesth. Analg. 2018, 127, 71–80. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. Practice guidelines for postanesthetic care. Anesthesiology 2002, 96, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Monitoring during Anaesthesia. Available online: www.anzca.edu.au (accessed on 20 February 2024).
- Committee on Standards and Practice parameters. Standards for Basic Anaesthetic Monitoring. Available online: www.asahq.org (accessed on 20 February 2024).
- Canadian Anesthesiologists Society. Guidelines to the Practice of Anaesthesia. Available online: www.cas.ca (accessed on 20 February 2024).
- Czech Society of Anaesthesiology and Intensive Care Medicine: Practice Parameters for the Safe and Effective Use of Neuromuscular Blocking Agents in Anaesthesia. Available online: www.akutne.cz (accessed on 20 February 2024).
- Rozporządzenie Ministra Zdrowia z Dnia 16 Grudnia 2016 r. w Sprawie Standardu Organizacyjnego Opieki Zdrowotnej w Dziedzinie Anestezjologii i Intensywnej Terapii. Dz.U. 2016 poz. 2218. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160002218 (accessed on 20 February 2024).
- Nemes, R.; Renew, J.R. Clinical Practice Guideline for management of neuromuscular blockade: What are the recommendations in the USA and other countries? Curr. Anesthesiol. Rep. 2020, 10, 9098. [Google Scholar] [CrossRef]
- Brull, S.J.; Longgrois, D.; Kranke, P.; Afshari, A.; Plaud, B.; Fuchs-Buder, T. Why a guideline on peri-operative managementof neuromuscular blockade? Why now? Eur. J. Anaesthesiol. 2023, 40, 75–77. [Google Scholar] [CrossRef]
- Brull, S.J.; Kopman, A. Measuring success of patient safety initiatives: The 2023 American Society of Anesthesiologists Practice Guidelines for Monitoring and antagonism of neuromuscular blockade. Anesthesiology 2023, 138, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kopman, A.F.; Lien, C.A.; Hunter, J.M.; Lopez, A.; Brull, S.J. A survey of current management of neuromuscular block in the United States and Europe. Anesth. Analg. 2010, 111, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, C.M.; Eskildsen, K.Z.; Gatke, M.R.; Staehr-Rye, A.K. Objective neuromuscular monitoring of neuromuscular blockade in Denmark: An online-based survey of current practice. Acta Anaesthesiol. Scand. 2017, 61, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Batistaki Ch Vagdatli, K.; Tsiotou, A.; Papaioannou, A.; Pandazi, A.; Matsota, P. A multicenter survey on the use of neuromuscular blockade in Greece. Does the real-world clinical practice indicate the necessity of guidelines? J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Carvahlo, H.; Verdonck, M.; Cools, W.; Geerts, L.; Forget, P.; Poelaert, J. Forty years of neuromuscular monitoring and postoperative residua curarisation: A meta-analysis and evaluation of confidence in network meta-analysis. Br. J. Anaesth. 2020, 125, 466–482. [Google Scholar] [CrossRef]
- Philips, S.; Steward, P.A.; Bilgin, A.B. A survey of the managenet of neuromuscular blockade monitoring in Australia and New Zealand. Anaesth. Intensive Care 2013, 41, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.L.D.; Staehr-Rye, A.K.; Mathiesen, O.; Hagi-Pedersen, D.; Gatke, M.R. A retrospective observational study of neuromuscular monitoring practice in 30,430 cases from six Danish hospitals. Anaesthesia 2020, 75, 1164–1172. [Google Scholar] [CrossRef]
- Greco, M.; Caruso, P.F.; Angelotti, G.; Aceto, R.; Coppalini, G.; Martinetti, N.; Albini, M.; Bash, L.D.; Carvello, M.; Piccioni, F.; et al. REVersal of nEuromusculAr bLocking Agents in Patients Undergoing General Anaesthesia (REVEAL Study). J. Clin. Med. 2023, 12, 563. [Google Scholar] [CrossRef]
- Motamed, C. Intraoperative monitoring of neuromuscular blockade. Life 2023, 13, 1184. [Google Scholar] [CrossRef]
- Thilen, S.R.; Hansen, B.E.; Ramaiah, R.; Kent, C.D.; Treggari, M.M.; Bhananker, S.M. Intraoperative neuromuscular monitoring site and residual paralysis. Anesthesiology 2012, 117, 964–972. [Google Scholar] [CrossRef]
- Brull, S.J.; Kopman, A.F. Current status of neuromuscular reversal and monitoring. Challenges and Opportunities. Anesthesiology 2017, 126, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Thilen, S.; Bhananker, S.M. Qualitative Neuromuscular Monitoring: How to optimize the use of peripheral nerve stimulator to reduce the risk of residua neuromuscular blockade. Curr. Anesth. Rep. 2016, 6, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Sorgenfrei, I.F.; Viby-Morgensen, J.; Swiatek, F.A. Does evidence lead to a change in clinical practice? Danish anaesthetists and nurse anaesthetist clinical practice and knowledge of postoperative residual curarization. Ugeskr. Laeger 2005, 167, 3878–3882. [Google Scholar] [PubMed]
- Murphy, G.S.; Brull, S.J. Quantitative Neuromuscular monitoring and postoperative outcomes: A narrative review. Anesthesiology 2022, 136, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.L.D.; Marty, A.P.; Wakatsuki, S.; Macario, A.; Tanaka, P.; Gatke, M.R.; Ostergaard, D. Barriers and aids to routine neuromuscular monitoring and consistent reversal practice- a qualitative study. Acta Anaesthiol Scand. 2020, 64, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Rodney, G.; Raju, P.K.; Ball, D.R. Not just monitoring; a strategy for managing neuromuscular blockade. Anaesthesia 2015, 70, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Brull, S.J.; Hunter, J.M.; Kopman, A.F.; Fülesdi, B.; Johnson, K.B.; Arkes, H.R. Anesthesiologists overconfidence in their perceived knowledge of neuromuscular monitoring and its relevance to all aspects of medical practice: An international survey. Anesth. Analg. 2019, 128, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- 5th National Audit Project of the Royal College of Anaesthetists and the Association of Anaesthetists of Great Britain and Ireland. Raport and findings. Chapter 19. Available online: https://www.nationalauditprojects.org.uk/NAP5report (accessed on 20 February 2024).
- Fuchs-Buder, T.; Brull, S.J.; Fagerlund, M.J.; Renew, J.R.; Cammu, G.; Murphy, G.S.; Warlé, M.; Vested, M.; Fülesdi, B.; Nemes, R.; et al. Good clinical research practice (GCRP) In pharmacodynamic studiem of neuromuscular bloking agents III: The 2023 Geneva revision. Acta Anaesthesiol. Scand. 2023, 67, 994–1017. [Google Scholar] [CrossRef] [PubMed]
- Bora, D.J.; Dasgupta, R. Various skin impedance models based on physiological stratification. IET Syst. Biol. 2020, 14, 147–159. [Google Scholar] [CrossRef]
- Webster, J.G. Skin Impedance. J. Electr. Bioimpedance 2014, 5, 1. [Google Scholar] [CrossRef]
- EN 61000-4-9:2016; Electromagnetic Compatibility (EMC)—Part 4–9: Testing and Measurement Techniques—Impulse Magnetic Field Immunity Test. Available online: https://www.en-standard.eu/bs-en-61000-4-9-2016-electromagnetic-compatibility-emc-testing-and-measurement-techniques-impulse-magnetic-field-immunity-test/ (accessed on 20 February 2024).
- EN 55011:2016/A11:2020; Industrial, Scientific and Medical Equipment—Radio-Frequency Disturbance Characteristics—Limits and Methods of Measurement. Available online: https://www.en-standard.eu/bs-en-55011-2016-a11-2020-industrial-scientific-and-medical-equipment-radio-frequency-disturbance-characteristics-limits-and-methods-of-measurement/ (accessed on 20 February 2024).
- EN 62304:2006/A1:2015; Medica Ldevice Software—Software Life-Cycleprocesses. Available online: https://www.iso.org/standard/64686.html (accessed on 20 February 2024).
- Torneiro, A.; Oliveira, E.; Rodrigues, N.F. Current Devices and Future Perspectives on Neuromuscular Blockade Monitoring: A Systematic Re-view. In Proceedings of the 2023 IEEE 11th International Conference on Serious Games and Applications for Health (SeGAH), Athens, Greece, 28–30 August 2023; pp. 1–7. [Google Scholar]
- Ozbey, N.B.; Abdullah, T.; Deligoz, O. Residual neuromuscular block in the postanesthesia care unit: Incidence, risk factors, and effect of neuromuscular monitoring and reversal agents. Turk. J. Med. Sci. 2022, 52, 1656–1664. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosciuczuk, U.; Dardzinska, A.; Kasperczuk, A.; Dzienis, P.; Tomaszuk, A.; Tarnowska, K.; Rynkiewicz-Szczepanska, E.; Kossakowska, A.; Pryzmont, M. Practice Guidelines for Monitoring Neuromuscular Blockade—Elements to Change to Increase the Quality of Anesthesiological Procedures and How to Improve the Acceleromyographic Method. J. Clin. Med. 2024, 13, 1976. https://doi.org/10.3390/jcm13071976
Kosciuczuk U, Dardzinska A, Kasperczuk A, Dzienis P, Tomaszuk A, Tarnowska K, Rynkiewicz-Szczepanska E, Kossakowska A, Pryzmont M. Practice Guidelines for Monitoring Neuromuscular Blockade—Elements to Change to Increase the Quality of Anesthesiological Procedures and How to Improve the Acceleromyographic Method. Journal of Clinical Medicine. 2024; 13(7):1976. https://doi.org/10.3390/jcm13071976
Chicago/Turabian StyleKosciuczuk, Urszula, Agnieszka Dardzinska, Anna Kasperczuk, Paweł Dzienis, Adam Tomaszuk, Katarzyna Tarnowska, Ewa Rynkiewicz-Szczepanska, Agnieszka Kossakowska, and Marta Pryzmont. 2024. "Practice Guidelines for Monitoring Neuromuscular Blockade—Elements to Change to Increase the Quality of Anesthesiological Procedures and How to Improve the Acceleromyographic Method" Journal of Clinical Medicine 13, no. 7: 1976. https://doi.org/10.3390/jcm13071976
APA StyleKosciuczuk, U., Dardzinska, A., Kasperczuk, A., Dzienis, P., Tomaszuk, A., Tarnowska, K., Rynkiewicz-Szczepanska, E., Kossakowska, A., & Pryzmont, M. (2024). Practice Guidelines for Monitoring Neuromuscular Blockade—Elements to Change to Increase the Quality of Anesthesiological Procedures and How to Improve the Acceleromyographic Method. Journal of Clinical Medicine, 13(7), 1976. https://doi.org/10.3390/jcm13071976






