Association of Peri-Implant Keratinized Mucosa Width and Mucosal Thickness with Early Bone Loss: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Selection
2.2. Peri-Implant Clinical Parameters
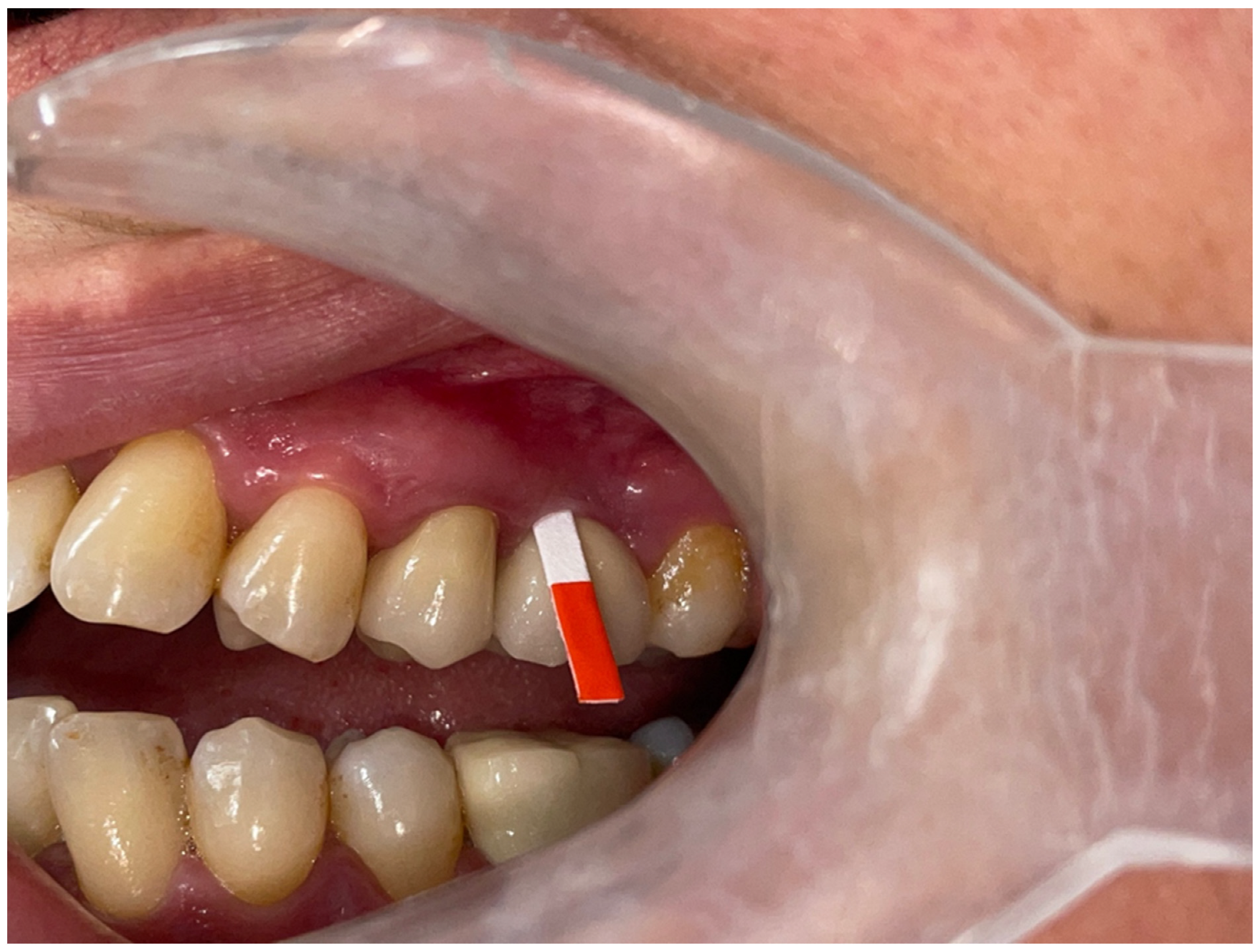
KMW and MT Measurements
2.3. Radiographic Examination and Measurements
2.4. Sample Collection and Determination
2.4.1. Collection of Peri-Implant Crevicular Fluid (PICF)
2.4.2. Determination of TNF-α, RANKL, and OPG
2.4.3. Determination of miRNA-27a
2.5. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Peri-Implant Clinical Parameters
3.3. Radiographic Examination
3.4. Immunological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Al Amri, M.D.; Al-Johany, S.S.; Al Baker, A.M.; Al Rifaiy, M.Q.; Abduljabbar, T.S.; Al-Kheraif, A.A. Soft tissue changes and crestal bone loss around platform-switched implants placed at crestal and subcrestal levels: 36-month results from a prospecstive split-mouth clinical trial. Clin. Oral Implant. Res. 2017, 28, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.J.; Yoon, J.; Misch, C.E.; Wang, H.L. The causes of early implant bone loss: Myth or science? J. Periodontol. 2002, 73, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Negri, B.; Calvo Guirado, J.L.; Mate Sanchez de Val, J.E.; Delgado Ruiz, R.A.; Ramirez Fernandez, M.P.; Barona Dorado, C. Peri-implant tissue reactions to immediate nonocclusal loaded implants with different collar design: An experimental study in dogs. Clin. Oral. Implant. Res. 2014, 25, e54–e63. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, J.; Chen, K.; Li, Z.; Chen, Z.; Huang, B. Clinical and radiographic results of crestal vs. subcrestal placement of implants in posterior areas: A split-mouth randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2023, 25, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lamazza, L.; Rapani, A.; Troiano, G.; Messina, M.; Antonelli, A.; Giudice, A.; Lombardi, T. Marginal bone changes around platform-switched conical connection implants placed 1 or 2 mm subcrestally: A multicenter crossover randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2023, 25, 398–408. [Google Scholar] [CrossRef]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hammerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sanchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 15), 32–49. [Google Scholar] [CrossRef]
- Zucchelli, G.; Tavelli, L.; Stefanini, M.; Barootchi, S.; Mazzotti, C.; Gori, G.; Wang, H.L. Classification of facial peri-implant soft tissue dehiscence/deficiencies at single implant sites in the esthetic zone. J. Periodontol. 2019, 90, 1116–1124. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Schwarz, F.; Sader, R. Influence of width of keratinized tissue on the prevalence of peri-implant diseases: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2022, 33 (Suppl. 23), 8–31. [Google Scholar] [CrossRef]
- Monje, A.; Roccuzzo, A.; Buser, D.; Wang, H.L. Influence of buccal bone wall thickness on the peri-implant hard and soft tissue dimensional changes: A systematic review. Clin. Oral Implant. Res. 2023, 34, 157–176. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Effects of occlusal forces on the peri-implant-bone interface stability. Periodontology 2000 2019, 81, 179–193. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Gonzalez-Martin, O.; Couso-Queiruga, E.; Wang, H.L. The peri-implant phenotype. J. Periodontol. 2020, 91, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Zigdon, H.; Machtei, E.E. The dimensions of keratinized mucosa around implants affect clinical and immunological parameters. Clin. Oral Implant. Res. 2008, 19, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Mailoa, J.; Arnett, M.; Chan, H.-L.; George, F.M.; Kaigler, D.; Wang, H.-L. The association between buccal mucosa thickness and periimplant bone loss and attachment loss: A cross-sectional study. Implant Dent. 2018, 27, 575–581. [Google Scholar] [CrossRef]
- Bonino, F.; Steffensen, B.; Natto, Z.; Hur, Y.; Holtzman, L.P.; Weber, H.P. Prospective study of the impact of peri-implant soft tissue properties on patient-reported and clinically assessed outcomes. J. Periodontol. 2018, 89, 1025–1032. [Google Scholar] [CrossRef]
- Buyukozdemir Askin, S.; Berker, E.; Akincibay, H.; Uysal, S.; Erman, B.; Tezcan, I.; Karabulut, E. Necessity of keratinized tissues for dental implants: A clinical, immunological, and radiographic study. Clin. Implant Dent. Relat. Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Boynueğri, D.; Nemli, S.K.; Kasko, Y.A. Significance of keratinized mucosa around dental implants: A prospective comparative study. Clin. Oral Implant. Res. 2013, 24, 928–933. [Google Scholar] [CrossRef]
- Frisch, E.; Ziebolz, D.; Vach, K.; Ratka-Kruger, P. The effect of keratinized mucosa width on peri-implant outcome under supportive postimplant therapy. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. 1), e236–e244. [Google Scholar] [CrossRef]
- Lim, H.C.; Wiedemeier, D.B.; Hammerle, C.H.F.; Thoma, D.S. The amount of keratinized mucosa may not influence peri-implant health in compliant patients: A retrospective 5-year analysis. J. Clin. Periodontol. 2019, 46, 354–362. [Google Scholar] [CrossRef]
- Monje, A.; Blasi, G. Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J. Periodontol. 2019, 90, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Sukuroglu, E.; Baltacioglu, E. Analyses of clinical and osteoimmunological parameters on keratinized mucosa around dental implants. Niger. J. Clin. Pract. 2019, 22, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Zhou, X.; Trombetta-eSilva, J.; Francis, M.; Gaharwar, A.K.; Atsawasuwan, P.; Diekwisch, T.G.H. MicroRNAs and Periodontal Homeostasis. J. Dent. Res. 2017, 96, 491–500. [Google Scholar] [CrossRef]
- Asa’ad, F.; Monje, A.; Larsson, L. Role of epigenetics in alveolar bone resorption and regeneration around periodontal and peri-implant tissues. Eur. J. Oral Sci. 2019, 127, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Garaicoa-Pazmino, C.; Dahlin, C.; Larsson, L. Expression of MicroRNAs in Periodontal and Peri-Implant Diseases: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 4147. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Mizuno, H.; Sugiura, Y.; Morita, M. Detection of Salivary miRNAs Reflecting Chronic Periodontitis: A Pilot Study. Molecules 2019, 24, 1034. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Q.; Chen, X.; Mi, W.; Wu, T.; Huang, H. MiR-27a targets DKK2 and SFRP1 to promote reosseointegration in the regenerative treatment of peri-implantitis. J. Bone Miner. Res. 2019, 34, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Loe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Silness, J.; Löe, H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Frost, N.A.; Mealey, B.L.; Jones, A.A.; Huynh-Ba, G. Periodontal Biotype: Gingival Thickness as It Relates to Probe Visibility and Buccal Plate Thickness. J. Periodontol. 2015, 86, 1141–1149. [Google Scholar] [CrossRef]
- Schwarz, F.; Claus, C.; Becker, K. Correlation between horizontal mucosal thickness and probing depths at healthy and diseased implant sites. Clin. Oral Implant. Res. 2017, 28, 1158–1163. [Google Scholar] [CrossRef]
- Tuter, G.; Kurtis, B.; Serdar, M. Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1. J. Periodontol. 2002, 73, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ravidà, A.; Arena, C.; Tattan, M.; Caponio, V.C.A.; Saleh, M.H.; Wang, H.L.; Troiano, G. The role of keratinized mucosa width as a risk factor for peri-implant disease: A systematic review, meta-analysis, and trial sequential analysis. Clin. Implant. Dent. Relat. Res. 2022, 24, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B.; Oshrain, R.L.; Gordon, J.M. Enzyme activity in human gingival crevicular fluid: Considerations in data reporting based on analysis of individual crevicular sites. J. Clin. Periodontol. 1986, 13, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Reis, I.; Todescan, F.F.; Ruiz, G.M.; da Silva, E.V.F.; Romito, G.A.; Spin-Neto, R.; Pannuti, C.M. Influence of supracrestal tissue height on peri-implant hard and soft tissues of single implants placed in the esthetic area using the one abutment-one time approach: A 1-year prospective interventional study. Quintessence Int. 2023, 54, 536–547. [Google Scholar] [CrossRef]
- Linkevicius, T.; Puisys, A.; Steigmann, M.; Vindasiute, E.; Linkeviciene, L. Influence of Vertical Soft Tissue Thickness on Crestal Bone Changes Around Implants with Platform Switching: A Comparative Clinical Study. Clin. Implant. Dent. Relat. Res. 2015, 17, 1228–1236. [Google Scholar] [CrossRef]
- Thoma, D.S.; Muhlemann, S.; Jung, R.E. Critical soft-tissue dimensions with dental implants and treatment concepts. Periodontology 2000 2014, 66, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Perussolo, J.; Souza, A.B.; Matarazzo, F.; Oliveira, R.P.; Araujo, M.G. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: A 4-year follow-up study. Clin. Oral Implant. Res. 2018, 29, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Cappare, P.; Gherlone, E. A 4-year evaluation of the peri-implant parameters of immediately loaded implants placed in fresh extraction sockets. J. Periodontol. 2010, 81, 1629–1634. [Google Scholar] [CrossRef]
- de Siqueira, R.A.C.; Savaget Goncalves Junior, R.; Dos Santos, P.G.F.; de Mattias Sartori, I.A.; Wang, H.L.; Fontao, F. Effect of different implant placement depths on crestal bone levels and soft tissue behavior: A 5-year randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 282–293. [Google Scholar] [CrossRef]
- Nohra, J.; Dagher, M.; Matni, G.; Mokbel, N.; Jobaili, E.; Naaman, N. Effect of Primary Stability and Soft- and Hard-Tissue Thickness on Marginal Bone Loss: A Prospective Pilot Study. Implant Dent. 2018, 27, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Spray, J.R.; Black, C.G.; Morris, H.F.; Ochi, S. The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Ann. Periodontol. 2000, 5, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kaklamanos, E.G.; Tsalikis, L. A review on peri-implant crevicular fluid assays potential in monitoring and predicting peri-implant tissue responses. J. Int. Acad. Periodontol. 2002, 4, 49–59. [Google Scholar] [PubMed]
- Xie, Y.F.; Shu, R.; Jiang, S.Y.; Liu, D.L.; Zhang, X.L. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int. J. Oral Sci. 2011, 3, 125–134. [Google Scholar] [CrossRef]
- Ogata, Y.; Matsui, S.; Kato, A.; Zhou, L.; Nakayama, Y.; Takai, H. MicroRNA expression in inflamed and noninflamed gingival tissues from Japanese patients. J. Oral Sci. 2014, 56, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Solakoglu, O.; Steinbach, B.; Gotz, W.; Heydecke, G.; Schwarzenbach, H. Characterization of circulating molecules and activities in plasma of patients after allogeneic and autologous intraoral bone grafting procedures: A prospective randomized controlled clinical trial in humans. BMC Oral Health 2022, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, K.; Yuan, J.; Huang, P.; Xu, X.; Li, C.; Qian, N.; Qi, J.; Shao, Z.; Deng, L.; et al. Estrogen inhibits osteoclasts formation and bone resorption via microRNA-27a targeting PPARgamma and APC. J. Cell. Physiol. 2018, 234, 581–594. [Google Scholar] [CrossRef]
- Munetsuna, E.; Yamada, H.; Ando, Y.; Yamazaki, M.; Tsuboi, Y.; Kondo, M.; Mizuno, G.; Ishikawa, H.; Sugimoto, K.; Osakabe, K.; et al. Association of subcutaneous and visceral fat with circulating microRNAs in a middle-aged Japanese population. Ann. Clin. Biochem. 2018, 55, 437–445. [Google Scholar] [CrossRef]
- Murri, M.; Insenser, M.; Fernandez-Duran, E.; San-Millan, J.L.; Escobar-Morreale, H.F. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J. Clin. Endocrinol. Metab. 2013, 98, E1835–E1844. [Google Scholar] [CrossRef]
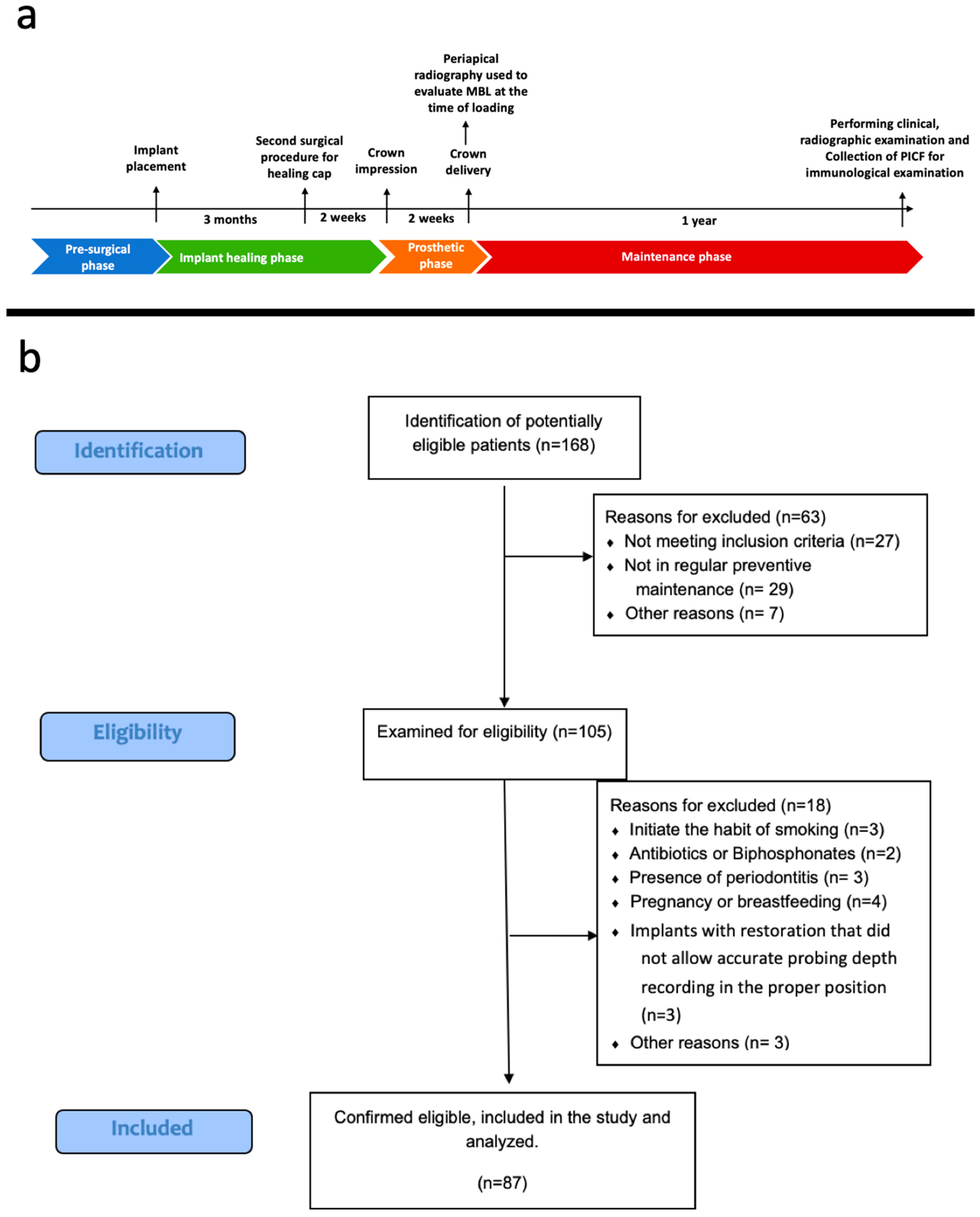

| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Variables | KMW Inadequate (<2 mm) (n = 19) | KMW Adequate (≥2 mm) (n = 68) | p | MT Thin (<2 mm) (n = 32) | MT Thick (≥2 mm) (n = 55) | p |
|---|---|---|---|---|---|---|
| Age † | 49.32 ± 9.96 | 47.88 ± 7.02 | 0.477 | 49.44 ± 9.30 | 47.47 ± 6.62 | 0.255 |
| Gender ‡ | ||||||
| Female | 11 (58.0%) | 47 (69.1%) | 0.246 | 23 (71.8%) | 12 (60.0%) | 0.152 |
| Male | 8 (42.0%) | 21 (30.8%) | 9 (28.1%) | 8 (40.0%) | ||
| Location † | ||||||
| Maxilla | 4 (21.1%) | 29 (42.6%) | 0.066 | 10 (31.3%) | 23 (41.8%) | 0.100 |
| Mandibula | 15 (78.9%) | 39 (57.4%) | 22 (68.7%) | 32 (58.2%) | ||
| Mean MBL (mm) † | 0.46 ± 0.73 | 0.59 ± 0.70 | 0.456 | 0.57 ± 68 | 0.13 ± 0.32 | 0.012 * |
| PI † | 1.00 ± 0.67 | 1.02 ± 0.56 | 0.923 | 1.06 ± 0.67 | 0.98 ± 0.53 | 0.539 |
| GI † | 1.02 ± 0.56 | 1.11 ± 0.66 | 0.301 | 1.38 ± 0.71 | 1.20 ± 0.78 | 0.299 |
| PD (mm) † | 2.10 ± 0.66 | 2.43 ± 0.79 | 0.098 | 2.40 ± 0.88 | 2.33 ± 0.71 | 0.687 |
| MT | KMW | TNF-α | RANKL | OPG | RANKL/OPG | miRNA-27a | Mean MBL | |
|---|---|---|---|---|---|---|---|---|
| MT | 1 | 0.522 * | −0.097 | 0.020 | 0.077 | −0.020 | −0.047 | −0.217 * |
| KMW | 1 | 0.023 | −0.036 | −0.007 | 0.009 | −0.186 | −0.170 | |
| TNF-α | 1 | 0.567 * | 0.565 * | −0.117 | −0.033 | 0.065 | ||
| RANKL | 1 | 0.654 * | 0.241 * | 0.001 | 0.083 | |||
| OPG | 1 | −0.339 * | −0.009 | −0.068 | ||||
| RANKL/OPG | 1 | −0.123 | 0.211 * | |||||
| MiRNA-27a | 1 | −0.155 | ||||||
| Mean MBL | 1 |
| KMW Adequate (≥2 mm) (n = 68) | KMW Inadequate (<2 mm) (n = 19) | p † | |
| TNF-α (pg/mL) | 8.24 ± 5.59 | 6.33 ± 2.89 | 0.155 |
| RANKL (pg/mL) | 10.75 ± 7.39 | 8.91 ± 5.80 | 0.321 |
| OPG (pg/mL) | 0.11 ± 0.09 | 0.10 ± 0.08 | 0.47 |
| RANKL/OPG | 106.70 ± 56.97 | 110.42 ± 45.90 | 0.794 |
| miRNA-27a | 183.71 ± 764.65 | 1.09 ± 1.36 | 0.001 * |
| MT Thick (≥2 mm) (n = 55) | MT Thin (<2 mm) (n = 32) | p † | |
| TNF-α (pg/mL) | 7.38 ± 3.70 | 8.60 ± 7.02 | 0.290 |
| RANKL (pg/mL) | 10.39 ± 6.93 | 10.26 ± 7.45 | 0.935 |
| OPG (pg/mL) | 0.11 ± 0.09 | 0.11 ± 0.08 | 0.837 |
| RANKL/OPG | 109.94 ± 60.35 | 103.34 ± 43.28 | 0.589 |
| miRNA-27a | 219.97 ± 834.82 | 1.81 ± 3.60 | 0.354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tastan Eroglu, Z.; Ozkan Sen, D.; Oncu, E. Association of Peri-Implant Keratinized Mucosa Width and Mucosal Thickness with Early Bone Loss: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 1936. https://doi.org/10.3390/jcm13071936
Tastan Eroglu Z, Ozkan Sen D, Oncu E. Association of Peri-Implant Keratinized Mucosa Width and Mucosal Thickness with Early Bone Loss: A Cross-Sectional Study. Journal of Clinical Medicine. 2024; 13(7):1936. https://doi.org/10.3390/jcm13071936
Chicago/Turabian StyleTastan Eroglu, Zeynep, Dilek Ozkan Sen, and Elif Oncu. 2024. "Association of Peri-Implant Keratinized Mucosa Width and Mucosal Thickness with Early Bone Loss: A Cross-Sectional Study" Journal of Clinical Medicine 13, no. 7: 1936. https://doi.org/10.3390/jcm13071936
APA StyleTastan Eroglu, Z., Ozkan Sen, D., & Oncu, E. (2024). Association of Peri-Implant Keratinized Mucosa Width and Mucosal Thickness with Early Bone Loss: A Cross-Sectional Study. Journal of Clinical Medicine, 13(7), 1936. https://doi.org/10.3390/jcm13071936





