Pathophysiology of Postoperative Hearing Disorders after Vestibular Schwannoma Resection: Insights from Auditory Brainstem Response and Otoacoustic Emissions
Abstract
1. Introduction
2. Materials and Methods
- Presence of only wave V: ABRs were considered as impaired but still present.
- Absence of any identifiable wave: ABRs were considered as absent.
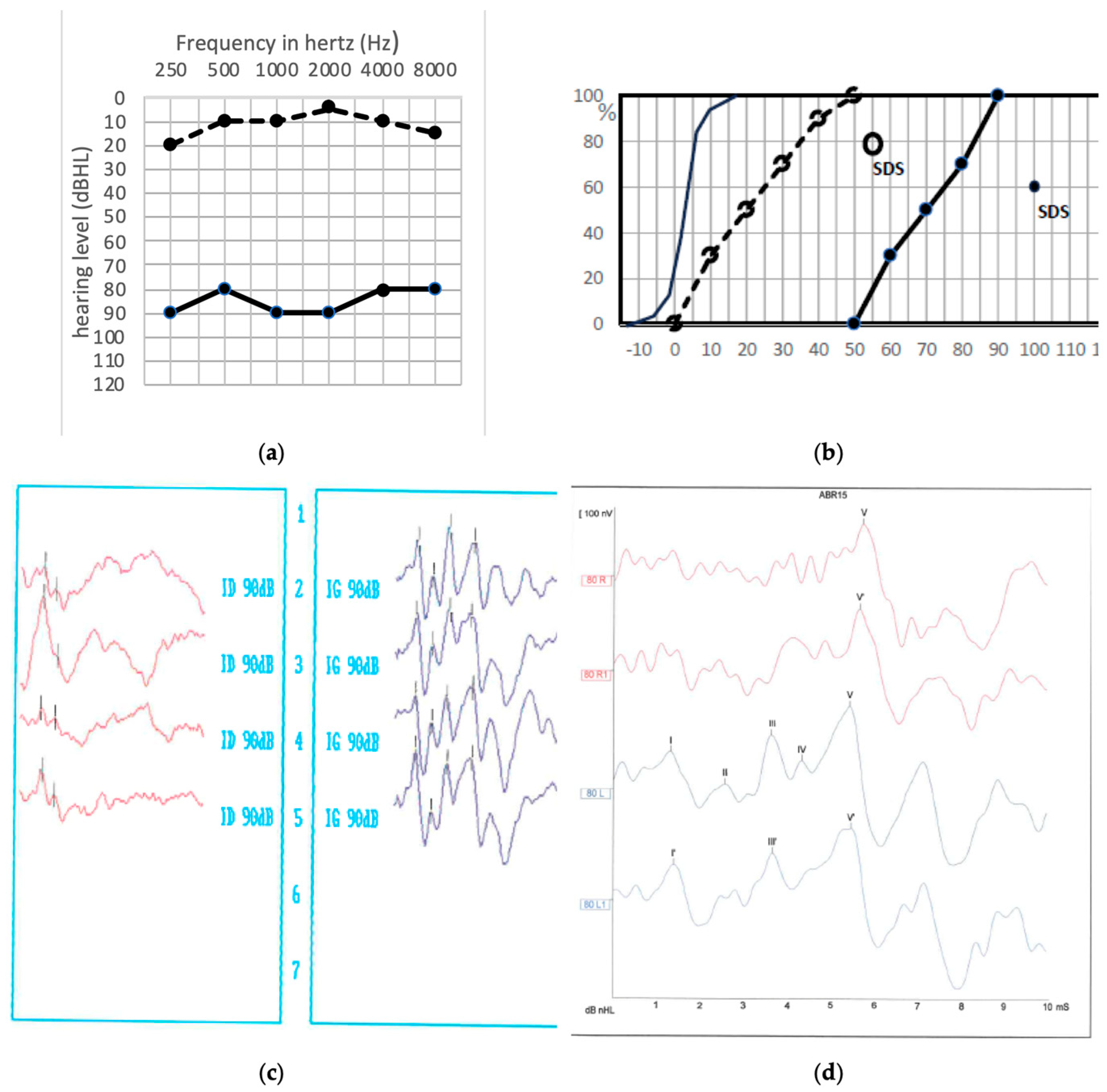
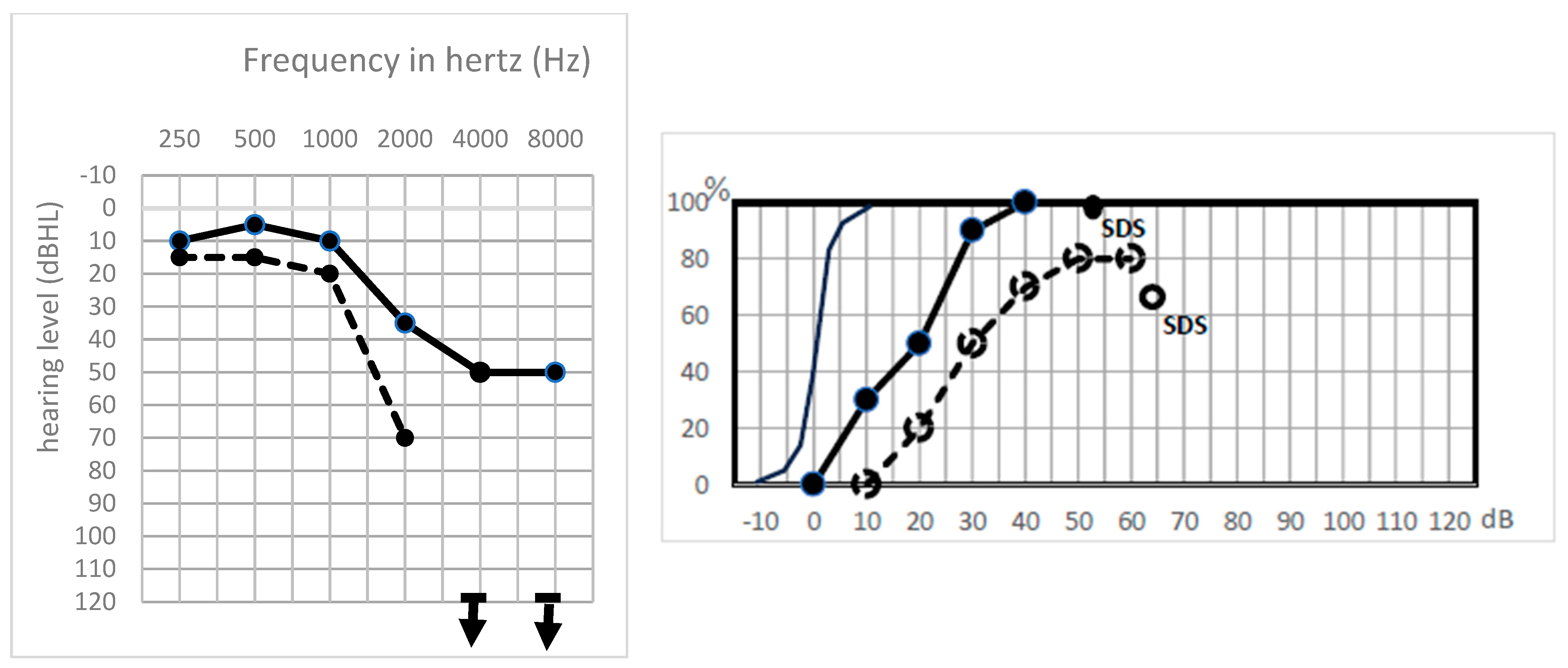
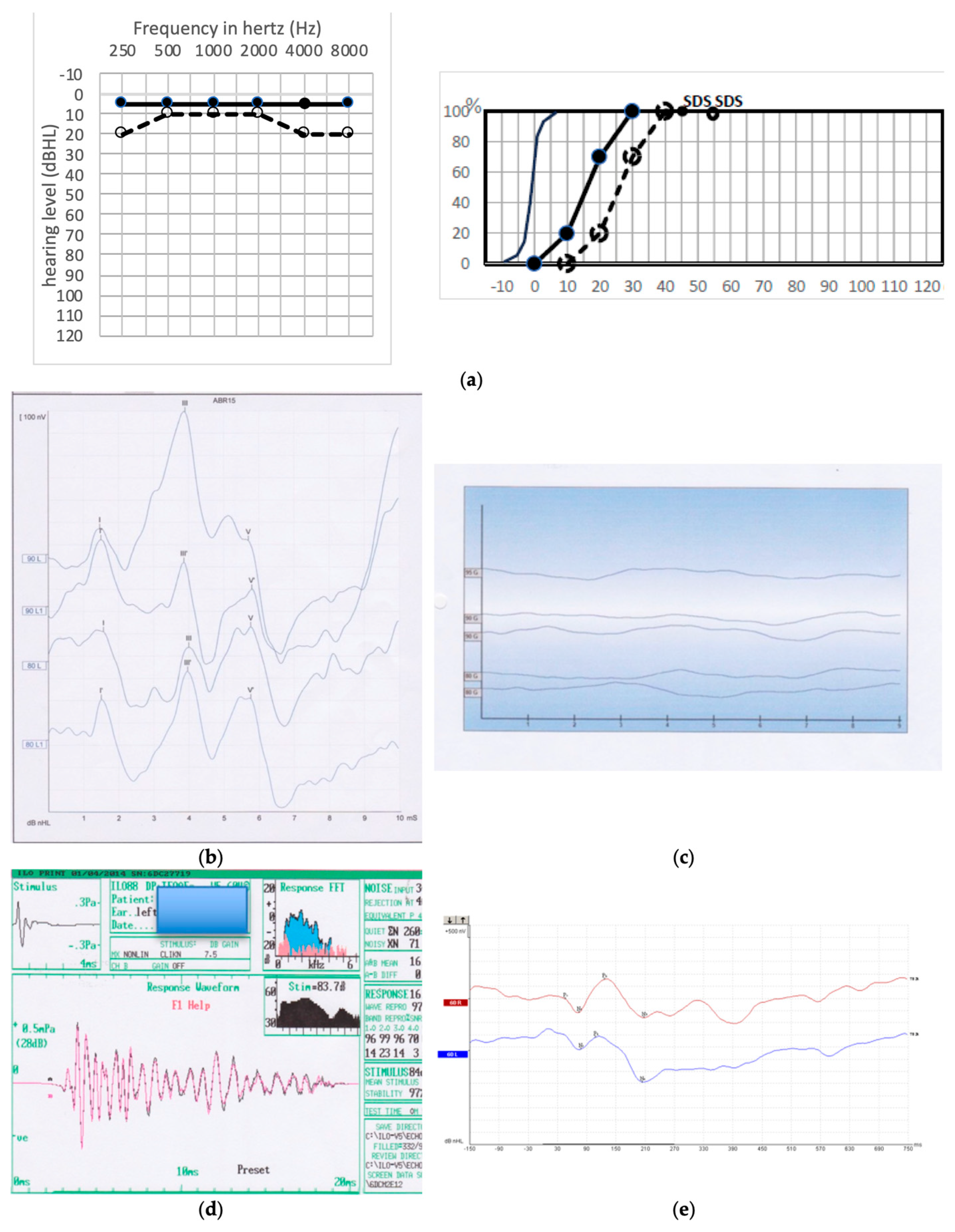
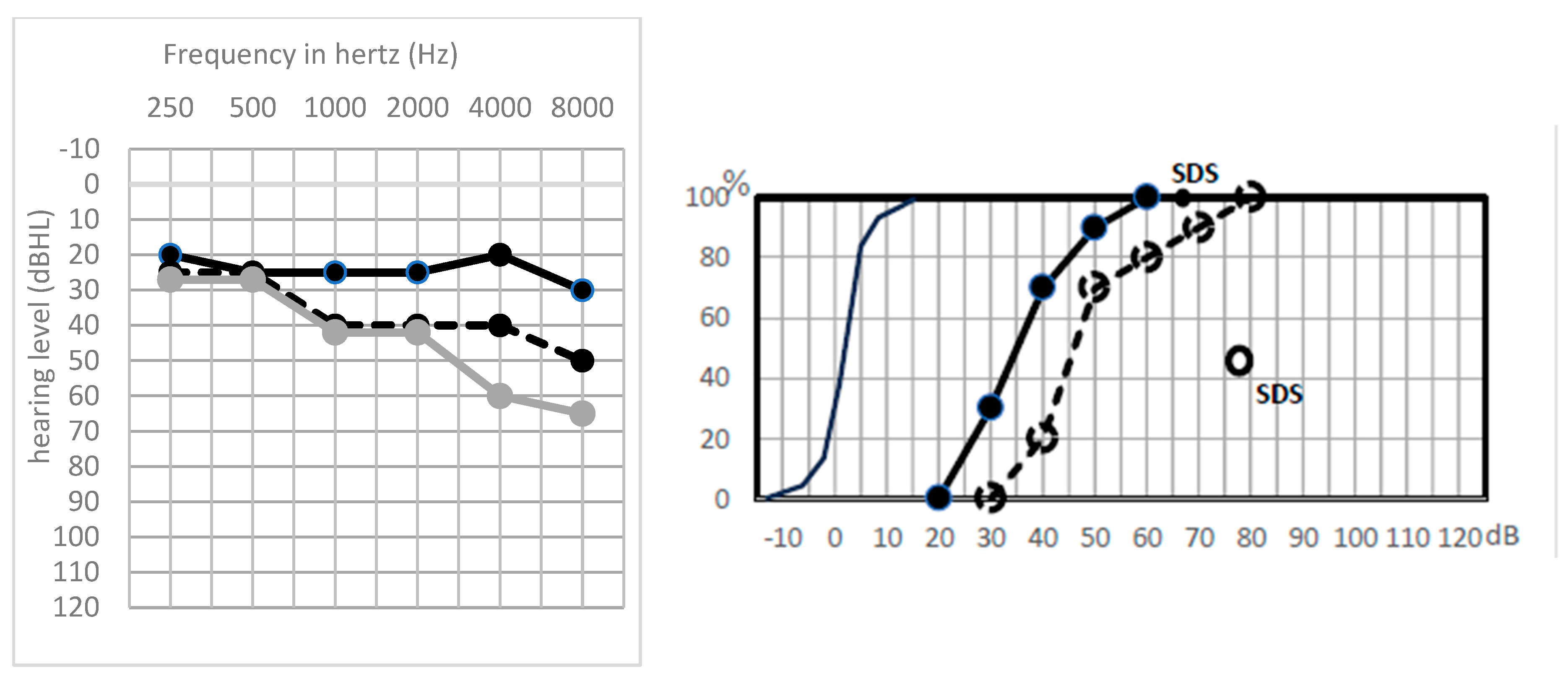
3. Results
Postoperative Auditory Status
- A global preserved (type GP) audiometry with ≤15 dB loss.
- A sharp hearing loss (type Sharp HL) in higher frequencies from 1 kHz or higher frequencies with preserved lower frequencies.
- An altered audiometry, but still with a useful hearing (at least AAO-HNS grade C).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samii, M.; Gerganov, V.; Samii, A. Improved Preservation of Hearing and Facial Nerve Function in Vestibular Schwannoma Surgery via the Retrosigmoid Approach in a Series of 200 Patients. J. Neurosurg. 2006, 105, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Matthies, C.; Samii, M. Management of Vestibular Schwannomas (Acoustic Neuromas): The Value of Neurophysiology for Evaluation and Prediction of Auditory Function in 420 Cases. Neurosurgery 1997, 40, 919–929; discussion 929–930. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, B.; Madero, B.; Tringali, S.; Dubernard, X.; Khalil, T.; Chays, A.; Bazin, A.; Mom, T.; Avan, P. Non-Invasive Intraoperative Monitoring of Cochlear Function by Cochlear Microphonics during Cerebellopontine-Angle Surgery. Eur. Arch. Otorhinolaryngol. 2018, 275, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Mom, T.; Montalban, A.; Khalil, T.; Gabrillargues, J.; Chazal, J.; Gilain, L.; Avan, P. Vasospasm of Labyrinthine Artery in Cerebellopontine Angle Surgery: Evidence Brought by Distortion-Product Otoacoustic Emissions. Eur. Arch. Otorhinolaryngol. 2014, 271, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, D.E.; Owens, R.M.; Friedman, R.A.; Hitselberger, W.E.; De la Cruz, A.; House, J.W.; Nelson, R.A.; Luxford, W.M.; Slattery, W.H.; Fayad, J.N. Prognostic Factors for Hearing Preservation in Vestibular Schwannoma Surgery. Am. J. Otol. 2000, 21, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Slattery, W.H.; Brackmann, D.E.; Hitselberger, W. Middle Fossa Approach for Hearing Preservation with Acoustic Neuromas. Am. J. Otol. 1997, 18, 596–601. [Google Scholar]
- Yates, P.D.; Jackler, R.K.; Satar, B.; Pitts, L.H.; Oghalai, J.S. Is It Worthwhile to Attempt Hearing Preservation in Larger Acoustic Neuromas? Otol. Neurotol. 2003, 24, 460–464. [Google Scholar] [CrossRef]
- Dornhoffer, J.L.; Helms, J.; Hoehmann, D.H. Hearing Preservation in Acoustic Tumor Surgery: Results and Prognostic Factors. Laryngoscope 1995, 105, 184–187. [Google Scholar] [CrossRef]
- Irving, R.M.; Jackler, R.K.; Pitts, L.H. Hearing Preservation in Patients Undergoing Vestibular Schwannoma Surgery: Comparison of Middle Fossa and Retrosigmoid Approaches. J. Neurosurg. 1998, 88, 840–845. [Google Scholar] [CrossRef]
- Stangerup, S.-E.; Caye-Thomasen, P. Epidemiology and Natural History of Vestibular Schwannomas. Otolaryngol. Clin. N. Am. 2012, 45, 257–268. [Google Scholar] [CrossRef]
- Avan, P.; Bonfils, P.; Gilain, L.; Mom, T. Physiopathological Significance of Distortion-Product Otoacoustic Emissions at 2f1-F2 Produced by High- versus Low-Level Stimuli. J. Acoust. Soc. Am. 2003, 113, 430–441. [Google Scholar] [CrossRef]
- Colletti, V.; Fiorino, F.G.; Sacchetto, L. Iatrogenic Impairment of Hearing during Surgery for Acoustic Neuroma. Skull Base Surg. 1996, 6, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sass, H.C.R.; Miyazaki, H.; West, N.; Hansen, S.; Møller, M.N.; Cayé-Thomasen, P. Extended Retrolabyrinthine Approach: Results of Hearing Preservation Surgery Using a New System for Continuous Near Real-Time Neuromonitoring in Patients with Growing Vestibular Schwannomas. Otol. Neurotol. 2019, 40, S72. [Google Scholar] [CrossRef] [PubMed]
- Mom, T.; Telischi, F.F.; Martin, G.K.; Stagner, B.B.; Lonsbury-Martin, B.L. Vasospasm of the Internal Auditory Artery: Significance in Cerebellopontine Angle Surgery. Am. J. Otol. 2000, 21, 735–742. [Google Scholar]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M. Principles of Neural Science, 3rd ed.; Kandel, E.R., Ed.; Elsevier: New York, NY, USA, 1991. [Google Scholar]
- Lang, J. Anatomy of the Brainstem and the Lower Cranial Nerves, Vessels, and Surrounding Structures. Am. J. Otol. 1985, 6, 20. [Google Scholar]
- Haller, S.; Etienne, L.; Kövari, E.; Varoquaux, A.D.; Urbach, H.; Becker, M. Imaging of Neurovascular Compression Syndromes: Trigeminal Neuralgia, Hemifacial Spasm, Vestibular Paroxysmia, and Glossopharyngeal Neuralgia. AJNR Am. J. Neuroradiol. 2016, 37, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Telischi, F.F.; Widick, M.P.; Lonsbury-Martin, B.L.; McCoy, M.J. Monitoring Cochlear Function Intraoperatively Using Distortion Product Otoacoustic Emissions. Am. J. Otol. 1995, 16, 597–608. [Google Scholar] [PubMed]
- Telischi, F.F.; Stagner, B.; Widick, M.P.; Balkany, T.J.; Lonsbury-Martin, B.L. Distortion-Product Otoacoustic Emission Monitoring of Cochlear Blood Flow. Laryngoscope 1998, 108, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Widick, M.P.; Telischi, F.F.; Lonsbury-Martin, B.L.; Stagner, B.B. Early Effects of Cerebellopontine Angle Compression on Rabbit Distortion-Product Otoacoustic Emissions: A Model for Monitoring Cochlear Function during Acoustic Neuroma Surgery. Otolaryngol. Head Neck Surg. 1994, 111, 407–416. [Google Scholar] [CrossRef]
- Ren, T.; Brown, N.J.; Zhang, M.; Nuttall, A.L.; Miller, J.M. A Reversible Ischemia Model in Gerbil Cochlea. Hear. Res. 1995, 92, 30–37. [Google Scholar] [CrossRef]
- Lambert, P.R.; Ruth, R.A. Simultaneous Recording of Noninvasive ECoG and ABR for Use in Intraoperative Monitoring. Otolaryngol. Head Neck Surg. 1988, 98, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Danner, C.; Mastrodimos, B.; Cueva, R.A. A Comparison of Direct Eighth Nerve Monitoring and Auditory Brainstem Response in Hearing Preservation Surgery for Vestibular Schwannoma. Otol. Neurotol. 2004, 25, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Hochet, B.; Daoudi, H.; Lefevre, E.; Nguyen, Y.; Bernat, I.; Sterkers, O.; Lahlou, G.; Kalamarides, M. Monitoring Cochlear Nerve Action Potential for Hearing Preservation in Medium/Large Vestibular Schwannoma Surgery: Tips and Pitfalls. J. Clin. Med. 2023, 12, 6906. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, H.; McDaniel, A.; Norrell, H.; Haberkamp, T. Hearing Preservation after Acoustic Neuroma Surgery with Intraoperative Direct Eighth Cranial Nerve Monitoring: Part II. A Classification of Results. Otolaryngol. Head Neck Surg. 1986, 95 Pt 1, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rosahl, S.K.; Tatagiba, M.; Gharabaghi, A.; Matthies, C.; Samii, M. Acoustic Evoked Response Following Transection of the Eighth Nerve in the Rat. Acta Neurochir. 2000, 142, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Roser, F.; Dormiani, M.; Samii, M.; Matthies, C. Intraoperative Auditory Brainstem Responses in Patients with Cerebellopontine Angle Meningiomas Involving the Inner Auditory Canal: Analysis of the Predictive Value of the Responses. J. Neurosurg. 2005, 102, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, B.; Romstöck, J.; Fahlbusch, R.; Buchfelder, M.; Strauss, C. Intraoperative Brainstem Auditory Evoked Potential Pattern and Perioperative Vasoactive Treatment for Hearing Preservation in Vestibular Schwannoma Surgery. J. Neurol. Neurosurg. Psychiatry 2008, 79, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Neu, M.; Strauss, C.; Romstöck, J.; Bischoff, B.; Fahlbusch, R. The Prognostic Value of Intraoperative BAEP Patterns in Acoustic Neurinoma Surgery. Clin. Neurophysiol. 1999, 110, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.M.; Harner, S.G.; Slavit, D.H.; Litchy, W.J.; Daube, J.R.; Beatty, C.W.; Ebersold, M.J. Effect of BAEP Monitoring on Hearing Preservation during Acoustic Neuroma Resection. Neurology 1992, 42, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Roberson, J.B.; Jackson, L.E.; McAuley, J.R. Acoustic Neuroma Surgery: Absent Auditory Brainstem Response Does Not Contraindicate Attempted Hearing Preservation. Laryngoscope 1999, 109, 904–910. [Google Scholar] [CrossRef]
- Aihara, N.; Murakami, S.; Takahashi, M.; Yamada, K. Preoperative Characteristics of Auditory Brainstem Response in Acoustic Neuroma with Useful Hearing: Importance as a Preliminary Investigation for Intraoperative Monitoring. Neurol. Med. Chir. 2014, 54, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Aihara, N.; Murakami, S.; Watanabe, N.; Takahashi, M.; Inagaki, A.; Tanikawa, M.; Yamada, K. Cochlear Nerve Action Potential Monitoring with the Microdissector in Vestibular Schwannoma Surgery. Skull Base 2009, 19, 325–332. [Google Scholar] [CrossRef][Green Version]
- Kawase, T.; Kanno, A.; Takata, Y.; Nakasato, N.; Kawashima, R.; Kobayashi, T. Positive Auditory Cortical Responses in Patients with Absent Brainstem Response. Clin. Neurophysiol. 2014, 125, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Satya-Murti, S.; Wolpaw, J.R.; Cacace, A.T.; Schaffer, C.A. Late Auditory Evoked Potentials Can Occur without Brain Stem Potentials. Electroencephalogr. Clin. Neurophysiol. 1983, 56, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; McGee, T.; Ferre, J.; Hoeppner, J.A.; Carrell, T.; Sharma, A.; Nicol, T. Mismatch Negativity in the Neurophysiologic/Behavioral Evaluation of Auditory Processing Deficits: A Case Study. Ear Hear. 1993, 14, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Picton, T.W.; Sininger, Y.; Hood, L.J.; Berlin, C.I. Auditory Neuropathy. Brain 1996, 119 Pt 3, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Michalewski, H.J.; Zeng, F.-G.; Fujikawa-Brooks, S.; Linthicum, F.; Kim, C.S.; Winnier, D.; Keats, B. Pathology and Physiology of Auditory Neuropathy with a Novel Mutation in the MPZ Gene (Tyr145->Ser). Brain 2003, 126 Pt 7, 1604–1619. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Isaacson, B.; Michalewski, H.J.; Zeng, F.-G.; Kong, Y.-Y.; Beale, P.; Paulson, G.W.; Keats, B.J.B.; Lesperance, M.M. A Dominantly Inherited Progressive Deafness Affecting Distal Auditory Nerve and Hair Cells. J. Assoc. Res. Otolaryngol. 2004, 5, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Rance, G.; Cone-Wesson, B.; Wunderlich, J.; Dowell, R. Speech Perception and Cortical Event Related Potentials in Children with Auditory Neuropathy. Ear Hear. 2002, 23, 239–253. [Google Scholar] [CrossRef]
- Michalewski, H.J.; Starr, A.; Nguyen, T.T.; Kong, Y.-Y.; Zeng, F.-G. Auditory Temporal Processes in Normal-Hearing Individuals and in Patients with Auditory Neuropathy. Clin. Neurophysiol. 2005, 116, 669–680. [Google Scholar] [CrossRef]
- Takata, Y.; Kawase, T.; Nakasato, N.; Kanno, A.; Kobayashi, T. Auditory Evoked Magnetic Fields in Patients with Absent Brainstem Responses Due to Auditory Neuropathy with Optic Atrophy. Clin. Neurophysiol. 2012, 123, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.; Hood, L.; Rose, K. On Renaming Auditory Neuropathy as Auditory Dys-Synchrony. Audiol. Today 2001, 13, 15–17. [Google Scholar]
- Arnesen, A.R.; Osen, K.K. The Cochlear Nerve in the Cat: Topography, Cochleotopy, and Fiber Spectrum. J. Comp. Neurol. 1978, 178, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Bauch, C.D.; Olsen, W.O. The Effect of 2000–4000 Hz Hearing Sensitivity on ABR Results. Ear Hear. 1986, 7, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Shepard, N.T.; Webster, J.C.; Baumen, M.; Schuck, P. Effect of Hearing Loss of Cochlear Origin on the Auditory Brain Stem Response. Ear Hear. 1992, 13, 173–180. [Google Scholar] [CrossRef]
- Kuk, F.; Keenan, D.; Korhonen, P.; Lau, C.-C. Efficacy of Linear Frequency Transposition on Consonant Identification in Quiet and in Noise. J. Am. Acad. Audiol. 2009, 20, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Giraudet, F.; Charles, P.; Mom, T.; Boespflug-Tanguy, O.; Dürr, A.; Deltenre, P.; Avan, P. Rapid Exhaustion of Auditory Neural Conduction in a Prototypical Mitochondrial Disease, Friedreich Ataxia. Clin. Neurophysiol. 2018, 129, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.I.; Morlet, T.; Hood, L.J. Auditory Neuropathy/Dyssynchrony: Its Diagnosis and Management. Pediatr. Clin. N. Am. 2003, 50, 331–340. [Google Scholar] [CrossRef]
- Starr, A.; McPherson, D.; Patterson, J.; Don, M.; Luxford, W.; Shannon, R.; Sininger, Y.; Tonakawa, L.; Waring, M. Absence of Both Auditory Evoked Potentials and Auditory Percepts Dependent on Timing Cues. Brain 1991, 114 Pt 3, 1157–1180. [Google Scholar] [CrossRef]
- El-Badry, M.M.; McFadden, S.L. Evaluation of Inner Hair Cell and Nerve Fiber Loss as Sufficient Pathologies Underlying Auditory Neuropathy. Hear. Res. 2009, 255, 84–90. [Google Scholar] [CrossRef]
- Morawski, K.; Namyslowski, G.; Lisowska, G.; Bazowski, P.; Kwiek, S.; Telischi, F.F. Intraoperative Monitoring of Cochlear Function Using Distortion Product Otoacoustic Emissions (DPOAEs) in Patients with Cerebellopontine Angle Tumors. Otol. Neurotol. 2004, 25, 818–825. [Google Scholar] [CrossRef]
- Mom, T.; Telischi, F.F.; Martin, G.K.; Lonsbury-Martin, B.L. Measuring the Cochlear Blood Flow and Distortion-Product Otoacoustic Emissions during Reversible Cochlear Ischemia: A Rabbit Model. Hear. Res. 1999, 133, 40–52. [Google Scholar] [CrossRef]
- Stangerup, S.-E.; Thomsen, J.; Tos, M.; Cayé-Thomasen, P. Long-Term Hearing Preservation in Vestibular Schwannoma. Otol. Neurotol. 2010, 31, 271–275. [Google Scholar] [CrossRef]
- Coughlin, A.R.; Willman, T.J.; Gubbels, S.P. Systematic Review of Hearing Preservation After Radiotherapy for Vestibular Schwannoma. Otol. Neurotol. 2018, 39, 273–283. [Google Scholar] [CrossRef]
- Sullivan, C.B.; Sun, D.Q.; Al-Qurayshi, Z.; Bathla, G.; Policeni, B.; Gantz, B.J.; Hansen, M.R. Relationship of a “Fundal Fluid Cap” and Vestibular Schwannoma Volume: Analysis of Preoperative Radiographic Findings and Outcomes. Otol. Neurotol. 2019, 40, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Nadol, J.B.; Diamond, P.F.; Thornton, A.R. Correlation of Hearing Loss and Radiologic Dimensions of Vestibular Schwannomas (Acoustic Neuromas). Am. J. Otol. 1996, 17, 312–316. [Google Scholar] [PubMed]
- Tringali, S.; Ferber-Viart, C.; Fuchsmann, C.; Buiret, G.; Zaouche, S.; Dubreuil, C. Hearing Preservation in Retrosigmoid Approach of Small Vestibular Schwannomas: Prognostic Value of the Degree of Internal Auditory Canal Filling. Otol. Neurotol. 2010, 31, 1469–1472. [Google Scholar] [CrossRef]
- Goddard, J.C.; Schwartz, M.S.; Friedman, R.A. Fundal Fluid as a Predictor of Hearing Preservation in the Middle Cranial Fossa Approach for Vestibular Schwannoma. Otol. Neurotol. 2010, 31, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Mohr, G.; Sade, B.; Dufour, J.-J.; Rappaport, J.M. Preservation of Hearing in Patients Undergoing Microsurgery for Vestibular Schwannoma: Degree of Meatal Filling. J. Neurosurg. 2005, 102, 1–5. [Google Scholar] [CrossRef]
- Dubrulle, F.; Ernst, O.; Vincent, C.; Vaneecloo, F.M.; Lejeune, J.P.; Lemaitre, L. Cochlear Fossa Enhancement at MR Evaluation of Vestibular Schwannoma: Correlation with Success at Hearing-Preservation Surgery. Radiology 2000, 215, 458–462. [Google Scholar] [CrossRef]
- Yamakami, I.; Yoshinori, H.; Saeki, N.; Wada, M.; Oka, N. Hearing Preservation and Intraoperative Auditory Brainstem Response and Cochlear Nerve Compound Action Potential Monitoring in the Removal of Small Acoustic Neurinoma via the Retrosigmoid Approach. J. Neurol. Neurosurg. Psychiatry 2009, 80, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Arts, H.A.; Telian, S.A.; El-Kashlan, H.; Thompson, B.G. Hearing Preservation and Facial Nerve Outcomes in Vestibular Schwannoma Surgery: Results Using the Middle Cranial Fossa Approach. Otol. Neurotol. 2006, 27, 234–241. [Google Scholar] [CrossRef] [PubMed]
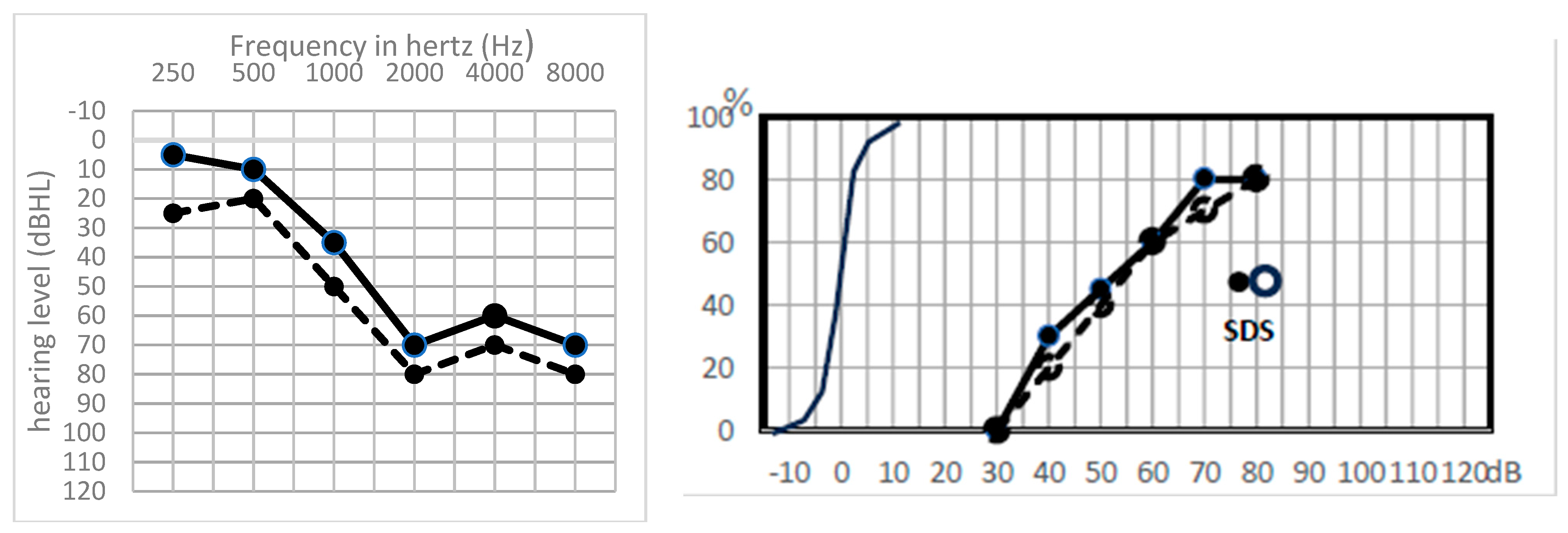
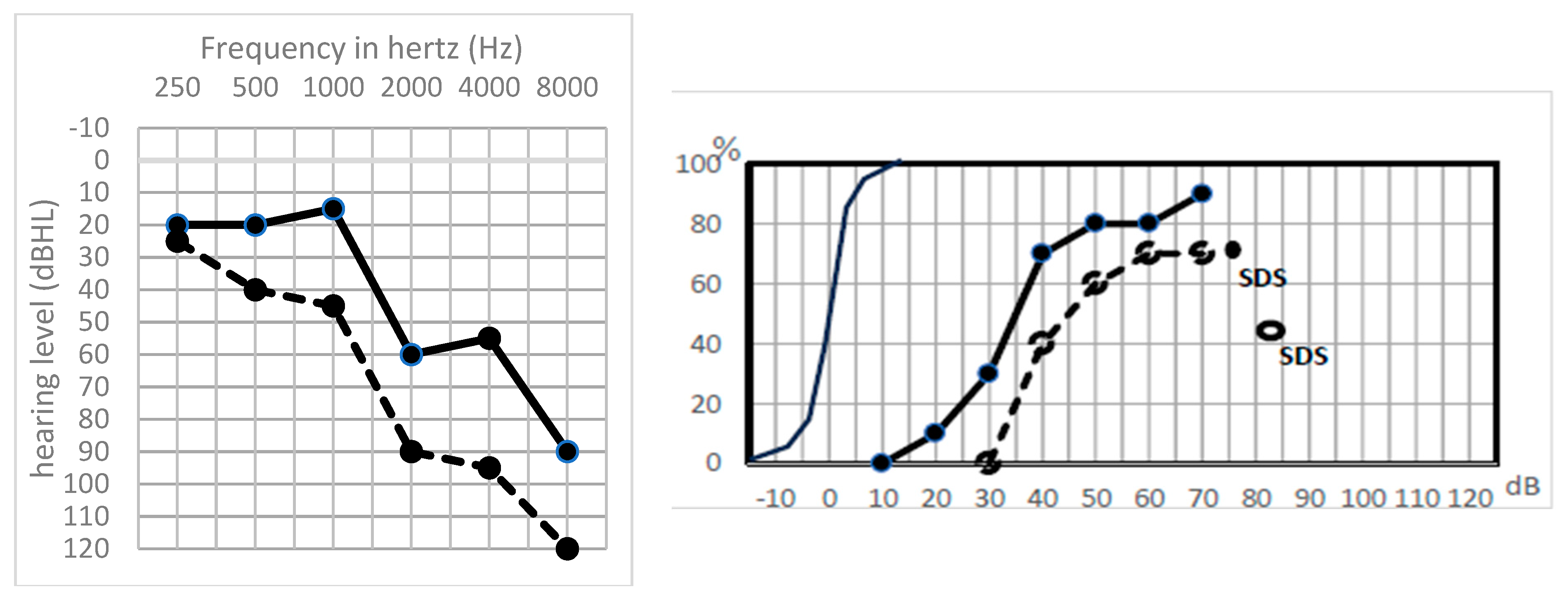
| n = 24 | PTA Type | TOAEs | ABRs | Hypothesis |
|---|---|---|---|---|
| Sharp HL (n = 6) | Total higher hearing frequency loss | Good or absent | Absent | Partial nerve severing |
| GP 1 (n = 2) | Flat preserved audiogram | Good | Absent | “Myelinopathy” |
| (GP 2) (n = 2) | Flat preserved audiogram | Good or absent | Increased latencies with auditory fatigue | “Neuropathy” |
| GP 3 (n = 10) | Flat preserved audiogram | Unchanged | Unchanged | Full preservation |
| GP 4 (n = 4) | Hearing loss in all frequencies | Absent | Good | Partial cochlear ischemia |
| Initial Group | Distant PTA Pattern | Individual Raw PTA Loss (dB) | Individual Initial Postop MaxIS | Individual Late Postop MaxIS |
|---|---|---|---|---|
| Sharp HL n = 4 | Total higher hearing frequency loss extending to the lower frequencies | 45/60/30/ 40 | 100/90/90/ 80 | 65/60/65/ 50 |
| GP 1 n = 2 | Global hearing loss in all frequencies | 40/60 | 100/100 | 50/0 |
| GP 2 n = 2 | unchanged | 15/ 20 | 100/100 | 35/70 |
| GP 3 n = 7 | Unchanged or loss mainly on higher frequencies | 0/0/10/ 60/20/20/20 | 70/100/100/ 100/70/100/100 | 60/10/35/ 0/55/25/35 |
| GP 4 n = 3 | Hearing loss mainly in high frequencies | 40/40/15 | 100/100/80/ 80 | 50/80/35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djennaoui, I.; Puechmaille, M.; Trillat, C.; Bécaud, J.; Saroul, N.; Khalil, T.; Avan, P.; Mom, T. Pathophysiology of Postoperative Hearing Disorders after Vestibular Schwannoma Resection: Insights from Auditory Brainstem Response and Otoacoustic Emissions. J. Clin. Med. 2024, 13, 1927. https://doi.org/10.3390/jcm13071927
Djennaoui I, Puechmaille M, Trillat C, Bécaud J, Saroul N, Khalil T, Avan P, Mom T. Pathophysiology of Postoperative Hearing Disorders after Vestibular Schwannoma Resection: Insights from Auditory Brainstem Response and Otoacoustic Emissions. Journal of Clinical Medicine. 2024; 13(7):1927. https://doi.org/10.3390/jcm13071927
Chicago/Turabian StyleDjennaoui, Idir, Mathilde Puechmaille, Chloé Trillat, Justine Bécaud, Nicolas Saroul, Toufic Khalil, Paul Avan, and Thierry Mom. 2024. "Pathophysiology of Postoperative Hearing Disorders after Vestibular Schwannoma Resection: Insights from Auditory Brainstem Response and Otoacoustic Emissions" Journal of Clinical Medicine 13, no. 7: 1927. https://doi.org/10.3390/jcm13071927
APA StyleDjennaoui, I., Puechmaille, M., Trillat, C., Bécaud, J., Saroul, N., Khalil, T., Avan, P., & Mom, T. (2024). Pathophysiology of Postoperative Hearing Disorders after Vestibular Schwannoma Resection: Insights from Auditory Brainstem Response and Otoacoustic Emissions. Journal of Clinical Medicine, 13(7), 1927. https://doi.org/10.3390/jcm13071927







