Use of a Triaxial Accelerometer to Measure Changes in Gait Sway and Related Motor Function after Corrective Spinal Fusion Surgery for Adult Spinal Deformity
Abstract
1. Introduction
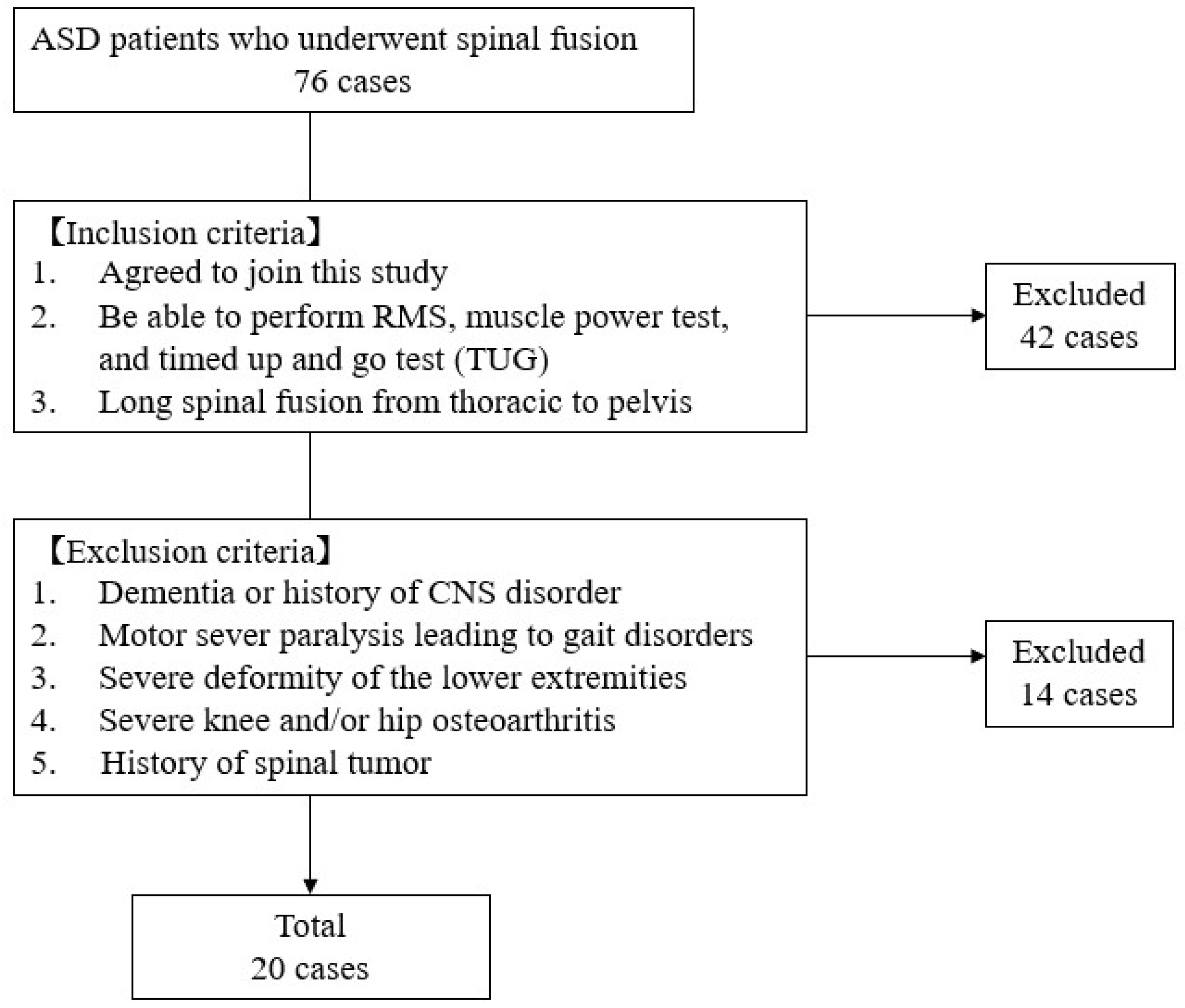
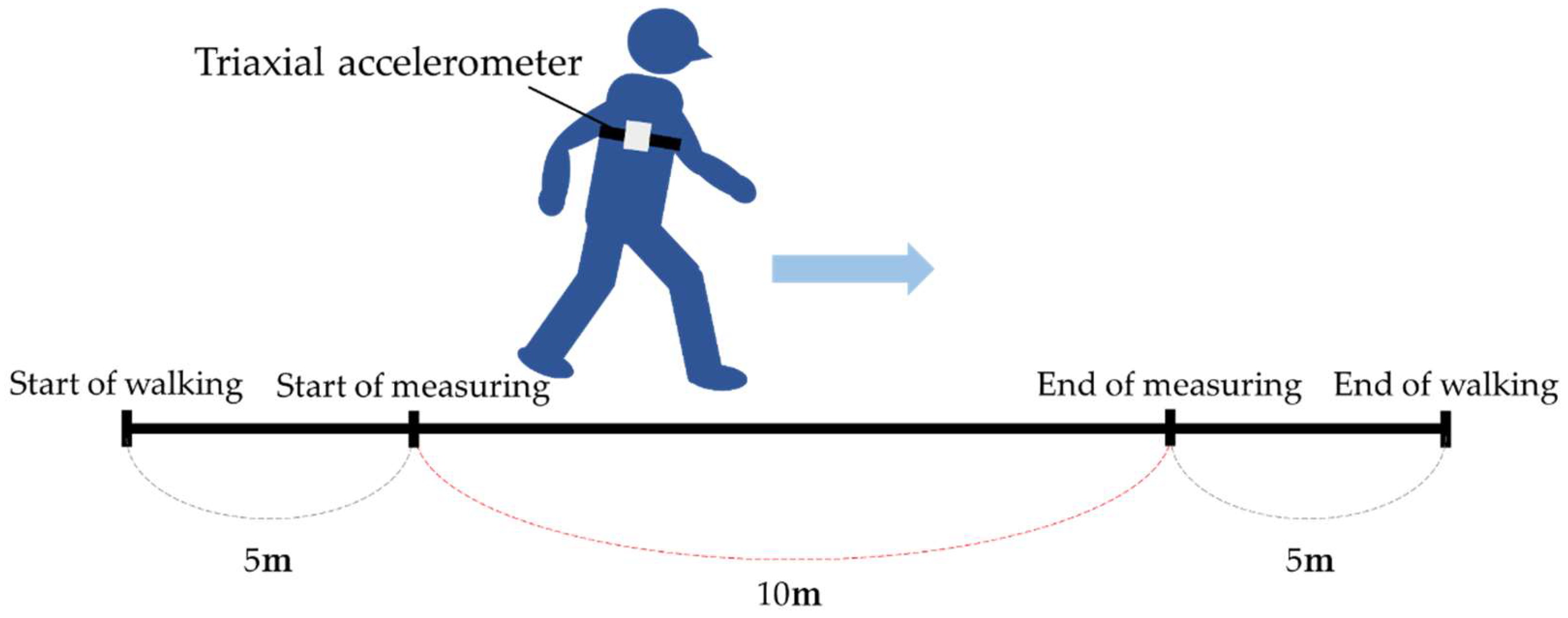
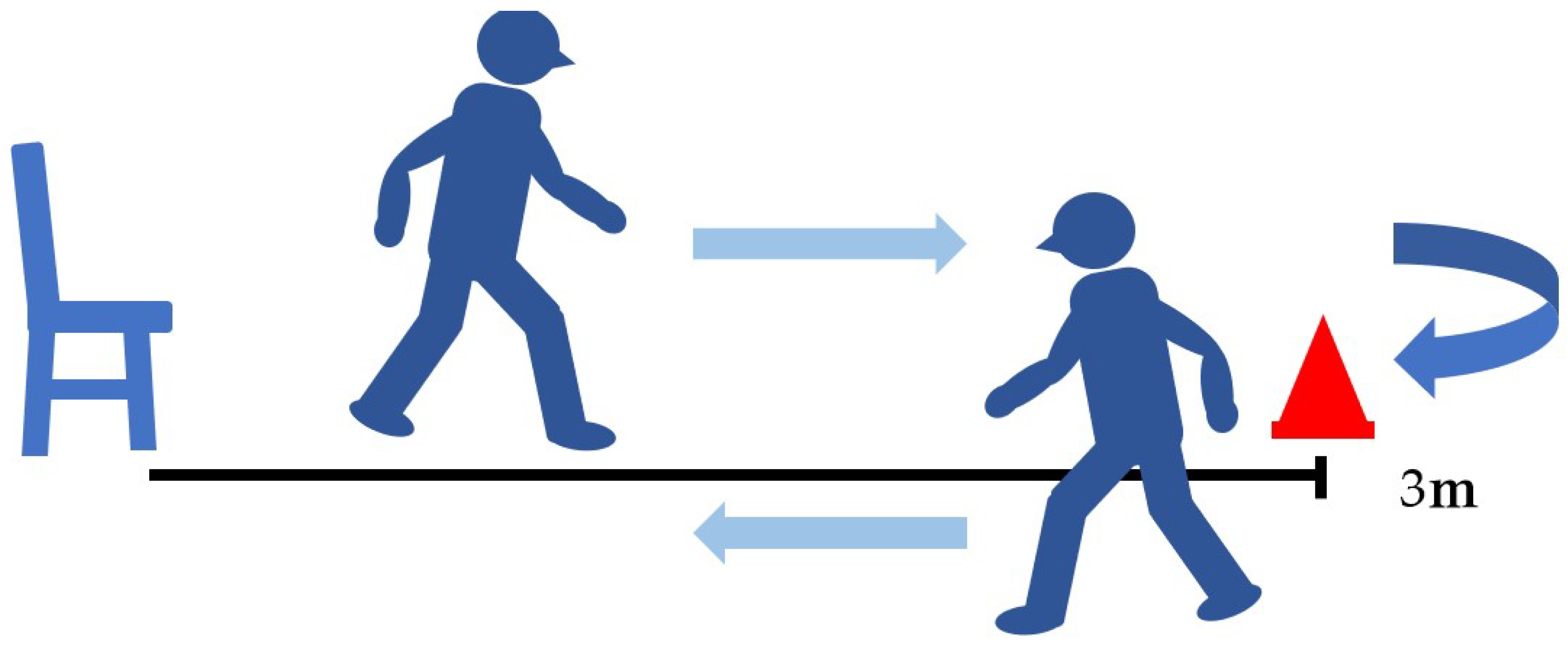
2. Materials and Methods
3. Measurement Outcome
3.1. Patients Demographic
3.2. Gait Analysis
3.3. Muscle Strength
3.4. Timed Up-and-Go Test (TUG)
3.5. Radiographic Measurements
4. Statistical Analysis
5. Results
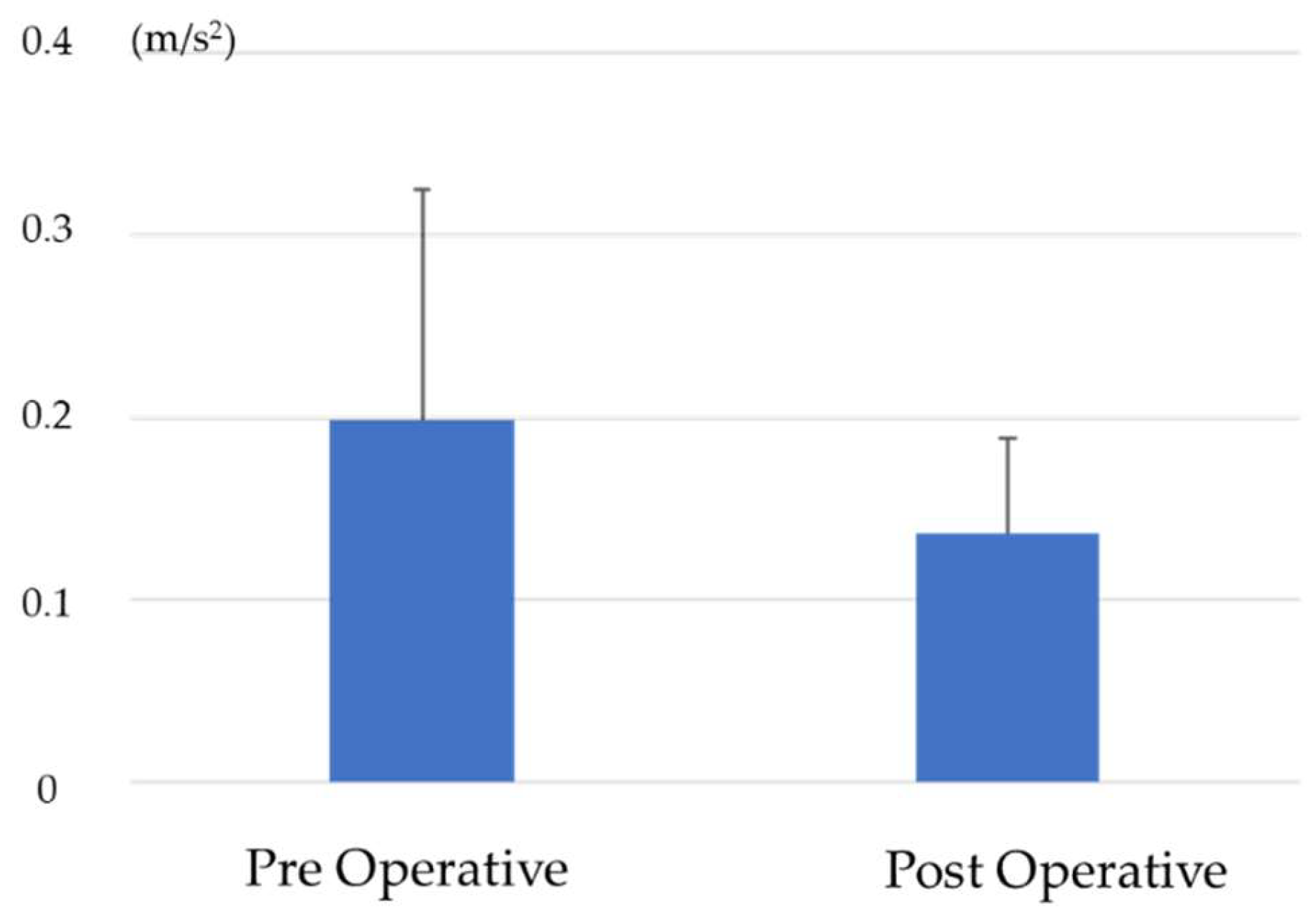
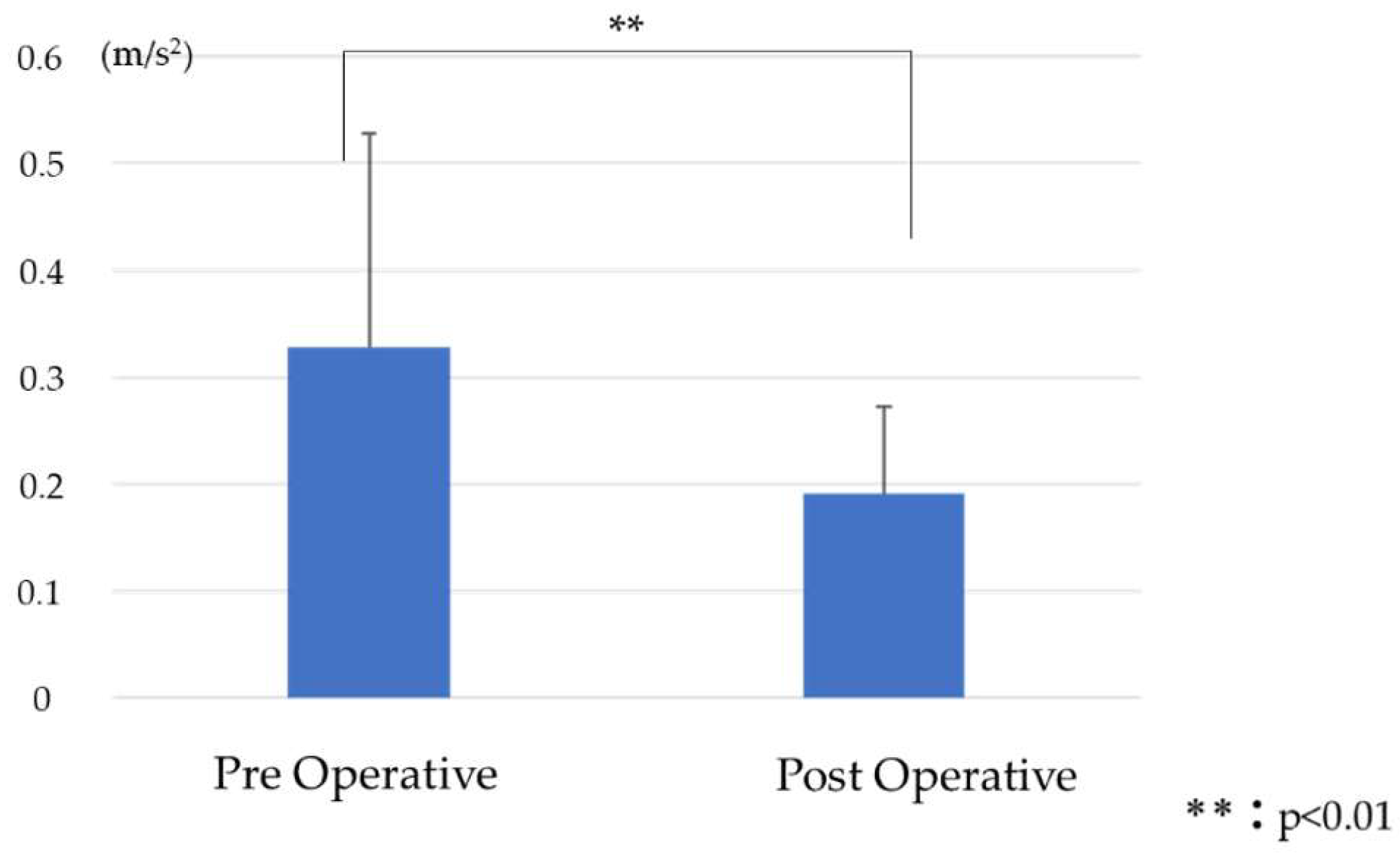
5.1. Comparison of RMS before and after Surgery
5.2. Comparison of Lower Extremity Muscle Strength and Timed Up-and-Go Test before and after Surgery
5.3. Comparison of Spinal Pelvic Parameters before and after Surgery
5.4. Correlation between Postoperative RMS and Improvement in Spinal Pelvic Parameters
5.5. Correlation between Postoperative RMS and Lower Extremity and Timed Up-and-Go Test
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konno, S.; Kikuchi, S.; Nagaosa, Y. The relationship between intramuscular pressure of the paraspinal muscles and low back pain. Spine 1994, 19, 2186–2188. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.D.; Bridwell, K.; Dimar, J.R.; Horton, W.; Berven, S.; Schwab, F. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). Spine 2005, 30, 2024–2029. [Google Scholar] [CrossRef]
- Kondo, R.; Yamato, Y.; Nagafusa, T.; Mizushima, T.; Hasegawa, T.; Kobayashi, S.; Togawa, D.; Oe, S.; Kurosu, K.; Matsuyama, Y. Effect of corrective long spinal fusion to the ilium on physical function in patients with adult spinal deformity. Eur. Spine J. 2017, 26, 2138–2145. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Tanaka, M.; Suthar, H.; Fujiwara, Y.; Uotani, K.; Arataki, S.; Yamauchi, T.; Sugyo, A.; Takamatsu, K.; Yasuda, Y.; et al. Chronological evaluation of gait ability and posture balance after adult spinal deformity surgery. Appl. Sci. 2022, 12, 4285. [Google Scholar] [CrossRef]
- Gottipati, P.; Fatone, S.; Koski, T.; Sugrue, P.A.; Ganju, A. Crouch gait in persons with positive sagittal spine alignment resolves with surgery. Gait Posture 2014, 39, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kadone, H.; Koda, M.; Abe, T.; Funayama, T.; Noguchi, H.; Mataki, K.; Nagashima, K.; Kumagai, H.; Shibao, Y.; et al. Thoracic kyphosis and pelvic anteversion in patients with adult spinal deformity increase while walking: Analyses of dynamic alignment change using a three-dimensional gait motion analysis system. Eur. Spine J. 2020, 29, 840–848. [Google Scholar] [CrossRef]
- Yagi, M.; Ohne, H.; Konomi, T.; Fujiyoshi, K.; Kaneko, S.; Takemitsu, M.; Machida, M.; Yato, Y.; Asazuma, T. Walking balance and compensatory gait mechanisms in surgically treated patients with adult spinal deformity. Spine J. 2017, 17, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Asada, T.; Miura, K.; Koda, M.; Kadone, H.; Funayama, T.; Takahashi, H.; Noguchi, H.; Shibao, Y.; Sato, K.; Eto, F.; et al. Can Proximal Junctional Kyphosis after Surgery for Adult Spinal Deformity Be Predicted by Preoperative Dynamic Sagittal Alignment Change with 3D Gait Analysis? A Case-Control Study. J. Clin. Med. 2022, 11, 5871. [Google Scholar] [CrossRef] [PubMed]
- Ghoussayni, S.; Stevens, C.; Durham, S.; Ewins, D. Assessment and validation of a simple automated method for the detection of gait events and intervals. Gait Posture 2004, 20, 266–272. [Google Scholar] [CrossRef]
- Hreljac, A.; Marshall, R.N. Algorithms to determine event timing during normal walking using kinematic data. J. Biomech. 2000, 33, 783–786. [Google Scholar] [CrossRef]
- Mills, P.M.; Barrett, R.S.; Morrison, S. Agreement between footswitch and ground reaction force techniques for identifying gait events: Inter-session repeatability and the effect of walking speed. Gait Posture 2007, 26, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Rau, P.P. Are cost-effective technologies feasible to measure gait in older adults? A systematic review of evidence-based literature. Arch. Gerontol. Geriatr. 2020, 87, 103970. [Google Scholar] [CrossRef] [PubMed]
- Mizuike, C.; Ohgi, S.; Morita, S. Analysis of stroke patient walking dynamics using a tri-axial accelerometer. Gait Posture 2009, 30, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Tamura, T.; Yoshida, M.; Suda, Y.; Kimura, Y.; Miyoshi, H.; Kijima, Y.; Higashi, Y.; Fujimoto, T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Hulleck, A.A.; Mohan, D.M.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture 2003, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chang, C.L.; Tai, T.W. Incidence and risk factors for hip fracture in elderly patients undergoing lumbar spine surgery: A nationwide database study with 11-year follow-up. Osteoporos. Int. 2018, 29, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Roussouly, P.; Gollogly, S.; Berthonnaud, E.; Dimnet, J. Classification of the normal variation in the sagittal alignment of the lumbar spine and pelvis in the standing position. Spine 2005, 30, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Brown, M. Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination and Performance Testing, 10th ed.; Elsevier: St. Louis, MO, USA, 2019; ISBN 978-0323569149. [Google Scholar]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Suwannarat, P.; Kaewsanmung, S.; Thaweewannakij, T.; Amatachaya, S. The use of functional performance tests by primary health-care providers to determine walking ability with and without awalking device in community-dwelling elderly. Physiother. Theory Pract. 2021, 37, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, C.I.; Berven, S.; Glassman, S.; Hamill, C.; Horton, W.; Ondra, S.; Schwab, F.; Shainline, M.; Fu, K.M.; et al. Improvement of back pain with operative and nonoperative treatment in adults with scoliosis. Neurosurgery 2009, 65, 86–93. [Google Scholar] [CrossRef]
- Miyazaki, J.; Murata, S.; Horie, J.; Uematsu, A.; Hortobágyi, T.; Suzuki, S. Lumbar lordosis angle (LLA) and leg strength predict walking ability in elderly males. Arch. Gerontol. Geriatr. 2013, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Tanaka, M.; Sake, N.; Latka, K.; Fujiwara, Y.; Arataki, S.; Yamauchi, T.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. The Most Significant Factor Affecting Gait and Postural Balance in Patients’ Activities of Daily Living Following Corrective Surgery for Deformity of the Adult Spine. Medicina 2022, 58, 1118. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Meena, U.; Tanaka, M.; Xiang, H.; Fujiwara, Y.; Arataki, S.; Taoka, T.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. Minimal Clinically Important Differences in Gait and Balance Ability in Patients Who Underwent Corrective Long Spinal Fusion for Adult Spinal Deformity. J. Clin. Med. 2023, 12, 6500. [Google Scholar] [CrossRef]
- Wada, O.; Asai, T.; Hiyama, Y.; Nitta, S.; Mizuno, K. Root mean square of lower trunk acceleration during walking in patients with unilateral total hip replacement. Gait Posture 2017, 58, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Asai, T.; Kubo, H.; Fukumoto, Y. Association of fear of falling with acceleration-derived gait indices in older adults with knee osteoarthritis. Aging Clin. Exp. Res. 2019, 31, 645–651. [Google Scholar] [CrossRef]
- Saida, T.; Kawada, M.; Kuroki, D.; Nakai, Y.; Miyazaki, T.; Kiyama, R.; Tsuneyoshi, Y. Accelerometer Measurement of Trunk Lateral Fluctuation During Walking Following Total Knee Arthroplasty in Patients With Osteoarthritis. J. Aging Phys. Act. 2020, 28, 669–674. [Google Scholar] [CrossRef]
- Dubousset, J. Reflections of an orthopaedic surgeon on patient care and research into the condition of scoliosis. J. Pediatr. Orthop. 2011, 31, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Burnfield, J.M. Gait Analysis: Normal and Pathological Function, 2nd ed.; SLACK: Thorofare, NJ, USA, 2010; ISBN 9781556427664. [Google Scholar]
- Kuo, A.D. The six determinants of gait and the inverted pendulum analogy: A dynamic walking perspective. Hum. Mov. Sci. 2007, 26, 617–656. [Google Scholar] [CrossRef] [PubMed]
- Diebo, B.G.; Ferrero, E.; Lafage, R.; Challier, V.; Liabaud, B.; Liu, S.; Vital, J.M.; Errico, T.J.; Schwab, F.J.; Lafage, V. Recruitment of compensatory mechanisms in sagittal spinal malalignment is age and regional deformity dependent: A full-standing axis analysis of key radiographical parameters. Spine 2015, 40, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Sarwahi, V.; Boachie-Adjei, O.; Backus, S.I.; Taira, G. Characterization of gait function in patients with postsurgical sagittal (flatback) deformity: A prospective study of 21 patients. Spine 2002, 27, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.P.; Figueiredo, M.C.; Abreu, S.; Sousa, H.; Machado, L.; Santos, R.; Vilas-Boas, J.P. The influence of gait cadence on the ground reaction forces and plantar pressures during load carriage of young adults. Appl. Ergon. 2015, 49, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Podschun, L.; Church, K.; Fleagle, A.; Hull, P.; Ohree, S.; Springfield, M.; Wood, S. Use of accelerometers in determining risk of falls in individuals post-stroke: A systematic review. Clin. Rehabil. 2023, 37, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Oddsson, L.; Grundström, H.; Thorstensson, A. The role of the psoas and iliacus muscles for stability and movement of the lumbar spine, pelvis and hip. Scand. J. Med. Sci. Sports 1995, 5, 10–16. [Google Scholar] [CrossRef]
- Penning, L. Psoas muscle and lumbar spine stability: A concept uniting existing controversies. Critical review and hypothesis. Eur. Spine J. 2000, 9, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Regev, G.J.; Kim, C.W.; Tomiya, A.; Lee, Y.P.; Ghofrani, H.; Garfin, S.R.; Lieber, R.L.; Ward, S.R. Psoas muscle architectural design, in vivo sarcomere length range, and passive tensile properties support its role as a lumbar spine stabilizer. Spine 2011, 36, E1666–E1674. [Google Scholar] [CrossRef]
- Fors, M.; Enthoven, P.; Abbott, A.; Öberg, B. Effects of pre-surgery physiotherapy on walking ability and lower extremity strength in patients with degenerative lumbar spine disorder: Secondary outcomes of the PREPARE randomised controlled trial. BMC Musculoskelet. Disord. 2019, 20, 468. [Google Scholar] [CrossRef]
- Granacher, U.; Lacroix, A.; Muehlbauer, T.; Roettger, K.; Gollhofer, A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology 2013, 59, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ilves, O.E.; Neva, M.H.; Häkkinen, K.; Dekker, J.; Kraemer, W.J.; Tarnanen, S.; Kyrölä, K.; Ylinen, J.; Piitulainen, K.; Järvenpää, S.; et al. Trunk Muscle Strength After Lumbar Spine Fusion: A 12-Month Follow-up. Neurospine 2019, 16, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Pérennou, D.; Marcelli, C.; Hérisson, C.; Simon, L. Adult lumbar scoliosis. Epidemiologic aspects in a low-back pain population. Spine 1994, 19, 123–128. [Google Scholar] [CrossRef] [PubMed]






| Patients | n = 20 |
|---|---|
| Age at surgery (year) | 71.2 ± 8.9 |
| Sex (male/female) | 0/20 |
| Height (cm) | 149.1 ± 6.9 |
| Weight (kg) | 50.9 ± 9.2 |
| Body Mass Index (kg/m2) | 22.6 ± 3.7 |
| Operation Time (min) | |
| 1st stage (OLIF) | 212.6 ± 48.9 |
| 2nd stage (posterior corrective fusion) | 270.9 ± 41.8 |
| Bleeding (mL) | |
| OLIF bleeding (mL) | 529.2 ± 349.4 |
| Posterior Bleeding (mL) | 808.2 ± 441.6 |
| Type of Posterior fusion (OPEN/MIS) | 8/12 |
| Upper instrumented vertebra (UIV) | T4:1, T10:19 |
| Preoperative | Postoperative | p Value | |
|---|---|---|---|
| HF (Kgf/kg) | 0.16 ± 0.04 | 0.1 ± 0.03 | 0.002 ** |
| KE (Kgf/kg) | 0.31 ± 0.07 | 0.28 ± 0.09 | 0.117 |
| TUG (sec) | 11.6 ± 4.2 | 11.7 ± 2.9 | 0.305 |
| Preoperative | Postoperative | p Value | |
|---|---|---|---|
| SVA (mm) | 121.8 ± 50.3 | 42.8 ± 28.7 | <0.001 ** |
| LL (degree) | 11.7 ± 9.1 | 42.8 ± 6.3 | <0.001 ** |
| PT (degree) | 36.2 ± 7.6 | 25.4 ± 7.4 | <0.001 ** |
| PI (degree) | 55.7 ± 7.9 | 55 ± 6.6 | 0.441 |
| PI-LL (degree) | 46.5 ± 11.8 | 15 ± 8.2 | <0.001 ** |
| Cobb (degree) | 29.4 ± 25.7 | 0.53 ± 2.23 | <0.001 ** |
| CSVL (mm) | 27.5 ± 24.6 | 6.7 ± 13.2 | <0.001 ** |
| SVA-RR | LL-RR | PT-RR | PI-RR | PI-LL-RR | Cobb-RR | CSVL-RR | |
|---|---|---|---|---|---|---|---|
| RMSV | −0.13 | 0.18 | −0.28 | −0.39 | −0.45 | 0.17 | 0.17 |
| RMSAP | −0.47 * | 0.21 | 0.05 | −0.12 | −0.18 | 0.21 | 0.16 |
| RMSML | −0.06 | −0.33 | −0.15 | 0.33 | 0.41 | 0.04 | 0.17 |
| HF | KE | TUG | |
|---|---|---|---|
| RMSV | −0.19 | −0.625 ** | 0.77 ** |
| RMSAP | −0.58 ** | −0.08 | −0.23 |
| RMSML | −0.37 | 0.23 | −0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, T.; Sake, N.; Tanaka, M.; Fujiwara, Y.; Arataki, S.; Taoka, T.; Kodama, Y.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. Use of a Triaxial Accelerometer to Measure Changes in Gait Sway and Related Motor Function after Corrective Spinal Fusion Surgery for Adult Spinal Deformity. J. Clin. Med. 2024, 13, 1923. https://doi.org/10.3390/jcm13071923
Sakaguchi T, Sake N, Tanaka M, Fujiwara Y, Arataki S, Taoka T, Kodama Y, Takamatsu K, Yasuda Y, Nakagawa M, et al. Use of a Triaxial Accelerometer to Measure Changes in Gait Sway and Related Motor Function after Corrective Spinal Fusion Surgery for Adult Spinal Deformity. Journal of Clinical Medicine. 2024; 13(7):1923. https://doi.org/10.3390/jcm13071923
Chicago/Turabian StyleSakaguchi, Tomoyoshi, Naveen Sake, Masato Tanaka, Yoshihiro Fujiwara, Shinya Arataki, Takuya Taoka, Yuya Kodama, Kazuhiko Takamatsu, Yosuke Yasuda, Masami Nakagawa, and et al. 2024. "Use of a Triaxial Accelerometer to Measure Changes in Gait Sway and Related Motor Function after Corrective Spinal Fusion Surgery for Adult Spinal Deformity" Journal of Clinical Medicine 13, no. 7: 1923. https://doi.org/10.3390/jcm13071923
APA StyleSakaguchi, T., Sake, N., Tanaka, M., Fujiwara, Y., Arataki, S., Taoka, T., Kodama, Y., Takamatsu, K., Yasuda, Y., Nakagawa, M., Utsunomiya, K., & Tomiyama, H. (2024). Use of a Triaxial Accelerometer to Measure Changes in Gait Sway and Related Motor Function after Corrective Spinal Fusion Surgery for Adult Spinal Deformity. Journal of Clinical Medicine, 13(7), 1923. https://doi.org/10.3390/jcm13071923








