Rosuvastatin-Based Lipid-Lowering Therapy for the Control of LDL Cholesterol in Patients at High Vascular Risk
Abstract
1. Introduction
2. Current Situation of Lipid Control
2.1. Europe
2.2. Spain
3. Role of Rosuvastatin in the Control of Cholesterol and the Reduction of Vascular Events
3.1. Efficacy
3.2. Safety/Interactions
3.3. Cost-Effectiveness
4. Role of Rosuvastatin-Based Combination Lipid-Lowering Therapy
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- Deaths According to Cause. Statistics National Institute. Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176780&menu=ultiDatos&idp=1254735573175 (accessed on 11 March 2024).
- García González, J.M. Contributions of cardiovascular mortality to Spanish life expectancy from 1980 to 2009. Rev. Esp. Cardiol. 2013, 66, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Grau, M.; O’Flaherty, M.; Ramos, R.; Elosua, R.; Violan-Fors, C.; Quesada, M.; Martí, R.; Sala, J.; Marrugat, J.; et al. Analyzing the coronary heart disease mortality decline in a Mediterranean population: Spain 1988–2005. Rev. Esp. Cardiol. 2011, 64, 988–996. [Google Scholar] [CrossRef]
- Ford, E.S.; Ajani, U.A.; Croft, J.B.; Critchley, J.A.; Labarthe, D.R.; Kottke, T.E.; Giles, W.H.; Capewell, S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N. Engl. J. Med. 2007, 356, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Mulita, F.; Lampropoulos, C.; Kehagias, D.; Verras, G.I.; Tchabashvili, L.; Kaplanis, C.; Liolis, E.; Iliopoulos, F.; Perdikaris, I.; Kehagias, I. Long-term nutritional deficiencies following sleeve gastrectomy: A 6-year single-centre retrospective study. Prz. Menopauzalny 2021, 20, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Verras, G.I.; Mulita, F.; Pouwels, S.; Parmar, C.; Drakos, N.; Bouchagier, K.; Kaplanis, C.; Skroubis, G. Outcomes at 10-Year Follow-Up after Roux-en-Y Gastric Bypass, Biliopancreatic Diversion, and Sleeve Gastrectomy. J. Clin. Med. 2023, 12, 4973. [Google Scholar] [CrossRef] [PubMed]
- Mulita, F.; Leivaditis, V.; Dimopoulos, P.; Ibra, A.; Iliopoulos, F.; Tasios, K.; Pitros, C.; Kaplanis, C.; Peteinaris, A.; Bouchagier, K.; et al. Correlation between gynecological tumors and atherosclerotic diseases. Arch. Med. Sci. Atheroscler. Dis. 2023, 8, e118–e122. [Google Scholar] [CrossRef] [PubMed]
- Pakhare, M.; Anjankar, A. Critical Correlation Between Obesity and Cardiovascular Diseases and Recent Advancements in Obesity. Cureus 2024, 16, e51681. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.J.; Durrington, P.; Hitman, G.A.; Welch, K.M.; et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Hovingh, G.K.; Mora, S.; Arsenault, B.J.; Amarenco, P.; Pedersen, T.R.; LaRosa, J.C.; Waters, D.D.; DeMicco, D.A.; Simes, R.J.; et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: A meta-analysis of statin trials. J. Am. Coll. Cardiol. 2014, 64, 485–494. [Google Scholar] [CrossRef]
- Masana, L.; Girona, J.; Ibarretxe, D.; Rodríguez-Calvo, R.; Rosales, R.; Vallvé, J.C.; Rodríguez-Borjabad, C.; Guardiola, M.; Rodríguez, M.; Guaita-Esteruelas, S.; et al. Clinical and pathophysiological evidence supporting the safety of extremely low LDL levels-The zero-LDL hypothesis. J. Clin. Lipidol. 2018, 12, 292–299. [Google Scholar] [CrossRef]
- de la Sierra, A.; Pintó, X.; Guijarro, C.; Miranda, J.L.; Callejo, D.; Cuervo, J.; Subirà, R.; Rubio, M. Prevalence, Treatment, and Control of Hypercholesterolemia in High Cardiovascular Risk Patients: Evidences from a Systematic Literature Review in Spain. Adv. Ther. 2015, 32, 944–961. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Haq, I.; Bilitou, A.; Manu, M.C.; Burden, A.; Aguiar, C.; Arca, M.; Connolly, D.L.; Eriksson, M.; Ferrières, J.; et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: The multinational observational SANTORINI study. Lancet Reg. Health Eur. 2023, 29, 100624. [Google Scholar] [CrossRef]
- Cosín-Sales, J.; Campuzano Ruiz, R.; Díaz Díaz, J.L.; Escobar Cervantes, C.; Fernández Olmo, M.R.; Gómez-Doblas, J.J.; Mostaza, J.M.; Pedro-Botet, J.; Plana Gil, N.; Valdivielso, P. Dyslipidemia observatory: Treatment of hypercholesterolemia in Spain, context and levers for improvement in clinical practice. Clin. Investig. Arterioscler. 2022, 34, 253–260. [Google Scholar] [CrossRef] [PubMed]
- SNAPSHOT study. Cross-sectional multinational epidemiological study of hypertensive patients with dyslipidemia and patient-reported outcomes. In Proceedings of the 28th SEH-LELHA 2023 Congress, Madrid, Spain, 28–29 September 2023.
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [PubMed]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Béjot, Y.; Cabrejo, L.; Cha, J.K.; Ducrocq, G.; Giroud, M.; et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med. 2020, 382, 9. [Google Scholar] [CrossRef] [PubMed]
- Pallarés-Carratalá, V.; Barrios, V.; Fierro-González, D.; Polo-García, J.; Cinza-Sanjurjo, S. Cardiovascular Risk in Patients with Dyslipidemia and Their Degree of Control as Perceived by Primary Care Physicians in a Survey-TERESA-Opinion Study. Int. J. Environ. Environ. Res. Public Health 2023, 20, 2388. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Danese, M.D.; Kutikova, L.; Catterick, D.; Sorio-Vilela, F.; Gleeson, M.; Kondapally Seshasai, S.R.; Brownrigg, J.; Ray, K.K. Association of a Combined Measure of Adherence and Treatment Intensity with Cardiovascular Outcomes in Patients with Atherosclerosis or Other Cardiovascular Risk Factors Treated with Statins and/or Ezetimibe. JAMA Netw. Open 2018, 1, e185554. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.L.; Sorio-Vilela, F.; Dornstauder, E.; Fraas, U.; Smieszek, T.; Zappacosta, S.; Laufs, U. Non-statin lipid-lowering therapy over time in very-high-risk patients: Effectiveness of fixed-dose statin/ezetimibe compared to separate pill combination on LDL-C. Clin. Res. Cardiol. 2022, 111, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Rosuvastatin and Ezetimibe. Summary of Product Characteristics. Available online: https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA2010-019-001_24082021170348.pdf (accessed on 16 January 2024).
- Barrios, V.; Escobar, C. Fixed-dose combination of rosuvastatin and ezetimibe: Treating hypercholesteremia according to cardiovascular risk. Expert Rev. Clin. Pharmacol. 2021, 14, 793–806. [Google Scholar] [CrossRef]
- Jones, P.H.; Davidson, M.H.; Stein, E.A.; Bays, H.E.; McKenney, J.M.; Miller, E.; Cain, V.A.; Blasetto, J.W. Comparison of the efficacy and safety of rosuvastatin vs. atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 2003, 92, 152–160. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Brandrup-Wognsen, G.; Palmer, M.; Barter, P.J. Meta-analysis of comparative efficacy of increasing dose of atorvastatin vs. rosuvastatin vs. simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am. J. Cardiol. 2010, 105, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, L.; Jia, X.; Pang, X.; Xiang, Q.; Zhao, X.; Ma, L.; Liu, Z.; Hu, K.; Wang, Z.; et al. Comparative Lipid-Lowering/Increasing Efficacy of 7 Statins in Patients with Dyslipidemia, Cardiovascular Diseases, or Diabetes Mellitus: Systematic Review and Network Meta-Analyses of 50 Randomized Controlled Trials. Cardiovasc. Ther. 2020, 2020, 3987065. [Google Scholar] [CrossRef] [PubMed]
- Jaam, M.; Al-Naimi, H.N.; Haddad, M.M.; Abushanab, D.; Al-Badriyeh, D. Comparative efficacy and safety among high-intensity statins. Syst. Rev. Meta-Anal. J. Comp. Eff. Res. 2023, 12, e220163. [Google Scholar] [CrossRef]
- Tse, M.L. Cluster of cases of high-dose rosuvastatin-associated rhabdomyolysis and recent reduction of rosuvastatin dose for Asians in other countries. Hong Kong Med. J. 2023, 29, 474. [Google Scholar] [CrossRef]
- Wu, H.F.; Hristeva, N.; Chang, J.; Liang, X.; Li, R.; Frassetto, L.; Benet, L.Z. Rosuvastatin Pharmacokinetics in Asian and White Subjects Wild Type for Both OATP1B1 and BCRP Under Control and Inhibited Conditions. J. Pharm. Sci. 2017, 106, 2751–2757. [Google Scholar] [CrossRef]
- Brewer, H.B. Benefit-Risk Assessment of Rosuvastatin 10 to 40 Milligrams. Am. J. Cardiol. 2003, 92, 23K–29K. [Google Scholar] [CrossRef]
- Stein, B.; Ward, T.; Hale, G.; Lyver, E. Safety of High-Intensity Statins in the Veteran Population: Atorvastatin 40 to 80 mg Compared with Rosuvastatin 20 to 40 mg. Ann. Pharmacother. 2020, 54, 405–413. [Google Scholar] [CrossRef]
- Cortese, F.; Gesualdo, M.; Cortese, A.; Carbonara, S.; Devito, F.; Zito, A.; Ricci, G.; Scicchitano, P.; Ciccone, M.M. Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol. Res. 2016, 107, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, X.; Lu, M.; Gao, Y. Rosuvastatin Improves Endothelial Dysfunction in Diabetes by Normalizing Endoplasmic Reticulum Stress via Calpain-1 Inhibition. Curr. Pharm. Des. 2023, 29, 2579–2590. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Thanopoulou, K.; Salagianni, M.; Siasos, G.; Oikonomou, E.; Perrea, D.D.; Nirakis, N.; Filis, K.; Tsioufis, K.; Tousoulis, D.; et al. Rosuvastatin Attenuates Progression of Atherosclerosis and Reduces Serum IL6 and CCL2 Levels in Apolipoprotein-E-deficient Mice. In Vivo 2023, 37, 994–1002. [Google Scholar] [CrossRef]
- Joseph, P.; Glynn, R.; Lonn, E.; Ramasundarahettige, C.; Eikelboom, J.; MacFadyen, J.; Ridker, P.; Yusuf, S. Rosuvastatin for the prevention of venous thromboembolism: A pooled analysis of the HOPE-3 and JUPITER randomized controlled trials. Cardiovasc. Res. 2022, 118, 897–903. [Google Scholar] [CrossRef]
- Vavlukis, A.; Vavlukis, M.; Mladenovska, K.; Dimovski, A.; Muñoz-García, N.; de Santisteban Villaplana, V.; Padro, T.; Badimon, L. Antioxidative Effects of Rosuvastatin in Low-to-Moderate Cardiovascular Risk Subjects. Prilozi 2022, 43, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Fonseca, F.A.; Genest, J.; Gotto, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; Nordestgaard, B.G.; et al. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: Justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (JUPITER). Circ. Cardiovasc. Qual. Outcomes 2009, 2, 616–623. [Google Scholar]
- Glynn, R.J.; Koenig, W.; Nordestgaard, B.G.; Shepherd, J.; Ridker, P.M. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann. Intern. Med. 2010, 152, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Glynn, R.J.; MacFadyen, J.G.; Ridker, P.M. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin JUPITER. Circulation 2010, 121, 143–150. [Google Scholar] [CrossRef]
- Ridker, P.M.; Macfadyen, J.G.; Nordestgaard, B.G.; Koenig, W.; Kastelein, J.J.; Genest, J.; Glynn, R.J. Rosuvastatin for primary prevention among individuals with elevated high-sensitivity c-reactive protein and 5% to 10% and 10% to 20% 10-year risk. Implications of the Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial for “intermediate risk”. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 447–452. [Google Scholar]
- Koenig, W.; Ridker, P.M. Rosuvastatin for primary prevention in patients with European systematic coronary risk evaluation risk = 5% or Framingham risk >20%: Post hoc analyses of the JUPITER trial requested by European health authorities. Eur. Heart J. 2011, 32, 75–83. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.; Cressman, M.; Glynn, R.J. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: A secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J. Am. Coll. Cardiol. 2010, 55, 1266–1273. [Google Scholar] [PubMed]
- Yusuf, S.; Bosch, J.; Dagenais, G.; Zhu, J.; Xavier, D.; Liu, L.; Pais, P.; López-Jaramillo, P.; Leiter, L.A.; Dans, A.; et al. HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without Cardiovascular Disease. N. Engl. J. Med. 2016, 374, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, M.B.; Amerena, J.; Bassand, J.P.; Hernández García, H.R.; Miller, S.S.; Sosef, F.F.; Palmer, M.K.; Bryzinski, B.S. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia–Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials 2006, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Nicholls, S.J.; Sipahi, I.; Libby, P.; Raichlen, J.S.; Ballantyne, C.M.; Davignon, J.; Erbel, R.; Fruchart, J.C.; Tardif, J.C.; et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA 2006, 295, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R., 3rd; Raichlen, J.S.; Riley, W.A.; Evans, G.W.; Palmer, M.K.; O’Leary, D.H.; Grobbee, D.E.; Bots, M.L. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: The METEOR Trial. JAMA 2007, 297, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Underhill, H.R.; Yuan, C.; Zhao, X.Q.; Kraiss, L.W.; Parker, D.L.; Saam, T.; Chu, B.; Takaya, N.; Liu, F.; Polissar, N.L.; et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: A high-resolution magnetic resonance imaging trial. Am. Heart J. 2008, 155, 584. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Hiro, T.; Yamagishi, M.; Daida, H.; Hirayama, A.; Saito, S.; Yamaguchi, T.; Matsuzaki, M. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: Multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ. J. 2009, 73, 2110–2117. [Google Scholar] [CrossRef]
- Hall, A.S.; Jackson, B.M.; Farrin, A.J.; Efthymiou, M.; Barth, J.H.; Copeland, J.; Bailey, K.M.; Romaine, S.P.; Balmforth, A.J.; McCormack, T.; et al. A randomized, controlled trial of simvastatin vs. rosuvastatin in patients with acute myocardial infarction: The secondary prevention of acute coronary events–reduction of cholesterol to key European targets trial. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 712–721. [Google Scholar] [CrossRef]
- Lablanche, J.M.; Leone, A.; Merkely, B.; Morais, J.; Alonso, J.; Santini, M.; Eha, J.; Demil, N.; Licour, M.; Tardif, J.C. Comparison of the efficacy of rosuvastatin vs. atorvastatin in reducing apolipoprotein B/apolipoprotein A-1 ratio in patients with acute coronary syndrome: Results of the CENTAURUS study. Arch. Cardiovasc. Dis. 2010, 103, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.M.; Libby, P.; Raichlen, J.S.; Uno, K.; Borgman, M.; Wolski, K.; et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 2011, 365, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Loscalzo, J.; Monyak, J.; Miller, E.; Raichlen, J. Comparison of lipid-modifying efficacy of rosuvastatin vs. atorvastatin in patients with acute coronary syndrome (from the LUNAR study). Am. J. Cardiol. 2012, 109, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Sun, X.; Shen, F.; Hong, B.; Wang, Z. Effects of high-dose rosuvastatin on ventricular remodelling and cardiac function in ST-segment elevation Myocardial infarction. Drug Des. Dev. Ther. 2020, 14, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Colangeli, V.; Borderi, M.; Beci, G.; Esposito, F.; Bon, I.; Re, M.C.; Viale, P. Rosuvastatin decreases serum inflammatory markers and slows atherosclerosis progression rate in treated HIV-infected patients with metabolic syndrome. Infect. Dis. 2021, 53, 81–88. [Google Scholar] [CrossRef]
- Zheng, H.; Li, H.; Wang, Y.; Li, Z.; Hu, B.; Li, X.; Fu, L.; Hu, H.; Nie, Z.; Zhao, B.; et al. Rosuvastatin Slows Progression of Carotid Intima-Media Thickness: The METEOR-China Randomized Controlled Study. Stroke 2022, 53, 3004–3013. [Google Scholar] [CrossRef]
- Rahhal, A.; Khir, F.; Orabi, B.; Chbib, S.; Al-Khalaila, O.; Abdelghani, M.S.; Osman, O.; Ashour, A.A.; Al-Awad, M.; Mahfouz, A.; et al. A Comparative Study of High-intensity Rosuvastatin Vs. Atorvastatin Therapy Post-acute Coronary Syndrome Using Real-world Data. Curr. Probl. Cardiol. 2022, 47, 100956. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hong, S.J.; Kang, W.C.; Hong, B.K.; Lee, J.Y.; Lee, J.B.; Cho, H.J.; Yoon, J.; Lee, S.J.; Ahn, C.M.; et al. Rosuvastatin vs. atorvastatin treatment in adults with coronary artery disease: Secondary analysis of the randomised LODESTAR trial. BMJ 2023, 383, e075837. [Google Scholar] [CrossRef]
- Perez-Calahorra, S.; Laclaustra, M.; Marco-Benedi, V.; Pinto, X.; Sanchez-Hernandez, R.M.; Plana, N.; Ortega, E.; Fuentes, F.; Civeira, F. Comparative efficacy between atorvastatin and rosuvastatin in the prevention of cardiovascular disease recurrence. Lipids Health Dis. 2019, 18, 216. [Google Scholar] [CrossRef]
- Pérez de Isla, L.; Arroyo-Olivares, R.; Muñiz-Grijalvo, O.; Diaz-Díaz, J.L.; Zambón, D.; Fuentes, F.; Sánchez Muñoz-Torrero, J.F.; Mediavilla, J.D.; González-Estrada, A.; Miramontes-González, J.P.; et al. Long-term effect of 2 intensive statin regimens on treatment and incidence of cardiovascular events in familial hypercholesterolemia: The SAFEHEART study. J. Clin. Lipidol. 2019, 13, 989–996. [Google Scholar] [CrossRef]
- Barrios, V.; Escobar, C. Rosuvastatin along the cardiovascular continuum: From JUPITER to AURORA. Expert Rev. Cardiovasc. Ther. 2009, 7, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Escobar, C. Rosuvastatin and diabetes: When the evidences talk. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ward, F.L.; John, R.; Bargman, J.M.; McQuillan, R.F. Renal Tubular Toxicity Associated with Rosuvastatin Therapy. Am. J. Kidney Dis. 2017, 69, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Fácila Rubio, L.; Pintó Sala, X.; Cinza Sanjurjo, S.; García Goñi, M.; Cortés Gil, X.; Martí Ragué, I.; Soler Martinez, M.; Aceituno Mata, S. Cost-effectiveness of rosuvastatin vs. atorvastatin, simvastatin pitavastatin, fluvastatin, pravastatin and lovastatin in the treatment of patients with moderate, high or very high cardiovascular risk in Spain. Rev. Esp. Econ. Salud 2018, 13, 678–691. [Google Scholar]
- Olmo-Quintana, V.; Zamora, B.; Pinto Sala, X.; Mart Rague, I.; Cortes Gil, X.; Gari Peris, C.; Aceituno Mata, S. Cost-effectiveness of the combination rosuvastatin/ezetimibe vs. the combinations of simvastatin and atorvastatin with ezetimibe to reduce the risk of cardiovascular events. Rev. Esp. Econ. Salud 2021, 16, 42–57. [Google Scholar]
- Mata, P.; Cortes, X.; Marti, I.; Saborit Canals, G.; Pomares, E. Analysis of cost-consequence of rosuvastatin vs. atorvastatin in the Spanish setting. Rev. Esp. Econ. Salud 2022, 17, 120–133. [Google Scholar]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Kim, C.H.; An, H.; Kim, S.H.; Shin, D. Pharmacokinetic and pharmacodynamic interaction between ezetimibe and rosuvastatin in healthy male subjects. Drug Des. Dev. Ther. 2017, 11, 3461–3469. [Google Scholar] [CrossRef]
- Rhee, M.Y.; Kim, K.J.; Kim, S.H.; Yoon, Y.W.; Rha, S.W.; Hong, S.J.; Kwak, C.H.; Kim, W.; Nam, C.W.; Park, T.H.; et al. Ezetimibe and rosuvastatin combination treatment can reduce the dose of rosuvastatin without compromising its lipid-lowering efficacy. Clin. Ther. 2019, 41, 2571–2592. [Google Scholar] [CrossRef]
- Escobar, C.; Anguita, M.; Arrarte, V.; Barrios, V.; Cequier, Á.; Cosín-Sales, J.; Egocheaga, I.; López de Sa, E.; Masana, L.; Pallarés, V.; et al. Recommendations to improve lipid control. Consensus document of the Spanish Society of Cardiology. Rev. Esp. Cardiol. 2020, 73, 161–167. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Weiss, R.; Moccetti, T.; Vogt, A.; Eber, B.; Sosef, F.; Duffield, E. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am. J. Cardiol. 2007, 99, 673–680. [Google Scholar] [CrossRef]
- Bays, H.E.; Davidson, M.H.; Massaad, R.; Flaim, D.; Lowe, R.S.; Tershakovec, A.M.; Jones-Burton, C. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg vs. up-titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study). Am. J. Cardiol. 2011, 108, 523–530. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Hoogeveen, R.C.; Raya, J.L.; Cain, V.A.; Palmer, M.K.; Karlson, B.W. Efficacy, safety and effect on biomarkers related to cholesterol and lipoprotein metabolism of rosuvastatin 10 or 20 mg plus ezetimibe 10 mg vs simvastatin 40 or 80 mg plus ezetimibe 10 mg in high-risk patients: Results of the GRAVITY randomized study. Atherosclerosis 2014, 232, 86–93. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, S.H.; Yoon, Y.W.; Rha, S.W.; Hong, S.J.; Kwak, C.H.; Kim, W.; Nam, C.W.; Rhee, M.Y.; Park, T.H.; et al. Effect of fixed-dose combinations of ezetimibe plus rosuvastatin in patients with primary hypercholesterolemia: MRS-ROZE (Multicenter Randomized Study of ROsuvastatin and eZEtimibe). Cardiovasc. Ther. 2016, 34, 371–382. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lee, S.H.; Kim, B.S.; Cho, Y.K.; Cho, H.J.; Cho, K.I.; Kim, S.Y.; Ryu, J.K.; Cho, J.M.; Park, J.I.; et al. Combination therapy of rosuvastatin and ezetimibe in patients with high cardiovascular risk. Clin. Ther. 2017, 39, 107–117. [Google Scholar] [CrossRef]
- Kim, W.; Yoon, Y.E.; Shin, S.H.; Bae, J.W.; Hong, B.K.; Hong, S.J.; Sung, K.C.; Han, S.H.; Kim, W.; Rhee, M.Y.; et al. Efficacy and safety of ezetimibe and rosuvastatin combination therapy vs. those of rosuvastatin monotherapy in patients with primary hypercholesterolemia. Clin. Ther. 2018, 40, 993–1013. [Google Scholar] [CrossRef]
- Hong, S.J.; Jeong, H.S.; Ahn, J.C.; Cha, D.H.; Won, K.H.; Kim, W.; Cho, S.K.; Kim, S.Y.; Yoo, B.S.; Sung, K.C.; et al. A phase III, multicenter, randomized, double-blind, active comparator clinical trial to compare the efficacy and safety of combination therapy with ezetimibe and rosuvastatin vs. rosuvastatin monotherapy in patients with hypercholesterolemia: I-ROSETTE (Ildong rosuvastatin & ezetimibe for hypercholesterolemia) randomized controlled trial. Clin. Ther. 2018, 40, 226–241.e4. [Google Scholar] [PubMed]
- Su, Q.; Liu, Y.; Zhang, G.; Xu, L.; Wang, M.; Mei, S.; Garon, G.; Wu, Y.; Lv, Q.; Ma, C. Efficacy and Safety of Single-Pill Combination of Rosuvastatin and Ezetimibe in Chinese Patients with Primary Hypercholesterolemia Inadequately Controlled by Statin Treatment (ROZEL): A Randomized, Double-Blind, Double Dummy, Active-Controlled Phase 3 Clinical Trial. Adv. Ther. 2023, 40, 5285–5299. [Google Scholar] [PubMed]
- Chilbert, M.R.; VanDuyn, D.; Salah, S.; Clark, C.M.; Ma, Q. Combination Therapy of Ezetimibe and Rosuvastatin for Dyslipidemia: Current Insights. Drug Des. Dev. Ther. 2022, 16, 2177–2186. [Google Scholar] [CrossRef]
- Strilchuk, L.; Tocci, G.; Fogacci, F.; Cicero, A.F.G. An overview of rosuvastatin/ezetimibe association for the treatment of hypercholesterolemia and mixed dyslipidemia. Expert Opin. Pharmacother. 2020, 21, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Karagiannis, A.; Athyros, V.G. Rosuvastatin and ezetimibe for the treatment of dyslipidemia and hypercholesterolemia. Expert Rev. Cardiovasc. Ther. 2021, 19, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Pintó, X.; Escobar, C.; Varona, J.F.; Gámez, J.M. Real-World Attainment of Low-Density Lipoprotein Cholesterol Goals in Patients at High Risk of Cardiovascular Disease Treated with High-Intensity Statins: The TERESA Study. J. Clin. Med. 2023, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Barrios, V.; Cequier, A.; Cosin-Sales, J.; Seijas, J.; Doblas, J.J.G.; Arrarte, V.; Tuñon, J.; Banach, M. Impact of the Spanish consensus for improving lipid control on patients admitted for an acute coronary syndrome. J. Clin. Lipidol. 2023, 17, 756–764. [Google Scholar] [CrossRef] [PubMed]
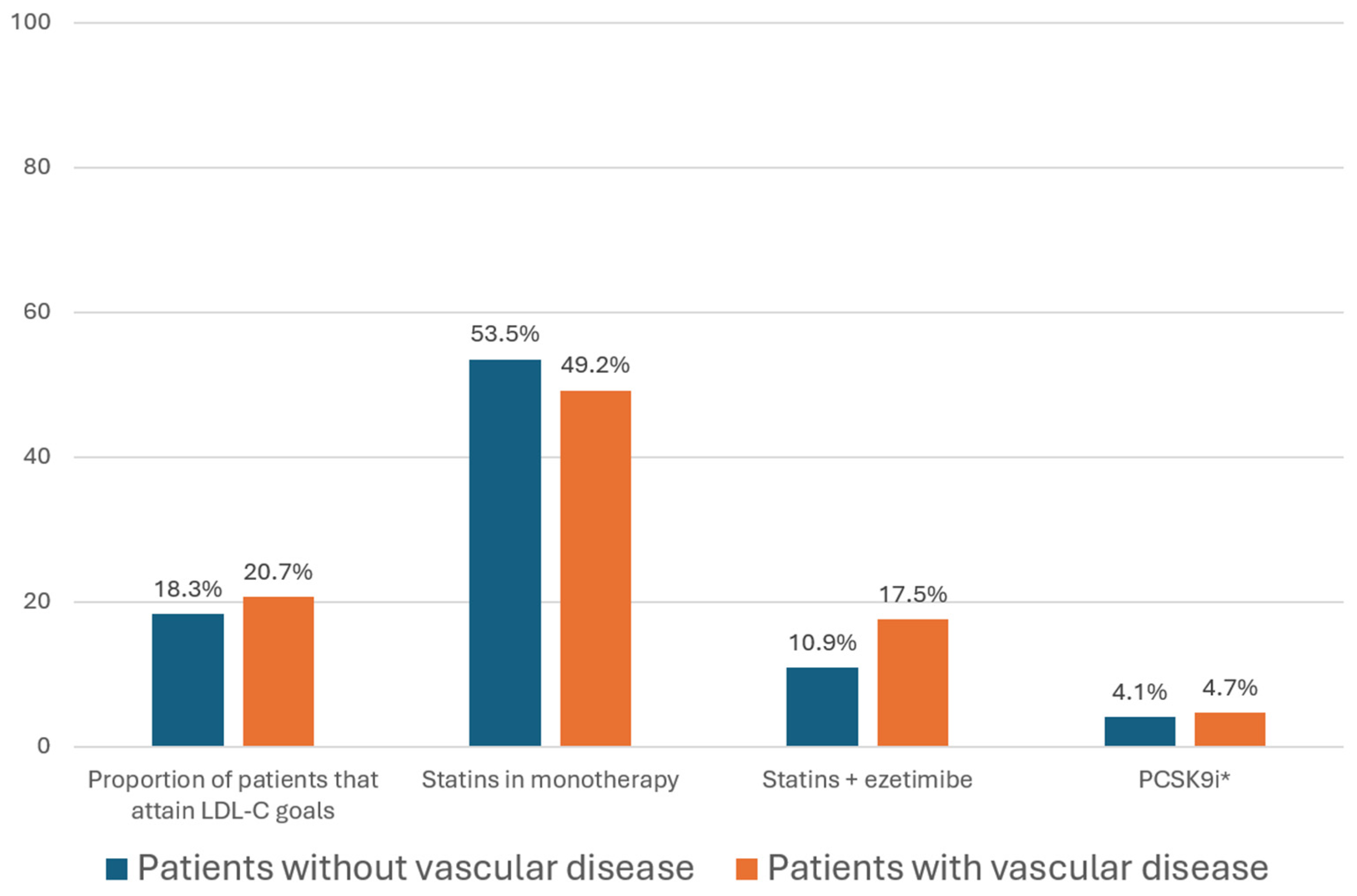
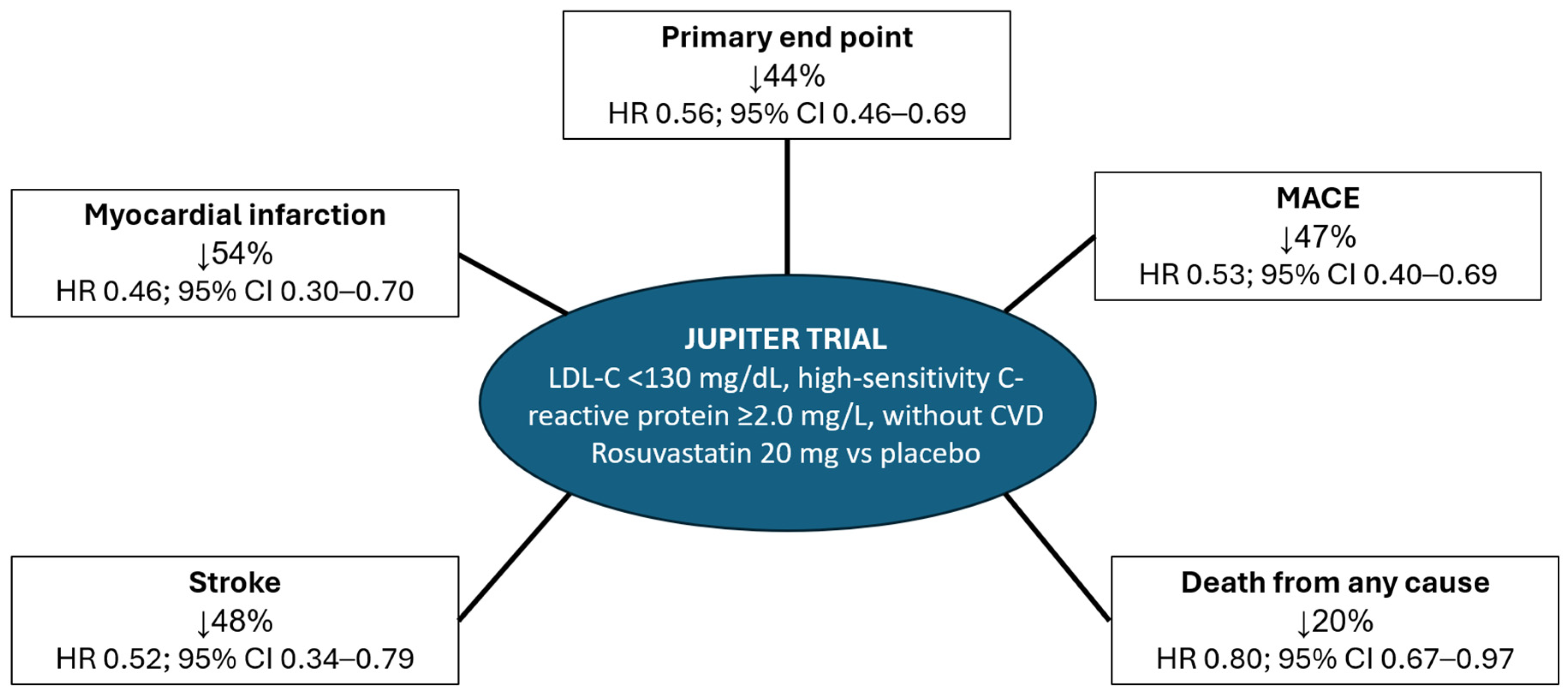
| Treatment | LDL-C Reduction | Cost per Cycle (2018) | Treatment | LDL-C Reduction | Cost per Cycle (2021) |
|---|---|---|---|---|---|
| Rosuvastatin 10 mg | 46% | 87.73€ | Rosu/EZE 10/10 mg | 56.7% | 340.5€ |
| Atorvastatin 40 mg | 49% | 150.78€ | Ator/EZE 40/10 mg | 58.8% | 396.03€ |
| Rosuvastatin 20 mg | 50% | 175.46€ | Rosu/EZE 20/10 mg | 59.8% | 422.23€ |
| Atorvastatin 80 mg | 50% | 306.24€ | Ator/EZE 80/10 mg | 59.9% | 530.94€ |
| Study (Year of Publication) | Treatments | LDL-C Reduction (%) | Proportion of Patients That Attain LDL-C Targets (%) |
|---|---|---|---|
| EXPLORER (2007) [74] | RSV/EZ 40 mg/10mg | −70.0 (p < 0.001) | 94.0 (p < 0.001) |
| RSV 40 mg | −57.0 | 79.1 | |
| GRAVITY (2014) [76] | RSV/EZE 10 mg/10 mg | −59.7 (p < 0.05 vs. SIM/EZE 40/10 mg) | 93.3 (p < 0.05 vs. SIM/EZE 40/10 mg) |
| RSV/EZE 20 mg/10 mg | −63.5 (p < 0.05 vs. SIM/EZE 40–80/10 mg) | 95.6 (p < 0.05 vs. SIM/EZE 40–80/10 mg) | |
| SIM/EZE 40 mg/10 mg | −55.2 | 87.4 | |
| SIM/EZE 80 mg/10 mg | −57.4 | 88.6 | |
| MRS-ROZE (2016) [77] | RSV/EZE 5–20 mg/10 mg | −59.1 (p < 0.001) | 94.1 (p ≤ 0.01) |
| RSV 5–20 mg | −49.4 | 86.3 | |
| ROSE (2017) [78] | RSV/EZE 5–20 mg/10 mg | −59.5 (p < 0.001) | 90.7 (p ≤ 0.01) |
| RSV 5–20 mg | −51.1 | 72.9 | |
| SP-RE-003 (2018) [79] | RSV/EZE 5–20 mg/10 mg | −56.5 (p ≤ 0.01) | 94.2 (p < 0.05) |
| RSV 5–20 mg | −45.2 | 86.6 | |
| I-ROSETTE (2018) [80] | RSV/EZE 5–20 mg/10 mg | −57.0 (p < 0.001) | 92.3 (p < 0.001) |
| RSV 5–20 mg | −44.4 | 79.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostaza, J.M.; Escobar, C. Rosuvastatin-Based Lipid-Lowering Therapy for the Control of LDL Cholesterol in Patients at High Vascular Risk. J. Clin. Med. 2024, 13, 1894. https://doi.org/10.3390/jcm13071894
Mostaza JM, Escobar C. Rosuvastatin-Based Lipid-Lowering Therapy for the Control of LDL Cholesterol in Patients at High Vascular Risk. Journal of Clinical Medicine. 2024; 13(7):1894. https://doi.org/10.3390/jcm13071894
Chicago/Turabian StyleMostaza, Jose María, and Carlos Escobar. 2024. "Rosuvastatin-Based Lipid-Lowering Therapy for the Control of LDL Cholesterol in Patients at High Vascular Risk" Journal of Clinical Medicine 13, no. 7: 1894. https://doi.org/10.3390/jcm13071894
APA StyleMostaza, J. M., & Escobar, C. (2024). Rosuvastatin-Based Lipid-Lowering Therapy for the Control of LDL Cholesterol in Patients at High Vascular Risk. Journal of Clinical Medicine, 13(7), 1894. https://doi.org/10.3390/jcm13071894







