Hormone Receptor-Positive/HER2-Positive Breast Cancer: Hormone Therapy and Anti-HER2 Treatment: An Update on Treatment Strategies
Abstract
1. Introduction
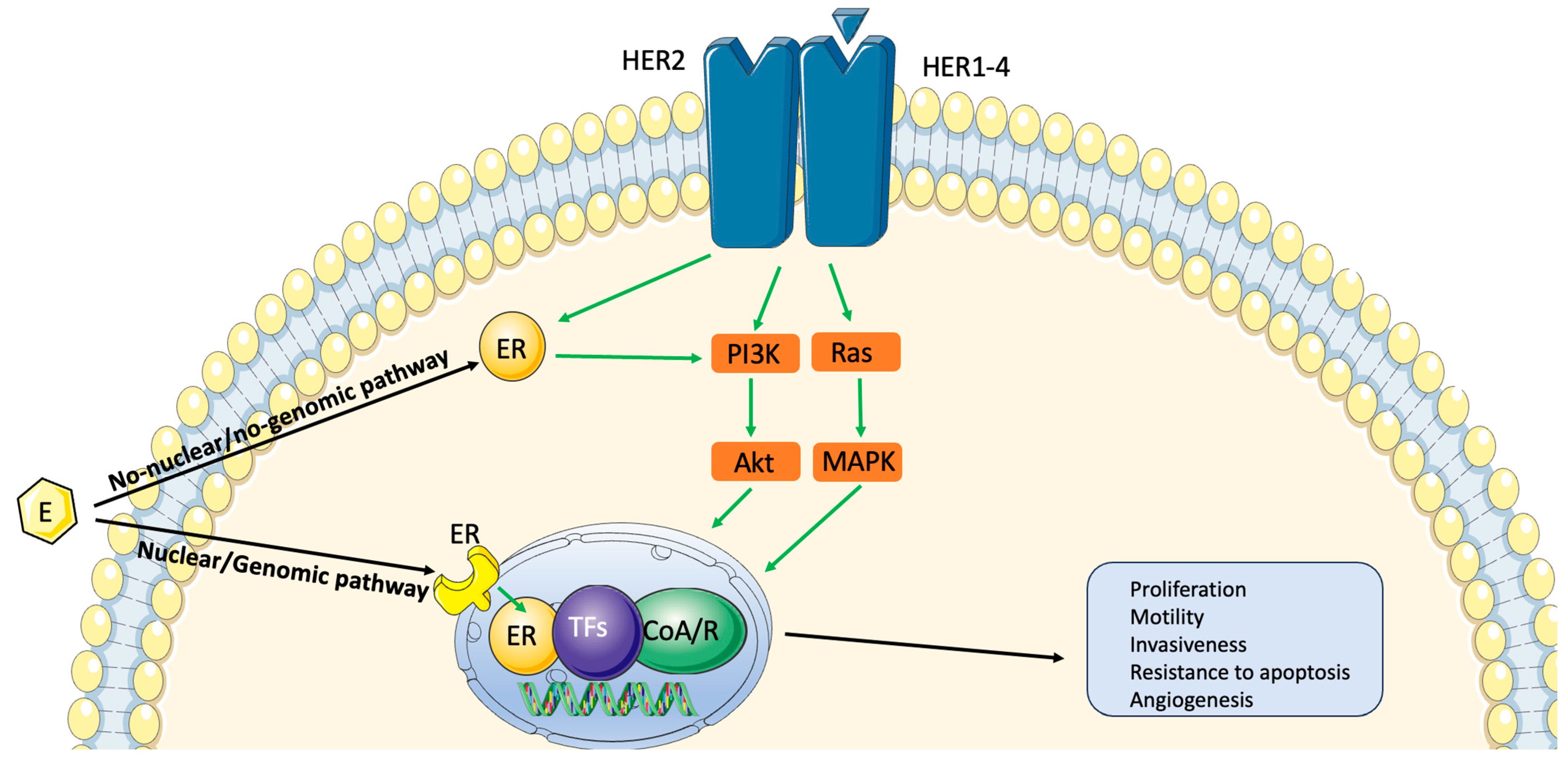
2. Biology and Prognosis of Hormone Receptor-Positive/HER2-Positive Breast Cancer
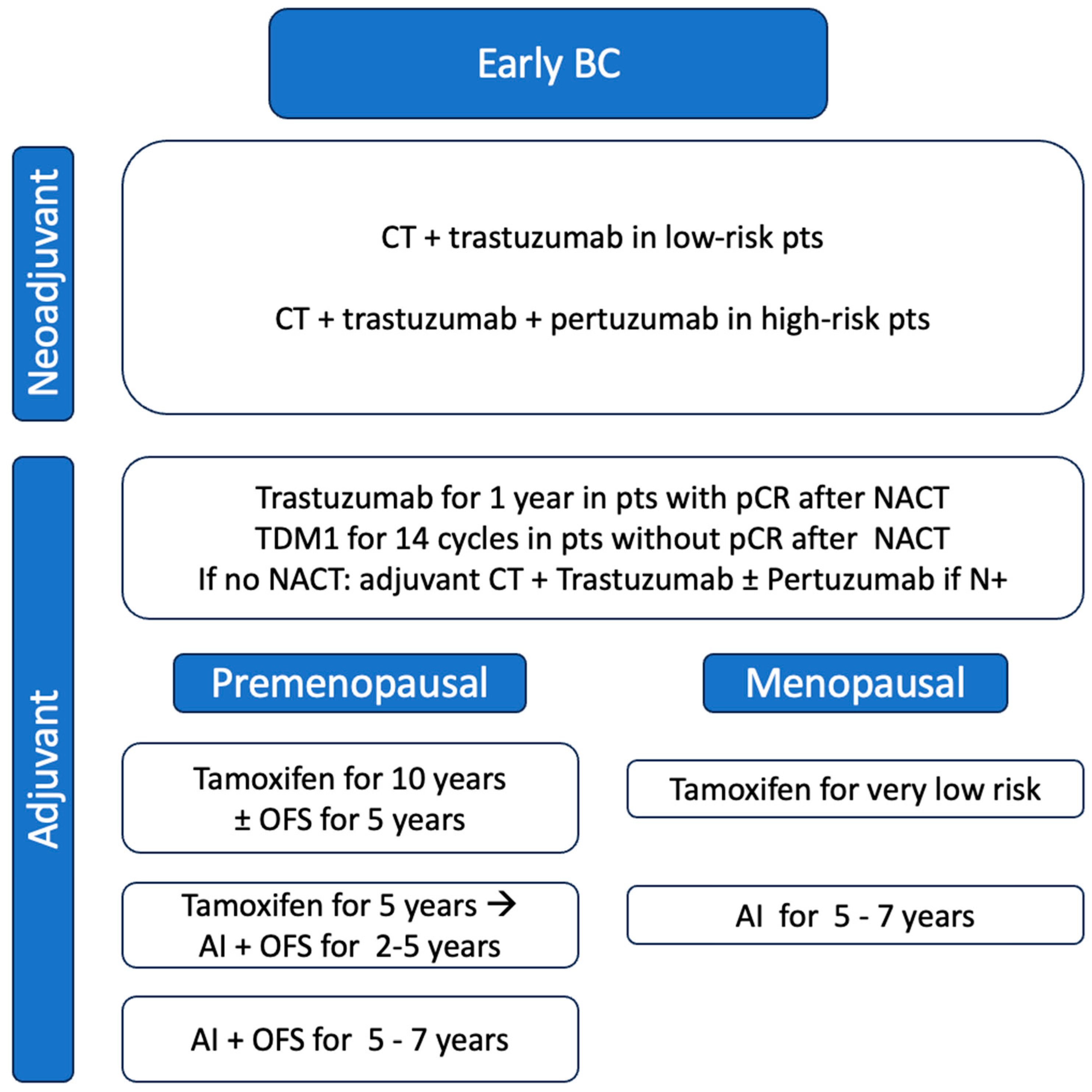
3. Early Stages
3.1. Neoadjuvant Setting
3.2. Adjuvant Setting
4. Metastatic Setting
| Trial | Study Phase | Treatment Line | Study Drugs | End-Points | Ref. |
|---|---|---|---|---|---|
| NCT03913234 | Phase Ib/II | First | Ribociclib+ Letrozole+ Trastuzumab | Primary: PFS Secondary: ORR, OS, QoL | [79] |
| YOUNGBC-22 (NCT05574881) | Phase II | First | Dalpiciclib+ Fulvestrant+ Trastuzumab+ Pertuzumab | Primary: PFS Secondary: ORR, CBR, DOR, OS, AEs | [80] |
| BREAST-Pyrotinib (NCT03910712) | Phase II | First | Aromatase inhibitor+ Trastuzumab +/− Pyrotinib | Primary: PFS Secondary: ORR, DOR, OS, TTR, CBR, QoL, AEs | [81] |
| NCT04088110 | Phase II | First | Trastuzumab+ Aromatase inhibitors+ Pyrotinib | Primary: PFS Secondary: ORR, DOR, OS | [82] |
| FAVOR trial (NCT04337658) | Phase III | First | Trastuzumab+ Pertuzumab + Fulvestrant vs. Capecitabina | Primary: PFS Secondary: CBR, OS, AEs | [83] |
| DETECT V/ CHEVENDO trial (NCT02344472) | Phase III | First | Trastuzumab+ Pertuzumab+ + CT vs. Ribociclib + ET | Primary: AEs Secondary: OS, PFS, ORR, incidence of CNS metastases, QoL, DCR | [77] |
| PATINA trial (NCT02947685) | Phase III | First line after induction therapy | Trastuzumab+ Pertuzuman+ ET +/− Palbociclib | Primary: PFS Secondary: ORR, DOR, OS, CBR, PROs, AEs, incidence of CNS metastases | [78,84] |
| NCT04034589 | Phase II | First line and beyond | Pyrotinib+ Fulvestrant | Primary: PFS Secondary: ORR, CBR, OS, AEs, QoL | [85] |
| NCT03054363 | Phase Ib/II | Second and beyond | Tucatinib+ Letrozole+ Palbociclib | Primary: PFS, AEs Secondary: PK | [86] |
| NCT04646759 | Phase III | Second and beyond | Pyrotinib + Fulvestrant vs. Capecitabine | Primary: PFS, incidence of G3 hand-foot syndrome Secondary: ORR, OS, CBR, QoL, Safety | [87] |
| MonarcHER (NCT02675231) | Phase III | Third line and beyond | Abemaciclib+ Trastuzumab vs. Abemaciclib+ Trastuzumab+ Fulvestrant vs. Trastuzumab+ SoC CT | Primary: PFS Secondary: ORR, DOR, OS, DCR, CBR, QoL | [76,88] |
5. A New Entity: HER2low BC
5.1. Biology of HER2low BC
5.2. Therapeutic Options for HER2low BC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nahleh, Z.A.; Jazieh, A.-R. Multitargeted Therapy in Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor-2–Positive Breast Cancer. Am. J. Clin. Oncol. 2005, 28, 631. [Google Scholar] [CrossRef]
- Female Breast Cancer Subtypes—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 18 June 2023).
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Nahta, R.; O’Regan, R.M. Therapeutic Implications of Estrogen Receptor Signaling in HER2-Positive Breast Cancers. Breast Cancer Res. Treat. 2012, 135, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Debien, V.; de Azambuja, E.; Piccart-Gebhart, M. Optimizing Treatment for HER2-Positive HR-Positive Breast Cancer. Cancer Treat. Rev. 2023, 115, 102529. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, M.; Trivedi, M.V.; Schiff, R. Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications. Breast Care 2013, 8, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L.; Sliwkowski, M.X.; Osborne, C.K.; Perez, E.A.; Puglisi, F.; Gianni, L. Treatment of HER2-Positive Breast Cancer: Current Status and Future Perspectives. Nat. Rev. Clin. Oncol. 2011, 9, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, J.; Liu, D.; Chen, W.; Lin, C.; Andriani, L.; Ding, S.; Huang, O.; He, J.; Chen, X.; et al. Combined Estrogen Receptor and Progesterone Receptor Level Can Predict Survival Outcome in Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer. Clin. Breast Cancer 2022, 22, e147–e156. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.-Y.; Jin, X.; Ma, D.; Xiao, Y.; Shao, Z.-M.; Jiang, Y.-Z. Molecular Portraits and Trastuzumab Responsiveness of Estrogen Receptor-Positive, Progesterone Receptor-Positive, and HER2-Positive Breast Cancer. Theranostics 2019, 9, 4935–4945. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Du, F.; Gao, S.-L.; Si, Y.-R.; Hu, N.-L.; Liu, D.-X.; Wang, X.; Yue, J.; Zheng, F.-C.; Kang, Y.-K.; et al. Combined Analysis of Receptor Expression Reflects Inter-and Intra-Tumor Heterogeneity in HR+/HER2+ Breast Cancer. Breast Cancer Res. Treat. 2022, 194, 221–230. [Google Scholar] [CrossRef]
- Viganò, L.; Locatelli, A.; Ulisse, A.; Galbardi, B.; Dugo, M.; Tosi, D.; Tacchetti, C.; Daniele, T.; Győrffy, B.; Sica, L.; et al. Modulation of the Estrogen/erbB2 Receptors Cross-Talk by CDK4/6 Inhibition Triggers Sustained Senescence in Estrogen Receptor- and ErbB2-Positive Breast Cancer. Clin. Cancer Res. 2022, 28, 2167–2179. [Google Scholar] [CrossRef]
- Brandão, M.; Caparica, R.; Malorni, L.; Prat, A.; Carey, L.A.; Piccart, M. What Is the Real Impact of Estrogen Receptor Status on the Prognosis and Treatment of HER2-Positive Early Breast Cancer? Clin. Cancer Res. 2020, 26, 2783–2788. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Edaily, S.; Iweir, S.; Salam, M.; Saleh, Y.; Sughayer, M.; Salama, O.; Mustafa, R.; Al-Masri, Y.; Bater, R.; et al. Effect of Level of Hormone-Receptor Expression on Treatment Outcomes of “Triple-Positive” Early-Stage Breast Cancer. Breast Cancer Res. Treat. 2021, 185, 459–467. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Gradishar, W.; O’Regan, R.; Gadi, V. Risk of Recurrence in Patients with HER2+ Early-Stage Breast Cancer: Literature Analysis of Patient and Disease Characteristics. Clin. Breast Cancer 2023, 23, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J. Natl. Compr. Canc. Netw. 2023, 21, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of Preoperative Chemotherapy on the Outcome of Women with Operable Breast Cancer. J. Clin. Oncol. 1998, 16, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Davidson, N.E. Primary Systemic Therapy in Operable Breast Cancer. J. Clin. Oncol. 2000, 18, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.S.D.; van der Hage, J.A.; van de Velde, C.J.H. Preoperative Chemotherapy for Women with Operable Breast Cancer. Cochrane Database Syst. Rev. 2007, 2007, CD005002. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Solinas, C.; Ceppi, M.; Lambertini, M.; Scartozzi, M.; Buisseret, L.; Garaud, S.; Fumagalli, D.; de Azambuja, E.; Salgado, R.; Sotiriou, C.; et al. Tumor-Infiltrating Lymphocytes in Patients with HER2-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy plus Trastuzumab, Lapatinib or Their Combination: A Meta-Analysis of Randomized Controlled Trials. Cancer Treat. Rev. 2017, 57, 8–15. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Manikhas, A.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Vazquez, F.; Byakhow, M.; Lichinitser, M.; et al. Neoadjuvant Chemotherapy with Trastuzumab Followed by Adjuvant Trastuzumab versus Neoadjuvant Chemotherapy Alone, in Patients with HER2-Positive Locally Advanced Breast Cancer (the NOAH Trial): A Randomised Controlled Superiority Trial with a Parallel HER2-Negative Cohort. Lancet 2010, 375, 377–384. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Moliterni, A.; Vazquez, F.; Byakhov, M.J.; Lichinitser, M.; et al. Neoadjuvant and Adjuvant Trastuzumab in Patients with HER2-Positive Locally Advanced Breast Cancer (NOAH): Follow-up of a Randomised Controlled Superiority Trial with a Parallel HER2-Negative Cohort. Lancet Oncol. 2014, 15, 640–647. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Huober, J.; Holmes, E.; Baselga, J.; de Azambuja, E.; Untch, M.; Fumagalli, D.; Sarp, S.; Lang, I.; Smith, I.; Boyle, F.; et al. Survival Outcomes of the NeoALTTO Study (BIG 1-06): Updated Results of a Randomised Multicenter Phase III Neoadjuvant Clinical Trial in Patients with HER2-Positive Primary Breast Cancer. Eur. J. Cancer 2019, 118, 169–177. [Google Scholar] [CrossRef]
- Harbeck, N.; Gluz, O.; Christgen, M.; Kates, R.E.; Braun, M.; Küemmel, S.; Schumacher, C.; Potenberg, J.; Kraemer, S.; Kleine-Tebbe, A.; et al. De-Escalation Strategies in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Early Breast Cancer (BC): Final Analysis of the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early BC HER2- and Hormone Receptor-Positive Phase II Randomized Trial-Efficacy, Safety, and Predictive Markers for 12 Weeks of Neoadjuvant Trastuzumab Emtansine with or without Endocrine Therapy (ET) Versus Trastuzumab Plus ET. J. Clin. Oncol. 2017, 35, 3046–3054. [Google Scholar] [CrossRef]
- Zeidman, M.; Schmidt, H.; Alberty-Oller, J.J.; Pisapati, K.V.; Ahn, S.; Mazumdar, M.; Ru, M.; Moshier, E.; Port, E. Trends in Neoadjuvant Chemotherapy versus Surgery-First in Stage I HER2-Positive Breast Cancer Patients in the National Cancer DataBase (NCDB). Breast Cancer Res. Treat. 2021, 187, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Bisagni, G.; Colleoni, M.; Del Mastro, L.; Zamagni, C.; Mansutti, M.; Zambetti, M.; Frassoldati, A.; De Fato, R.; Valagussa, P.; et al. Neoadjuvant Treatment with Trastuzumab and Pertuzumab plus Palbociclib and Fulvestrant in HER2-Positive, ER-Positive Breast Cancer (NA-PHER2): An Exploratory, Open-Label, Phase 2 Study. Lancet Oncol. 2018, 19, 249–256. [Google Scholar] [CrossRef]
- Biganzoli, L.; Brain, E.; Malorni, L.; Risi, E.; Regan, M.M. Phase II Randomized Trial of Neoadjuvant Trastuzumab and Pertuzumab (TP) with Either Palbociclib + Letrozole (Pal+L) or Paclitaxel (Pac) for Elderly Patients with Estrogen Receptor & HER2 Positive (ER+/HER2+) Breast Cancer (BC) (International Breast Cancer Study Group IBCSG 55-17, TOUCH). Ann. Oncol. 2019, 30, v96. [Google Scholar] [CrossRef]
- MedSIR Chemotherapy-Free pCR-Guided Strategy with Subcutaneous Trastuzumab-Pertuzumab and T-DM1 in HER2-Positive Early Breast Cancer (PHERGAIN-2); clinicaltrials.gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04733118 (accessed on 15 November 2023).
- Xue, J.; Niu, N.; Wang, S.; Chen, G.; Qiu, F.; Zhao, Y.; Xing, F.; Zheng, X.; Tu, W.; Li, K.; et al. Neoadjuvant Pyrotinib plus Trastuzumab, Dalpiciclib, and Letrozole for Triple-Positive Breast Cancer: A Pilot Trial. JCO 2023, 41, e12603. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Sledge, G.; Geyer, C.E.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. JCO 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 Years’ Follow-up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Piccart, M.; Procter, M.; Fumagalli, D.; de Azambuja, E.; Clark, E.; Ewer, M.S.; Restuccia, E.; Jerusalem, G.; Dent, S.; Reaby, L.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years’ Follow-Up. JCO 2021, 39, 1448–1457. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.; Holmes, E.; Baselga, J.; de Azambuja, E.; Dueck, A.C.; Viale, G.; Zujewski, J.A.; Goldhirsch, A.; Armour, A.; Pritchard, K.I.; et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J. Clin. Oncol. 2016, 34, 1034–1042. [Google Scholar] [CrossRef]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after Trastuzumab-Based Adjuvant Therapy in Patients with HER2-Positive Breast Cancer (ExteNET): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after Trastuzumab-Based Adjuvant Therapy in HER2-Positive Breast Cancer (ExteNET): 5-Year Analysis of a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Gao, S.-L.; Wang, D.-Y.; Wang, X.; Zhang, B.; Du, F.; Ju, J.; Yue, J.; Kang, Y.-K.; Wang, X.; Xu, B.-H.; et al. Prognostic Factors and Adjuvant Systemic Therapy for Patients with HER2-Positive T1N0 Breast Cancer: Evidence from a Real-World Study with Long-Term Follow-Up. Breast Cancer Res. Treat. 2023, 197, 569–582. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ji, J.; Tian, M.; Esteva, F.J.; Hortobagyi, G.N.; Yeung, S.-C.J. Long-Term Survival Analysis of Adjuvant Chemotherapy with or without Trastuzumab in Patients with T1, Node-Negative HER2-Positive Breast Cancer. Clin. Cancer Res. 2019, 25, 7388–7395. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; et al. Relevance of Breast Cancer Hormone Receptors and Other Factors to the Efficacy of Adjuvant Tamoxifen: Patient-Level Meta-Analysis of Randomised Trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase Inhibitors versus Tamoxifen in Early Breast Cancer: Patient-Level Meta-Analysis of the Randomised Trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Kim, H.A.; Ahn, S.H.; Nam, S.J.; Park, S.; Ro, J.; Im, S.A.; Jung, Y.S.; Yoon, J.H.; Hur, M.H.; Choi, Y.J.; et al. The Role of the Addition of Ovarian Suppression to Tamoxifen in Young Women with Hormone-Sensitive Breast Cancer Who Remain Premenopausal or Regain Menstruation after Chemotherapy (ASTRRA): Study Protocol for a Randomized Controlled Trial and Progress. BMC Cancer 2016, 16, 319. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Wang, M.; Zhao, F.; Fetting, J.H.; Cella, D.; Wagner, L.I.; Martino, S.; Ingle, J.N.; Sparano, J.A.; Solin, L.J.; et al. Phase III Comparison of Tamoxifen versus Tamoxifen plus Ovarian Function Suppression in Premenopausal Women with Node-Negative, Hormone Receptor-Positive Breast Cancer (E-3193, INT-0142): A Trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2014, 32, 3948–3958. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase Inhibitors versus Tamoxifen in Premenopausal Women with Oestrogen Receptor-Positive Early-Stage Breast Cancer Treated with Ovarian Suppression: A Patient-Level Meta-Analysis of 7030 Women from Four Randomised Trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Peleg Hasson, S.; Brezis, M.R.; Shachar, E.; Shachar, S.S.; Wolf, I.; Sonnenblick, A. Adjuvant Endocrine Therapy in HER2-Positive Breast Cancer Patients: Systematic Review and Meta-Analysis. ESMO Open 2021, 6, 100088. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Bisagni, G.; Bartolini, S.; Frassoldati, A.; Vicini, R.; Balduzzi, S.; D’amico, R.; Conte, P.; Guarneri, V. Type of Adjuvant Endocrine Therapy and Disease-Free Survival in Patients with Early HR-Positive/HER2-Positive BC: Analysis from the Phase III Randomized ShortHER Trial. npj Breast Cancer 2023, 9, 6. [Google Scholar] [CrossRef]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Gray, R.G.; Rea, D.; Handley, K.; Bowden, S.J.; Perry, P.; Earl, H.M.; Poole, C.J.; Bates, T.; Chetiyawardana, S.; Dewar, J.A.; et al. aTTom: Long-Term Effects of Continuing Adjuvant Tamoxifen to 10 Years versus Stopping at 5 Years in 6,953 Women with Early Breast Cancer. JCO 2013, 31, 5. [Google Scholar] [CrossRef]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-Term Effects of Continuing Adjuvant Tamoxifen to 10 Years versus Stopping at 5 Years after Diagnosis of Oestrogen Receptor-Positive Breast Cancer: ATLAS, a Randomised Trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Gnant, M.; Harbeck, N.S. Gallen/Vienna 2023: Optimization of Treatment for Patients with Primary Breast Cancer—A Brief Summary of the Consensus Discussion. Breast Care 2023, 18, 213–222. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing Local and Systemic Therapies for Women with Early Breast Cancer: The St. Gallen International Consensus Guidelines for Treatment of Early Breast Cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Buono, G.; Arpino, G.; Del Mastro, L.; Fabi, A.; Generali, D.; Puglisi, F.; Zambelli, A.; Cinieri, S.; Nuzzo, F.; Di Lauro, V.; et al. Extended Adjuvant Endocrine Treatment for Premenopausal Women: A Delphi Approach to Guide Clinical Practice. Front. Oncol. 2022, 12, 1032166. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, K.C.; Henry, N.L. Adjuvant Endocrine Therapy in Premenopausal Women with Breast Cancer. Clin. Adv. Hematol. Oncol. 2015, 13, 663–672. [Google Scholar]
- Goss, P.E.; Ingle, J.N.; Pritchard, K.I.; Robert, N.J.; Muss, H.; Gralow, J.; Gelmon, K.; Whelan, T.; Strasser-Weippl, K.; Rubin, S.; et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N. Engl. J. Med. 2016, 375, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA): End-of-Study Results from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Miles, D.; Ciruelos, E.; Schneeweiss, A.; Puglisi, F.; Peretz-Yablonski, T.; Campone, M.; Bondarenko, I.; Nowecki, Z.; Errihani, H.; Paluch-Shimon, S.; et al. Final Results from the PERUSE Study of First-Line Pertuzumab plus Trastuzumab plus a Taxane for HER2-Positive Locally Recurrent or Metastatic Breast Cancer, with a Multivariable Approach to Guide Prognostication. Ann. Oncol. 2021, 32, 1245–1255. [Google Scholar] [CrossRef]
- Schwarzberg, L.S.; Franco, S.X.; Florance, A.; O’Rourke, L.; Maltzman, J.; Johnston, S. Lapatinib plus Letrozole as First-Line Therapy for HER-2+ Hormone Receptor–Positive Metastatic Breast Cancer. Oncologist 2010, 15, 327. [Google Scholar] [CrossRef][Green Version]
- Kaufman, B.; Mackey, J.R.; Clemens, M.R.; Bapsy, P.P.; Vaid, A.; Wardley, A.; Tjulandin, S.; Jahn, M.; Lehle, M.; Feyereislova, A.; et al. Trastuzumab plus Anastrozole versus Anastrozole Alone for the Treatment of Postmenopausal Women with Human Epidermal Growth Factor Receptor 2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: Results from the Randomized Phase III TAnDEM Study. J. Clin. Oncol. 2009, 27, 5529–5537. [Google Scholar] [CrossRef]
- Huober, J.; Fasching, P.A.; Barsoum, M.; Petruzelka, L.; Wallwiener, D.; Thomssen, C.; Reimer, T.; Paepke, S.; Azim, H.A.; Ragosch, V.; et al. Higher Efficacy of Letrozole in Combination with Trastuzumab Compared to Letrozole Monotherapy as First-Line Treatment in Patients with HER2-Positive, Hormone-Receptor-Positive Metastatic Breast Cancer—Results of the eLEcTRA Trial. Breast 2012, 21, 27–33. [Google Scholar] [CrossRef]
- Hua, X.; Bi, X.-W.; Zhao, J.-L.; Shi, Y.-X.; Lin, Y.; Wu, Z.-Y.; Zhang, Y.-Q.; Zhang, L.-H.; Zhang, A.-Q.; Huang, H.; et al. Trastuzumab Plus Endocrine Therapy or Chemotherapy as First-Line Treatment for Patients with Hormone Receptor-Positive and HER2-Positive Metastatic Breast Cancer (SYSUCC-002). Clin. Cancer Res. 2022, 28, 637–645. [Google Scholar] [CrossRef]
- Johnston, S.; Basik, M.; Hegg, R.; Lausoontornsiri, W.; Grzeda, L.; Clemons, M.; Dreosti, L.; Mann, H.; Stuart, M.; Cristofanilli, M. Inhibition of EGFR, HER2, and HER3 Signaling with AZD8931 in Combination with Anastrozole as an Anticancer Approach: Phase II Randomized Study in Women with Endocrine-Therapy-Naïve Advanced Breast Cancer. Breast Cancer Res. Treat. 2016, 160, 91–99. [Google Scholar] [CrossRef]
- Watanabe, S.; Yonesaka, K.; Tanizaki, J.; Nonagase, Y.; Takegawa, N.; Haratani, K.; Kawakami, H.; Hayashi, H.; Takeda, M.; Tsurutani, J.; et al. Targeting of the HER2/HER3 Signaling Axis Overcomes Ligand-Mediated Resistance to Trastuzumab in HER2-Positive Breast Cancer. Cancer Med. 2019, 8, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Faratian, D.; Zweemer, A.J.M.; Nagumo, Y.; Sims, A.H.; Muir, M.; Dodds, M.; Mullen, P.; Um, I.; Kay, C.; Hasmann, M.; et al. Trastuzumab and Pertuzumab Produce Changes in Morphology and Estrogen Receptor Signaling in Ovarian Cancer Xenografts Revealing New Treatment Strategies. Clin. Cancer Res. 2011, 17, 4451–4461. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, M.; Ferrero, J.-M.; de la Haba-Rodriguez, J.; Poole, C.; De Placido, S.; Osborne, C.K.; Hegg, R.; Easton, V.; Wohlfarth, C.; Arpino, G. First-Line Trastuzumab Plus an Aromatase Inhibitor, with or without Pertuzumab, in Human Epidermal Growth Factor Receptor 2–Positive and Hormone Receptor–Positive Metastatic or Locally Advanced Breast Cancer (PERTAIN): A Randomized, Open-Label Phase II Trial. JCO 2018, 36, 2826–2835. [Google Scholar] [CrossRef]
- Arpino, G.; de la Haba Rodríguez, J.; Ferrero, J.-M.; De Placido, S.; Osborne, C.K.; Klingbiel, D.; Revelant, V.; Wohlfarth, C.; Poppe, R.; Rimawi, M.F.; et al. Pertuzumab, Trastuzumab, and an Aromatase Inhibitor for HER2-Positive and Hormone Receptor-Positive Metastatic or Locally Advanced Breast Cancer: PERTAIN Final Analysis. Clin. Cancer Res. 2023, 29, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Hegg, R.; Im, S.-A.; Park, I.H.; Burdaeva, O.; Kurteva, G.; Press, M.F.; Tjulandin, S.; Iwata, H.; Simon, S.D.; et al. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade with Lapatinib Plus Trastuzumab in Combination with an Aromatase Inhibitor in Postmenopausal Women with HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: Updated Results of ALTERNATIVE. J. Clin. Oncol. 2021, 39, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a Selective Cyclin D Kinase 4/6 Inhibitor, Preferentially Inhibits Proliferation of Luminal Estrogen Receptor-Positive Human Breast Cancer Cell Lines in Vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Cox, D.; Knudsen, E.S. CDK4/6 Inhibition Provides a Potent Adjunct to Her2-Targeted Therapies in Preclinical Breast Cancer Models. Gene. Cancer 2014, 5, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos, E.; Villagrasa, P.; Pascual, T.; Oliveira, M.; Pernas, S.; Paré, L.; Escrivá-de-Romaní, S.; Manso, L.; Adamo, B.; Martínez, E.; et al. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res. 2020, 26, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Wardley, A.M.; Zambelli, S.; Hilton, J.F.; Troso-Sandoval, T.A.; Ricci, F.; Im, S.-A.; Kim, S.-B.; Johnston, S.R.; Chan, A.; et al. Abemaciclib plus Trastuzumab with or without Fulvestrant versus Trastuzumab plus Standard-of-Care Chemotherapy in Women with Hormone Receptor-Positive, HER2-Positive Advanced Breast Cancer (monarcHER): A Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2020, 21, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Friedl, T.; Fehm, T.; Romashova, T.; Fasching, P.; Schneeweiss, A.; Müller, V.; Taran, F.-A.; Polasik, A.; Tzschaschel, M.; et al. Abstract OT2-07-01: DETECT V/CHEVENDO—Comparison of Dual HER2-Targeted Therapy with Trastuzumab plus Pertuzumab in Combination with Chemo- or Endocrine Therapy in Addition with CDK4/6 Inhibition in Patients with HER2-Positive and Hormone-Receptor Positive Metastatic Breast Cancer. Cancer Res. 2019, 79, OT2-07-01. [Google Scholar] [CrossRef]
- Loibl, S.; Metzger, O.; Mandrekar, S.J.; Mundhenke, C.; Seiler, S.; Valagussa, P.; DeMichele, A.; Lim, E.; Tripathy, D.; Winer, E.P.; et al. PATINA: A Randomized, Open Label, Phase III Trial to Evaluate the Efficacy and Safety of Palbociclib + Anti-HER2 Therapy + Endocrine Therapy (ET) vs. Anti-HER2 Therapy + ET after Induction Treatment for Hormone Receptor Positive (HR+)/HER2-Positive Metastatic Breast Cancer (MBC). Ann. Oncol. 2018, 29, viii121. [Google Scholar] [CrossRef]
- Phase IB & II Study of Ribociclib with Trastuzumab Plus Letrozole in Postmenopausal HR+, HER2+ Advanced Breast Cancer Patients—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03913234 (accessed on 10 July 2023).
- Dalpiciclib, Fulvestrant, Trastuzumab and Pertuzumab in HR Positive, HER2 Positive Metastatic Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05574881 (accessed on 10 July 2023).
- Pyrotinib Combined with Trastuzumab and AI in the First-Line Treatment of HER2 Positive/ HR Positive MBC—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03910712 (accessed on 10 July 2023).
- Pyrotinib Combined with Trastuzumab Plus Aromatase Inhibitor in Treatment of Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04088110 (accessed on 10 July 2023).
- Anti-HER2 Therapy + Fulvestrant/Capecitabine in Women with HR+, HER2+, Non-Visceral Metastases Stage IV Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04337658 (accessed on 10 July 2023).
- Randomized, Open Label, Clinical Study of the Targeted Therapy, Palbociclib, to Treat Metastatic Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02947685 (accessed on 10 July 2023).
- Pyrotinib in Combination with Fulvestrant in Patients with HER2 Positive, HR-Positive Metastatic Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04034589 (accessed on 10 July 2023).
- Tucatinib, Palbociclib and Letrozole in Metastatic Hormone Receptor Positive and HER2-Positive Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03054363 (accessed on 10 July 2023).
- Fulvestrant or Capecitabine Combined with Pyrotinib in HR+/HER2+ Metastatic Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04646759 (accessed on 10 July 2023).
- A Study of Abemaciclib (LY2835219) in Women with HR+, HER2+ Locally Advanced or Metastatic Breast Cancer—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02675231 (accessed on 10 July 2023).
- Venetis, K.; Crimini, E.; Sajjadi, E.; Corti, C.; Guerini-Rocco, E.; Viale, G.; Curigliano, G.; Criscitiello, C.; Fusco, N. HER2 Low, Ultra-Low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer. Front. Mol. Biosci. 2022, 9, 834651. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, Pathological, and PAM50 Gene Expression Features of HER2-Low Breast Cancer. npj Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Tarantino, P.; Jin, Q.; Tayob, N.; Jeselsohn, R.M.; Schnitt, S.J.; Vincuilla, J.; Parker, T.; Tyekucheva, S.; Li, T.; Lin, N.U.; et al. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol. 2022, 8, 1177–1183. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Rinnerthaler, G.; Tinchon, C.; Petzer, A.; Balic, M.; Heibl, S.; Schmitt, C.; Zabernigg, A.F.; Egle, D.; Sandholzer, M.; et al. Landscape of HER2-Low Metastatic Breast Cancer (MBC): Results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021, 23, 112. [Google Scholar] [CrossRef]
- de Calbiac, O.; Lusque, A.; Mailliez, A.; Bachelot, T.; Uwer, L.; Mouret-Reynier, M.-A.; Emile, G.; Jouannaud, C.; Gonçalves, A.; Patsouris, A.; et al. Comparison of Management and Outcomes in ERBB2-Low vs ERBB2-Zero Metastatic Breast Cancer in France. JAMA Netw. Open 2022, 5, e2231170. [Google Scholar] [CrossRef]
- Prat, A.; Perou, C.M. Deconstructing the Molecular Portraits of Breast Cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Tarantino, P.; Gandini, S.; Nicolò, E.; Trillo, P.; Giugliano, F.; Zagami, P.; Vivanet, G.; Bellerba, F.; Trapani, D.; Marra, A.; et al. Evolution of Low HER2 Expression between Early and Advanced-Stage Breast Cancer. Eur. J. Cancer 2022, 163, 35–43. [Google Scholar] [CrossRef]
- Horisawa, N.; Adachi, Y.; Takatsuka, D.; Nozawa, K.; Endo, Y.; Ozaki, Y.; Sugino, K.; Kataoka, A.; Kotani, H.; Yoshimura, A.; et al. The Frequency of Low HER2 Expression in Breast Cancer and a Comparison of Prognosis between Patients with HER2-Low and HER2-Negative Breast Cancer by HR Status. Breast Cancer 2022, 29, 234–241. [Google Scholar] [CrossRef]
- Jacot, W.; Maran-Gonzalez, A.; Massol, O.; Sorbs, C.; Mollevi, C.; Guiu, S.; Boissière-Michot, F.; Ramos, J. Prognostic Value of HER2-Low Expression in Non-Metastatic Triple-Negative Breast Cancer and Correlation with Other Biomarkers. Cancers 2021, 13, 6059. [Google Scholar] [CrossRef]
- Schlam, I.; Tolaney, S.M.; Tarantino, P. How I Treat HER2-Low Advanced Breast Cancer. Breast 2023, 67, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. HER2-Low Breast Cancer: Molecular Characteristics and Prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.-U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and Molecular Characteristics of HER2-Low-Positive Breast Cancer: Pooled Analysis of Individual Patient Data from Four Prospective, Neoadjuvant Clinical Trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.Y.C.; Ong, W.S.; Lee, K.H.; Lim, A.H.; Park, S.; Park, Y.H.; Lin, C.-H.; Lu, Y.-S.; Ono, M.; Ueno, T.; et al. HER2 Expression, Copy Number Variation and Survival Outcomes in HER2-Low Non-Metastatic Breast Cancer: An International Multicentre Cohort Study and TCGA-METABRIC Analysis. BMC Med. 2022, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.-S.; Lusque, A.; Mailliez, A.; Bachelot, T.; Uwer, L.; Reynier, M.A.M.; Levy, C.; Jouannaud, C.; Gonçalves, A.; Patsouris, A.; et al. 291P HER2-Low Metastatic Breast Cancer (MBC): Management and Prognosis of a New Breast Cancer Entity in a Real-World Setting. Ann. Oncol. 2021, 32, S491. [Google Scholar] [CrossRef]
- Musolino, A.; Serra, O.; Pellegrino, B.; Tommasi, C.; Zanoni, D.; Cortesi, L.; Canino, F.; Piacentini, F.; Sgargi, P.; Michiara, M. Prognostic Role of HER2 Expression in Patients with ER-Positive/HER2-Negative Breast Cancer: Results from a Population-Based Cancer Registry Study. JCO 2023, 41, 547. [Google Scholar] [CrossRef]
- Musolino, A.; Serra, O.; Pellegrino, B.; Tommasi, C.; Zanoni, D.; Faccini, F.; Michiara, M.; Sgargi, P.; Carrozzi, G.; Ferretti, S.; et al. 286P Prognostic Role of HER2 Expression in Patients with ER-Positive/HER2-Negative Breast Cancer: Results from a Population-Based Cancer Registry Study. Ann. Oncol. 2023, 34, S298–S299. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer Jr, C.E.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and with IHC 1+ or 2+. JCO 2020, 38, 444–453. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. JCO 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- ESMO EMA Recommends Extending Indications for Trastuzumab Deruxtecan to Include Treatment of Advanced HER2-Mutated NSCLC. Available online: https://www.esmo.org/oncology-news/ema-recommends-extending-indications-for-trastuzumab-deruxtecan-to-include-treatment-of-advanced-her2-mutated-nsclc (accessed on 23 March 2024).
- US Food and Drug Administration. FDA Approves Fam-Trastuzumab Deruxtecan-Nxki for HER2-Low Breast Cancer; FDA: Silver Spring, MD, USA, 2022.
- AstraZeneca A Phase 3, Randomized, Multi-Center, Open-Label Study of Trastuzumab Deruxtecan (T-DXd) Versus Investigator’s Choice Chemotherapy in HER2-Low, Hormone Receptor Positive Breast Cancer Patients Whose Disease Has Progressed on Endocrine Therapy in the Metastatic Setting (DESTINY-Breast06); clinicaltrials.gov. 2023. Available online: https://www.dana-farber.org/clinical-trials/20-536 (accessed on 15 November 2023).
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.; Dalenc, F.; Gómez Pardo, P.; et al. Primary Results from TROPiCS-02: A Randomized Phase 3 Study of Sacituzumab Govitecan (SG) versus Treatment of Physician’s Choice (TPC) in Patients (Pts) with Hormone Receptor–Positive/HER2-Negative (HR+/HER2-) Advanced Breast Cancer. JCO 2022, 40, LBA1001. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.M.; Dalenc, F.; Gómez Pardo, P.; et al. Final Overall Survival (OS) Analysis from the Phase 3 TROPiCS-02 Study of Sacituzumab Govitecan (SG) in Patients (Pts) with Hormone Receptor–Positive/HER2-Negative (HR+/HER2–) Metastatic Breast Cancer (mBC). JCO 2023, 41, 1003. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Bardia, A.; Punie, K.; Kalinsky, K.; Cortés, J.; O’Shaughnessy, J.; Carey, L.A.; Rugo, H.S.; Yoon, O.K.; Pan, Y.; et al. 168P Sacituzumab Govitecan (SG) Efficacy in Patients with Metastatic Triple-Negative Breast Cancer (mTNBC) by HER2 Immunohistochemistry (IHC) Status: Findings from the Phase III ASCENT Study. Ann. Oncol. 2022, 33, S200–S201. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: A Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab Vedotin: A Novel Antibody-Drug Conjugates for Cancer Therapy. Drug Deliv. 2022, 29, 1335–1344. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, Q.; Feng, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P.; et al. RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with HER2-Positive and HER2-Low Expressing Advanced or Metastatic Breast Cancer: A Pooled Analysis of Two Studies. JCO 2021, 39, 1022. [Google Scholar] [CrossRef]
- RemeGen Co., Ltd. Randomized, Controlled, Multicenter Phase III Clinical Study Evaluating the Efficacy and Safety of RC48-ADC for the Treatment of Locally Advanced or Metastatic Breast Cancer With Low Expression of HER2; clinicaltrials.gov. 2021. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04400695 (accessed on 23 March 2024).
- Jiang, Z.; Sun, T.; Wang, X.; Liu, Q.; Yan, M.; Tong, Z.; Geng, C.; Tang, J.; Yin, Y.; Yu, G.; et al. A Multiple Center, Open-Label, Single-Arm, Phase II Clinical Trial of MRG002, an HER2-Targeted Antibody-Drug Conjugate, in Patients with HER2-Low Expressing Advanced or Metastatic Breast Cancer. JCO 2022, 40, 1102. [Google Scholar] [CrossRef]
- Schmid, P.; Im, S.-A.; Armstrong, A.; Park, Y.H.; Chung, W.-P.; Nowecki, Z.; Lord, S.; Wysocki, P.J.; Lu, Y.-S.; Dry, H.; et al. BEGONIA: Phase 1b/2 Study of Durvalumab (D) Combinations in Locally Advanced/Metastatic Triple-Negative Breast Cancer (TNBC)—Initial Results from Arm 1, D+paclitaxel (P), and Arm 6, D+trastuzumab Deruxtecan (T-DXd). JCO 2021, 39, 1023. [Google Scholar] [CrossRef]
- Daiichi Sankyo, Inc. A Phase 1b, Multicenter, Two-Part, Open-Label Study of Trastuzumab Deruxtecan (DS-8201a), An Anti-Human Epidermal Growth Factor Receptor-2 (HER2)-Antibody Drug Conjugate (ADC), In Combination With Pembrolizumab, An Anti-PD-1 Antibody, For Subjects With Locally Advanced/Metastatic Breast Or Non-Small Cell Lung Cancer (NSCLC); clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT02564900 (accessed on 23 March 2024).
- Clive, K.S.; Tyler, J.A.; Clifton, G.T.; Holmes, J.P.; Ponniah, S.; Peoples, G.E.; Mittendorf, E.A. The GP2 Peptide: A HER2/Neu-Based Breast Cancer Vaccine. J. Surg. Oncol. 2012, 105, 452–458. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Barnscher, S.D.; Davies, R.H.; Hammond, P.W.; Hernandez, A.; Wickman, G.R.; Fung, V.K.C.; Ding, T.; Garnett, G.; Galey, A.S.; et al. ZW49, A HER2-Targeted Biparatopic Antibody Drug Conjugate for the Treatment of HER2-Expressing Cancers. Cancer Res. 2018, 78, 3914. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.-Y.; Hanna, D.L.; Kang, Y.-K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a Novel Bispecific Antibody, for the Treatment of Locally Advanced or Metastatic HER2-Expressing or HER2-Amplified Cancers: A Phase 1, Dose-Escalation and Expansion Study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef]
- Zymeworks Inc. Phase I Trial of ZW25 in Patients with Locally Advanced (Unresectable) and/or Metastatic HER2-Expressing Cancers; clinicaltrials.gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02892123 (accessed on 15 November 2023).
- Skidmore, L.; Sakamuri, S.; Knudsen, N.A.; Hewet, A.G.; Milutinovic, S.; Barkho, W.; Biroc, S.L.; Kirtley, J.; Marsden, R.; Storey, K.; et al. ARX788, a Site-Specific Anti-HER2 Antibody-Drug Conjugate, Demonstrates Potent and Selective Activity in HER2-Low and T-DM1-Resistant Breast and Gastric Cancers. Mol. Cancer Ther. 2020, 19, 1833–1843. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, D.; Shen, W.; Xiao, Q.; Gu, Y.; O’Shaughnessy, J.; Hu, X. Phase I Trial of a Novel Anti-HER2 Antibody-Drug Conjugate, ARX788, for the Treatment of HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2022, 28, 4212–4221. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, J.; Liu, R.; Gao, S.; Qing, Y.; Yi, S.; Yuan, J.; Chen, H.; Fan, B.; Zheng, H.; et al. Phase I Study of A166 in Patients with HER2-Expressing Locally Advanced or Metastatic Solid Tumors. JCO 2021, 39, 1024. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Gao, S.; Li, W.; Chen, Y.; Meng, Y.; Liu, C.; Jin, W.; Wu, J.; Wang, Y.; et al. Phase I Study of A166, an Antibody–drug Conjugate in Advanced HER2-Expressing Solid Tumours. NPJ Breast Cancer 2023, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Calvo, E.; Lee, K.S.; Moreno, V.; Park, Y.H.; Rha, S.Y.; Chalasani, P.; Zhong, W.; Zhou, L.; Pirie-Shepherd, S.; et al. Safety and Tolerability of a Novel Anti-HER2 Antibody-Drug Conjugate (PF-06804103) in Patients with HER2-Expressing Solid Tumors: A Phase 1 Dose Escalation Study. Mol. Cancer Ther. 2023, 22, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Cheng, Y.; Liu, Y.; Chang, J.; Wang, Z.; Wu, C.; Wang, M.; Hui, A.-M.; Wu, Z.; et al. Abstract P4-01-07: FS-1502, an Anti-HER2 ADC, in Patients with HER2-Expressing Advanced Solid Tumors: A Phase 1a Dose-Escalation Study. Cancer Res. 2023, 83, P4-01–07. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Ardavanis, A.; Symanowski, J.; Murray, J.L.; Shumway, N.M.; Litton, J.K.; Hale, D.F.; Perez, S.A.; Anastasopoulou, E.A.; Pistamaltzian, N.F.; et al. Primary Analysis of a Prospective, Randomized, Single-Blinded Phase II Trial Evaluating the HER2 Peptide AE37 Vaccine in Breast Cancer Patients to Prevent Recurrence. Ann. Oncol. 2016, 27, 1241–1248. [Google Scholar] [CrossRef]
| Drug | Clinical Trial | Phase Trial | Action and Rational | Ref |
|---|---|---|---|---|
| GP2 peptide | Preclinical | HER2-derived peptide vaccine | [122] | |
| ZW49 | Preclinical | Biparatopic ADC, which combines ZW25 with linker-drug conjugated via disulfides containing a cleavable linker and novel auristatin payload | [123] | |
| Zanidatamab (ZW25) | NCT02892123 | Phase I | Humanized, bispecific, Ig G isotype 1-like, mAb directed against the juxtamembrane extracellular domain and the dimerization domain of HER2. | [124,125] |
| ARX788 | ACE-PanTumor-01 | Phase I/II | ADC with anti-HER2 mAb and a potent tubulin inhibitor payload AS269 | [126,127] |
| A166 | CTR20181301 | Phase I | ADC composed of a cytotoxic drug (Duostatin-5, anti-microtubule agent) with site-specific conjugation to trastuzumab via a stable protease-cleavable valine citrulline linker | [128,129] |
| PF-06804103 | NCT03284723 | Phase I | Anti-HER2 ADC with auristatin payload | [130] |
| FS-1502 | NCT03944499 | Phase I | ADC against HER2 with a cleavable β-glucuronide linker and an antimitotic agent (monomethyl auristatin F) | [131] |
| AE37 + GM-CSF | NCT00524277 | Phase I | Peptide vaccine | [132] |
| ARX788 | ACE-PanTumor-01 | Phase I/II | ADC with anti-HER2 mAb and a potent tubulin inhibitor payload AS269 | [126,127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommasi, C.; Airò, G.; Pratticò, F.; Testi, I.; Corianò, M.; Pellegrino, B.; Denaro, N.; Demurtas, L.; Dessì, M.; Murgia, S.; et al. Hormone Receptor-Positive/HER2-Positive Breast Cancer: Hormone Therapy and Anti-HER2 Treatment: An Update on Treatment Strategies. J. Clin. Med. 2024, 13, 1873. https://doi.org/10.3390/jcm13071873
Tommasi C, Airò G, Pratticò F, Testi I, Corianò M, Pellegrino B, Denaro N, Demurtas L, Dessì M, Murgia S, et al. Hormone Receptor-Positive/HER2-Positive Breast Cancer: Hormone Therapy and Anti-HER2 Treatment: An Update on Treatment Strategies. Journal of Clinical Medicine. 2024; 13(7):1873. https://doi.org/10.3390/jcm13071873
Chicago/Turabian StyleTommasi, Chiara, Giulia Airò, Fabiana Pratticò, Irene Testi, Matilde Corianò, Benedetta Pellegrino, Nerina Denaro, Laura Demurtas, Mariele Dessì, Sara Murgia, and et al. 2024. "Hormone Receptor-Positive/HER2-Positive Breast Cancer: Hormone Therapy and Anti-HER2 Treatment: An Update on Treatment Strategies" Journal of Clinical Medicine 13, no. 7: 1873. https://doi.org/10.3390/jcm13071873
APA StyleTommasi, C., Airò, G., Pratticò, F., Testi, I., Corianò, M., Pellegrino, B., Denaro, N., Demurtas, L., Dessì, M., Murgia, S., Mura, G., Wekking, D., Scartozzi, M., Musolino, A., & Solinas, C. (2024). Hormone Receptor-Positive/HER2-Positive Breast Cancer: Hormone Therapy and Anti-HER2 Treatment: An Update on Treatment Strategies. Journal of Clinical Medicine, 13(7), 1873. https://doi.org/10.3390/jcm13071873









