Rare Breast Cancer Histotypes—A Retrospective Study and Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics (Table 2)
| Characteristic | Invasive Carcinoma (NOS) | Mucinous | Invasive Lobular | Papillary | Mixed Invasive (NOS) and Lobular | Tubular/Cribriform |
|---|---|---|---|---|---|---|
| Number of Patients | 3594 | 141 | 139 | 82 | 67 | 45 |
| Age (years) | 51.1 ± 12.7 | 54.6 ± 16.3 * (p = 0.013) | 52.6 ± 11.6 | 58.2 ± 15.2 * (p < 0.001) | 52.7 ± 12.7 | 54.5 ± 13.3 |
| Follow-Up Time (months) | 111.2 ± 65.4 | 115.7 ± 61.6 | 101.4 ± 58.7 | 115.2 ± 57.8 | 139.0 ± 74.2 * (p = 0.003) | 137.5 ± 58.1 |
| Default Rate | 7.3% | 3.6% | 7.2% | 7.3% | 10.4% | 8.9% |
3.2. Disease Characteristics (Table 3)
| Characteristics | Invasive Carcinoma (NOS) | Mucinous | Invasive Lobular | Papillary | Mixed Invasive (NOS) and Lobular | Tubular/Cribriform |
|---|---|---|---|---|---|---|
| Mode of Detection | ||||||
| Palpable breast mass | 82.6% | 84.4% | 74.9% | 87.8% | 86.6% | 77.8% |
| Screen-detected | 6.0% | 5.7% | 12.2% | 2.4% | 4.5% | 13.3% |
| Incidental (CT/PET-CT) | 0.4% | 0% | 0.7% | 1.2% | 0% | 0% |
| Staging | ||||||
| Stage I | 35.8% | 46.1% * (p = 0.0125) | 25.9% * (p = 0.0167) | 37.8% | 22.4% * (p = 0.0232) | 68.9% * (p < 0.0001) |
| Stage II | 39.7% | 41.1% | 38.1% | 11.0% * (p < 0.001) | 43.3% | 24.4% * (p = 0.037) |
| Stage III | 17.6% | 5.7% * (p = 0.002) | 30.9% * (p = 0.0001) | 1.2% * (p = 0.0001) | 25.4% | 2.2% * (p = 0.0068) |
| Stage IV | 3.1% | 1.4% | 2.2% | 1.2% | 4.5% | 0% |
| Lymph node-positive | 43.7% | 12.8% * (p < 0.0001) | 49.6% | 3.7% * (p < 0.0001) | 59.7% * (p = 0.009) | 17.8% * (p = 0.005) |
| Mode of Surgery | ||||||
| Breast conservative Therapy | 25.2% | 36.1% | 16.6% | 41.4% | 20.9% | 35.6% |
| Median tumor Size (cm) | 2 | 2 | 2.5 | 1.3 | 2.5 | 1 |
| Axillary Dissection | ||||||
| Overall | 81.7% | 74.5% | 79.1% | 56.1% | 92.5% | 82.2% |
| 2004 Onward | 73.9% | 60% | 70.1% | 50% | 80% | 66.7% |
| Recurrence | ||||||
| Overall | 9.5% | 6.4% | 9.4% | 9.8% | 4.5% | 2.2% |
| Chest wall/breast | 6.5% | 5.7% | 6.5% | 8.5% | 4.5% | 2.2% |
| Axilla | 3.0% | 0.7% | 1.4% | 2.4% | 0% | 0% |
| Supraclavicular Fossa | 2.1% | 0% | 3.6% | 0% | 0% | 0% |
| Adjuvant Therapy | ||||||
| Radiotherapy | 56.1% | 45.4% * (p = 0.0121) | 59.0% | 35.4% * (p = 0.0002) | 58.2% | 40.0% * (p = 0.0307) |
| Chemotherapy | 55.8% | 24.8% * (p < 0.0001) | 54.0% | 12.2% * (p = 0.0001) | 53.7% | 13.3% * (p < 0.0001) |
| Hormonal therapy | 62.1% | 78.0% * (p = 0.0001) | 66.9% | 43.9% * (p = 0.0001) | 67.2% | 66.7% |
| Targeted therapy | 8.5% | 1.4% * (p = 0.0026) | 5.8% | 0% | 4.5% | 0% |
3.3. Adjuvant Therapies (Table 3)
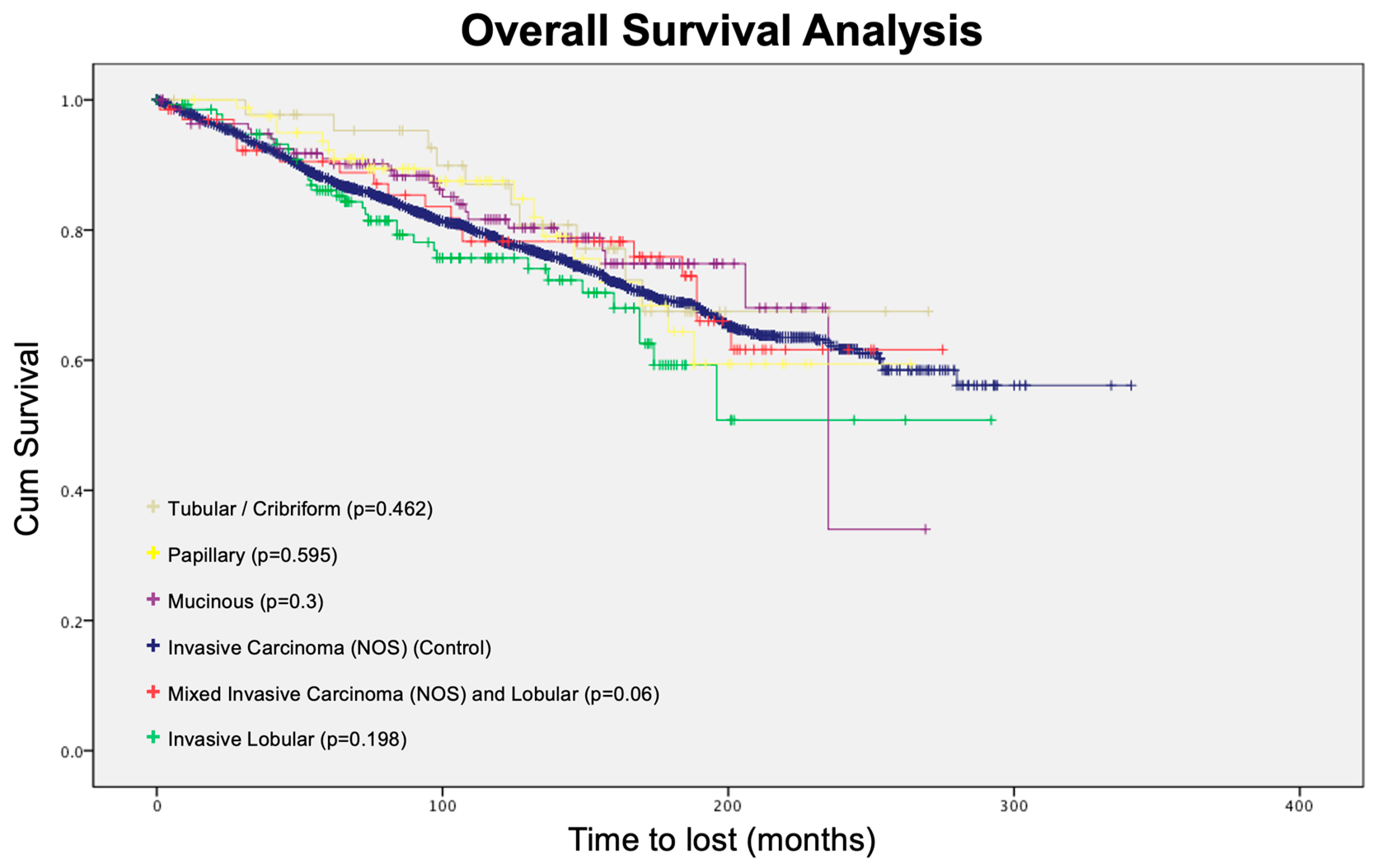
3.4. Survival Analysis
4. Discussion
4.1. Literature Review
4.1.1. Mucinous Carcinoma
4.1.2. Invasive Lobular Carcinoma
4.1.3. Papillary Carcinoma
4.1.4. Tubular/Cribriform Carcinoma
4.1.5. Mixed Ductal/Lobular Carcinoma
4.2. Implications for Practice
4.3. Axillary Dissection Rate
4.4. Limitations of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. (Eds.) World Cancer Report 2020; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Overview of Hong Kong Cancer Statistics of 2021. Available online: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202021.pdf (accessed on 23 December 2023).
- WHO. Classification of Tumours: Breast Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; Volume 2. [Google Scholar]
- Ginter, P.S.; Tang, X.; Shin, S.J. A review of mucinous lesions of the breast. Breast J. 2020, 26, 1168–1178. [Google Scholar] [CrossRef]
- Di Saverio, S.; Gutierrez, J.; Avisar, E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res. Treat. 2008, 111, 541–547. [Google Scholar] [CrossRef]
- Limaiem, F.; Ahmad, F. Mucinous Breast Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Acevedo, C.; Amaya, C.; Lopez-Guerra, J.L. Rare breast tumors: Review of the literature. Rep. Pract. Oncol. Radiother. 2014, 19, 267–274. [Google Scholar] [CrossRef][Green Version]
- Lei, L.; Yu, X.; Chen, B.; Chen, Z.; Wang, X. Clinicopathological Characteristics of Mucinous Breast Cancer: A Retrospective Analysis of a 10-Year Study. PLoS ONE 2016, 11, e0155132. [Google Scholar] [CrossRef]
- Jenkins, S.; Kachur, M.E.; Rechache, K.; Wells, J.M.; Lipkowitz, S. Rare Breast Cancer Subtypes. Curr. Oncol. Rep. 2021, 23, 54. [Google Scholar] [CrossRef]
- Sas-Korczynska, B.; Mitus, J.; Stelmach, A.; Rys, J.; Majczyk, A. Mucinous breast cancer—Clinical characteristics and treatment results in patients treated at the Oncology Centre in Krakow between 1952 and 2002. Contemp. Oncol. 2014, 18, 120–123. [Google Scholar] [CrossRef]
- Dixon, J.M.; Anderson, T.J.; Page, D.L.; Lee, D.; Duffy, S.W.; Stewart, H.J. Infiltrating lobular carcinoma of the breast: An evaluation of the incidence and consequence of bilateral disease. Br. J. Surg. 1983, 70, 513–516. [Google Scholar] [CrossRef]
- Mouabbi, J.A.; Hassan, A.; Lim, B.; Hortobagyi, G.N.; Tripathy, D.; Layman, R.M. Invasive lobular carcinoma: An understudied emergent subtype of breast cancer. Breast Cancer Res. Treat. 2022, 193, 253–264. [Google Scholar] [CrossRef]
- Li, C.I.; Uribe, D.J.; Daling, J.R. Clinical characteristics of different histologic types of breast cancer. Br. J. Cancer 2005, 93, 1046–1052. [Google Scholar] [CrossRef]
- Arpino, G.; Bardou, V.J.; Clark, G.M.; Elledge, R.M. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004, 6, R149–R156. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Powe, D.G.; Green, A.R.; Habashy, H.; Grainge, M.J.; Robertson, J.F.; Blamey, R.; Gee, J.; Nicholson, R.I.; et al. Invasive lobular carcinoma of the breast: Response to hormonal therapy and outcomes. Eur. J. Cancer 2008, 44, 73–83. [Google Scholar] [CrossRef]
- Korhonen, T.; Kuukasjarvi, T.; Huhtala, H.; Alarmo, E.L.; Holli, K.; Kallioniemi, A.; Pylkkanen, L. The impact of lobular and ductal breast cancer histology on the metastatic behavior and long term survival of breast cancer patients. Breast 2013, 22, 1119–1124. [Google Scholar] [CrossRef]
- Pal, S.K.; Lau, S.K.; Kruper, L.; Nwoye, U.; Garberoglio, C.; Gupta, R.K.; Paz, B.; Vora, L.; Guzman, E.; Artinyan, A.; et al. Papillary carcinoma of the breast: An overview. Breast Cancer Res. Treat. 2010, 122, 637–645. [Google Scholar] [CrossRef]
- Reiner, A.; Reiner, G.; Spona, J.; Schemper, M.; Holzner, J.H. Histopathologic characterization of human breast cancer in correlation with estrogen receptor status. A comparison of immunocytochemical and biochemical analysis. Cancer 1988, 61, 1149–1154. [Google Scholar] [CrossRef]
- Soomro, S.; Shousha, S.; Taylor, P.; Shepard, H.M.; Feldmann, M. c-erbB-2 expression in different histological types of invasive breast carcinoma. J. Clin. Pathol. 1991, 44, 211–214. [Google Scholar] [CrossRef]
- Louwman, M.W.; Vriezen, M.; van Beek, M.W.; Nolthenius-Puylaert, M.C.; van der Sangen, M.J.; Roumen, R.M.; Kiemeney, L.A.; Coebergh, J.W. Uncommon breast tumors in perspective: Incidence, treatment and survival in the Netherlands. Int. J. Cancer 2007, 121, 127–135. [Google Scholar] [CrossRef]
- McDivitt, R.W.; Boyce, W.; Gersell, D. Tubular carcinoma of the breast. Clinical and pathological observations concerning 135 cases. Am. J. Surg. Pathol. 1982, 6, 401–411. [Google Scholar] [CrossRef]
- Min, Y.; Bae, S.Y.; Lee, H.C.; Lee, J.H.; Kim, M.; Kim, J.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Lee, J.E.; et al. Tubular carcinoma of the breast: Clinicopathologic features and survival outcome compared with ductal carcinoma in situ. J. Breast Cancer 2013, 16, 404–409. [Google Scholar] [CrossRef][Green Version]
- Cabral, A.H.; Recine, M.; Paramo, J.C.; McPhee, M.M.; Poppiti, R.; Mesko, T.W. Tubular carcinoma of the breast: An institutional experience and review of the literature. Breast J. 2003, 9, 298–301. [Google Scholar] [CrossRef]
- Mo, C.H.; Ackbarkhan, Z.; Gu, Y.Y.; Chen, G.; Pang, Y.Y.; Dang, Y.W.; Feng, Z.B. Invasive cribriform carcinoma of the breast: A clinicopathological analysis of 12 cases with review of literature. Int. J. Clin. Exp. Pathol. 2017, 10, 9917–9924. [Google Scholar] [PubMed]
- Venable, J.G.; Schwartz, A.M.; Silverberg, S.G. Infiltrating cribriform carcinoma of the breast: A distinctive clinicopathologic entity. Hum. Pathol. 1990, 21, 333–338. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Lin, Z.; Zhang, X.; Liu, F.; Wang, Y.; Liu, H.; Yang, Y.; Niu, Y. Invasive cribriform carcinoma in a Chinese population: Comparison with low-grade invasive ductal carcinoma-not otherwise specified. Int. J. Clin. Exp. Pathol. 2013, 6, 445–457. [Google Scholar]
- Colleoni, M.; Rotmensz, N.; Maisonneuve, P.; Mastropasqua, M.G.; Luini, A.; Veronesi, P.; Intra, M.; Montagna, E.; Cancello, G.; Cardillo, A.; et al. Outcome of special types of luminal breast cancer. Ann. Oncol. 2012, 23, 1428–1436. [Google Scholar] [CrossRef]
- Cong, Y.; Qiao, G.; Zou, H.; Lin, J.; Wang, X.; Li, X.; Li, Y.; Zhu, S. Invasive cribriform carcinoma of the breast: A report of nine cases and a review of the literature. Oncol. Lett. 2015, 9, 1753–1758. [Google Scholar] [CrossRef]
- Metzger-Filho, O.; Ferreira, A.R.; Jeselsohn, R.; Barry, W.T.; Dillon, D.A.; Brock, J.E.; Vaz-Luis, I.; Hughes, M.E.; Winer, E.P.; Lin, N.U. Mixed Invasive Ductal and Lobular Carcinoma of the Breast: Prognosis and the Importance of Histologic Grade. Oncologist 2019, 24, e441–e449. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo-Donovan, D.D.; Dickson-Witmer, D.; Petrelli, N.J. Sentinel lymph node biopsy in breast cancer: A history and current clinical recommendations. Surg. Oncol. 2012, 21, 196–200. [Google Scholar] [CrossRef] [PubMed]

| Total Number of Patients (n = 4393) | ||
|---|---|---|
| Surgical Resection Group (n = 4202) | ||
| Histological Type | Frequency | % |
| Invasive carcinoma (NOS) | 3594 | 85.5 |
| Mucinous | 141 | 3.36 |
| Invasive lobular | 139 | 3.31 |
| Papillary | 82 | 1.95 |
| Mixed invasive (NOS) and lobular | 67 | 1.59 |
| Pure tubular/cribriform | 45 | 1.07 |
| Medullary | 39 | 0.93 |
| Other subtypes | 62 | 1.48 |
| Palliative Group (n = 191) | ||
| Histological Type | Frequency | % |
| Invasive carcinoma (NOS) | 161 | 84.3 |
| Invasive lobular | 9 | 4.71 |
| Invasive adenocarcinoma | 7 | 3.66 |
| Mucinous | 3 | 1.57 |
| Papillary | 3 | 1.57 |
| Other subtypes | 6 | 3.14 |
| Variables | Invasive Carcinoma (NOS) | Mucinous | Invasive Lobular | Papillary | Mixed Invasive (NOS) and Lobular | Tubular/Cribriform |
|---|---|---|---|---|---|---|
| Estrogen receptor-positive | 50.1% | 69.4% * (p < 0.0001) | 59.1% * (p = 0.0373) | 52.4% | 73.1% * (p = 0.0002) | 71.2% * (p = 0.0049) |
| Progesterone receptor-positive | 40.9% | 60.9% * (p < 0.0001) | 41.8% | 50.1% | 70.2% * (p < 0.0001) | 66.8% * (p = 0.0005) |
| HER2-positive | 19.7% | 5.0% * (p < 0.0001) | 17.0% | 4.9% * (p = 0.0008) | 3.0% * (p = 0.0006) | 0% |
| Variables | Mucinous | Invasive Lobular | Papillary | Tubular/Cribriform |
|---|---|---|---|---|
| Age | ||||
| Cohort data (mean) | 54.6 ± 16.3 | 52.6 ± 11.6 | 58.2 ± 15.2 | 54.5 ± 13.3 |
| Literature (mean) | 71 | 63.4 ± 12.7 | 65.7 ± 13.2 | 57.0 ± 13.8 |
| Literature (median) | - | - | 53 | - |
| ER-Positive | ||||
| Cohort data | 69.4% | 59.1% | 52.4% | 71.2% |
| Literature | 94% | 92.7% | 100% | 92.9%/100% |
| PR-Positive | ||||
| Cohort data | 60.9% | 41.8% | 50.1% | 66.8% |
| Literature | 80% | 67.4% | 80% | 87%/69% |
| HER2-Positive | ||||
| Cohort data | 5% | 17% | 4.9% | 0% |
| Literature | - | 7–10.7% | - | 2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, A.H.K.; Co, M.T.H.; Kwong, A. Rare Breast Cancer Histotypes—A Retrospective Study and Literature Review. J. Clin. Med. 2024, 13, 643. https://doi.org/10.3390/jcm13030643
Lam AHK, Co MTH, Kwong A. Rare Breast Cancer Histotypes—A Retrospective Study and Literature Review. Journal of Clinical Medicine. 2024; 13(3):643. https://doi.org/10.3390/jcm13030643
Chicago/Turabian StyleLam, Allan Hoi Kin, Michael Tiong Hong Co, and Ava Kwong. 2024. "Rare Breast Cancer Histotypes—A Retrospective Study and Literature Review" Journal of Clinical Medicine 13, no. 3: 643. https://doi.org/10.3390/jcm13030643
APA StyleLam, A. H. K., Co, M. T. H., & Kwong, A. (2024). Rare Breast Cancer Histotypes—A Retrospective Study and Literature Review. Journal of Clinical Medicine, 13(3), 643. https://doi.org/10.3390/jcm13030643




