Autonomic Function in Obese Children and Adolescents: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Terms
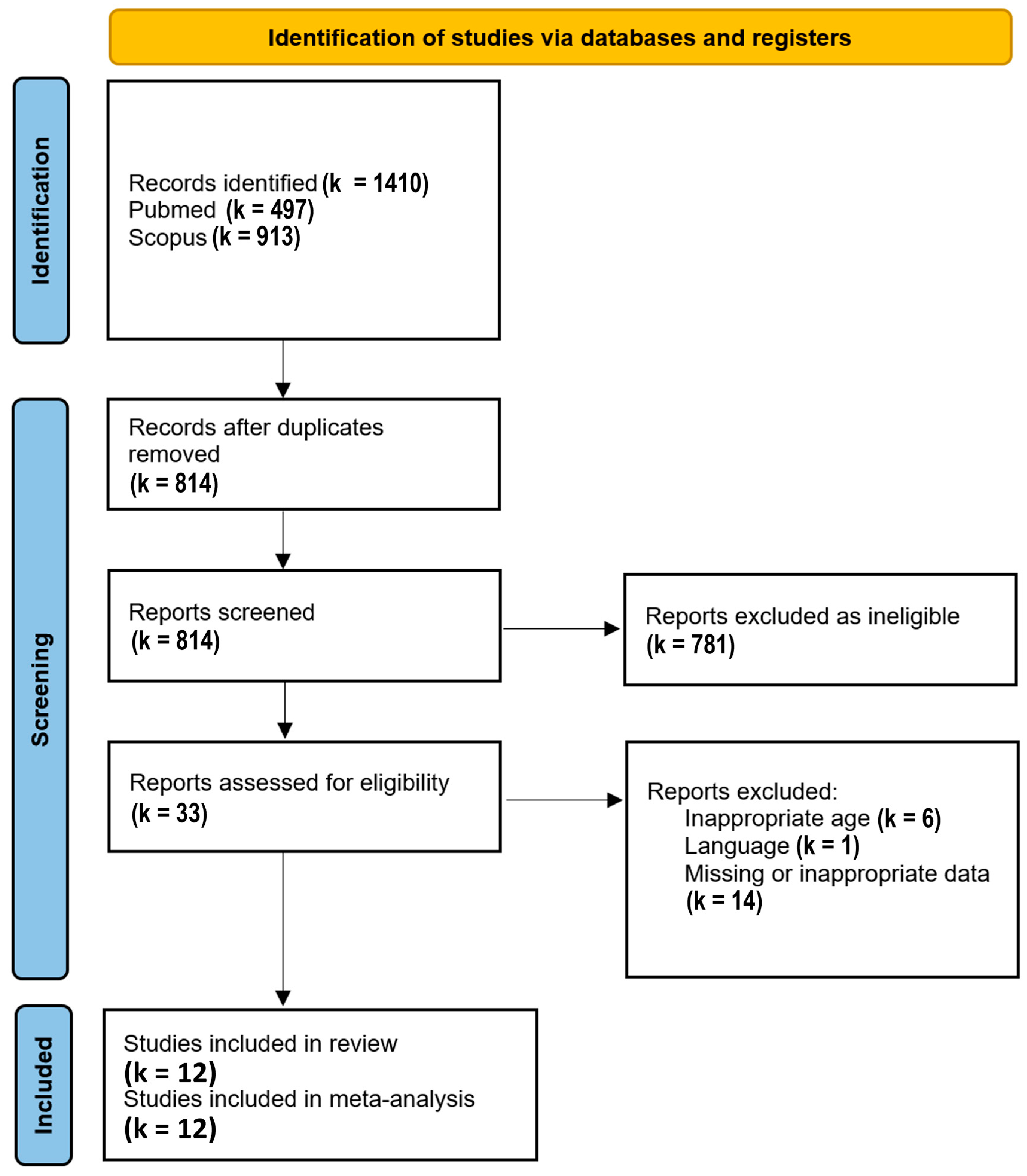
2.2. Search Strategy and Inclusion Criteria
2.3. Autonomic Variables
2.4. Evaluation of the Studies
2.5. Heterogeneity
2.6. Standardized Mean Differences
3. Results
3.1. Clinical Characteristics
3.2. Heterogeneity
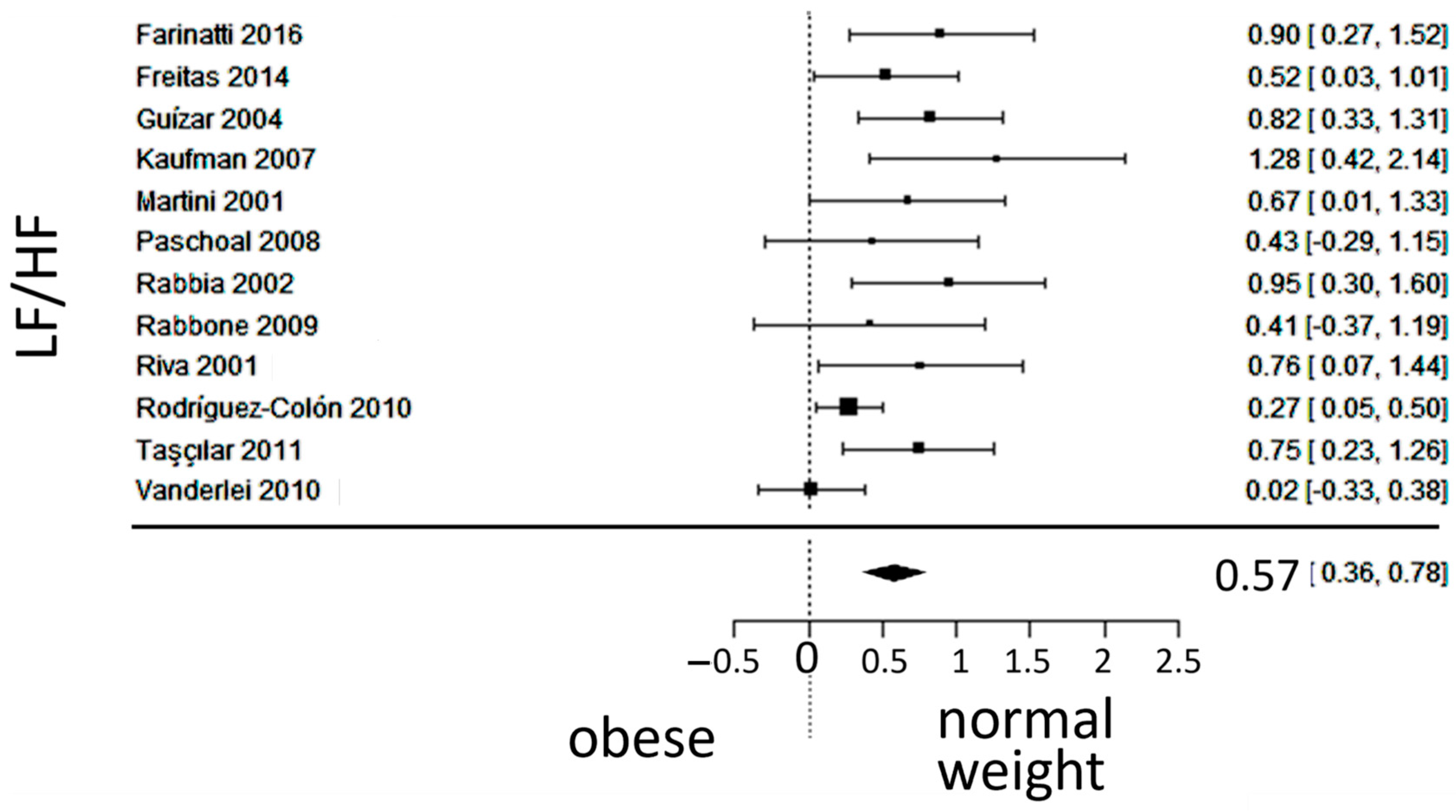
3.3. Autonomic (Sympatho-Vagal) Balance
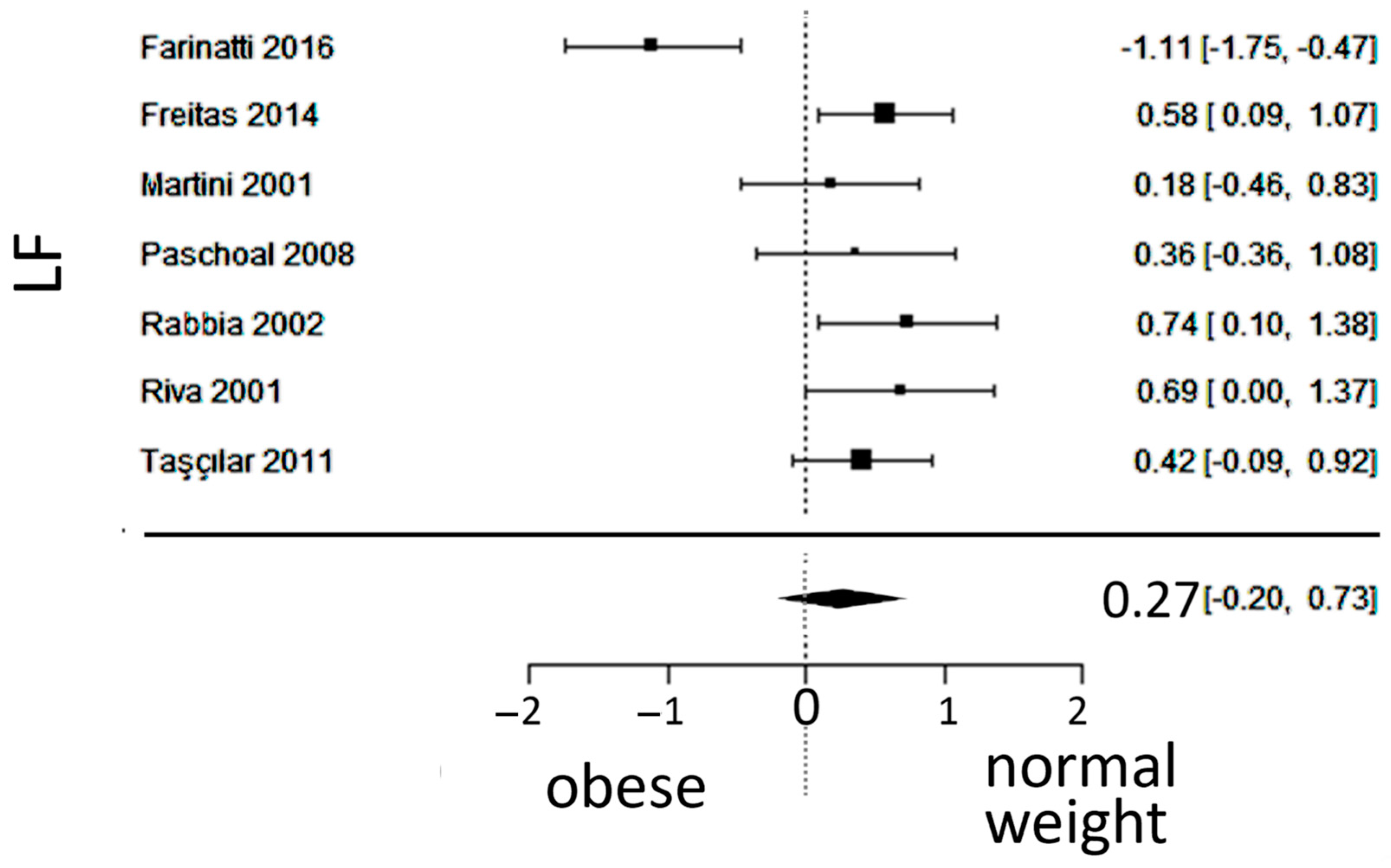
3.4. Sympathetic Activity
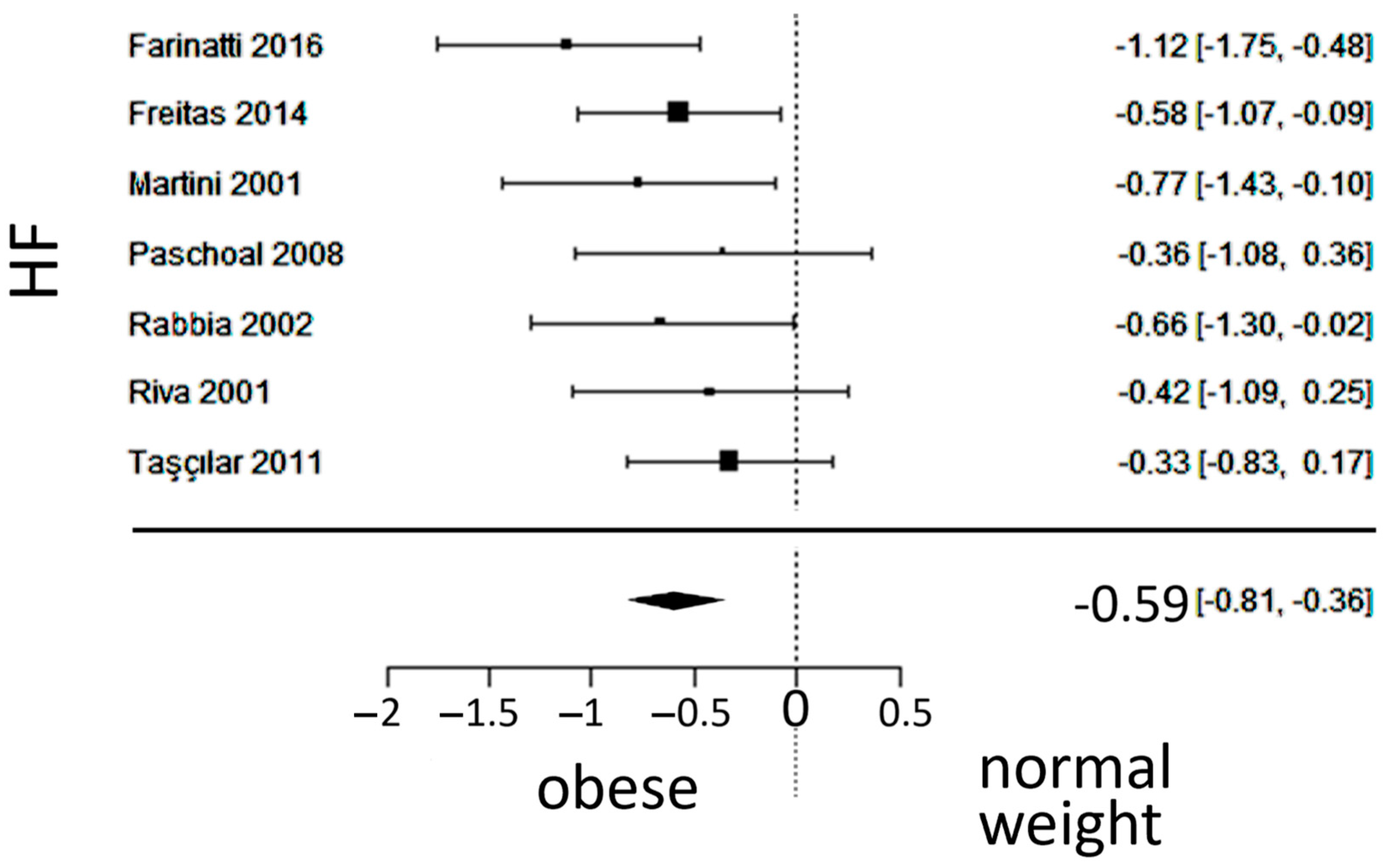
3.5. Vagal Activity
4. Discussion
4.1. Autonomic Balance
4.2. Sympathetic Activity
4.3. Vagal Activity
4.4. Limitations
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katta, N.; Loethen, T.; Lavie, C.J.; Alpert, M.A. Obesity and coronary heart disease: Epidemiology, pathology, and coronary artery imaging. Curr. Probl. Cardiol. 2021, 46, 100655. [Google Scholar] [CrossRef] [PubMed]
- Peterson, H.R.; Rothschild, M.; Weinberg, C.R.; Fell, R.D.; McLeish, K.R.; Pfeifer, M.A. Body fat and the activity of the autonomic nervous system. N. Engl. J. Med. 1988, 318, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Hsiao, T.J.; Lo, H.M.; Kuo, C.D. Abdominal obesity is associated with autonomic nervous derangement in healthy Asian obese subjects. Clin. Nutr. 2008, 27, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Moreira, A.; Moreira, P.; Delgado, L.; Silva, D. Effects of weight changes in the autonomic nervous system: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 110–126. [Google Scholar] [CrossRef]
- Wang, Y.; Anesi, J.; Maier, M.C.; Myers, M.A.; Oqueli, E.; Sobey, C.G.; Drummond, G.R.; Denton, K.M. Sympathetic Nervous System and Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 13132. [Google Scholar] [CrossRef]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef]
- Imakita, M.; Yutani, C.; Sakurai, I.; Sumiyoshi, A.; Watanabe, T.; Mitsumata, M.; Kusumi, Y.; Katayama, S.; Mano, M.; Baba, S.; et al. The second nationwide study of atherosclerosis in infants, children, and young adults in Japan. Comparison with the first study carried out 13 years ago. Ann. N. Y. Acad. Sci. 2000, 902, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Brisbois, T.D.; Farmer, A.P.; McCargar, L.J. Early markers of adult obesity: A review. Obes. Rev. 2012, 13, 347–367. [Google Scholar] [CrossRef]
- Singh, A.S.; Mulder, C.; Twisk, J.W.; van Mechelen, W.; Chinapaw, M.J. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Veliath, S.; Krishnamurthy, N.; Thenmozhi, M. Comparison of Cardiovascular Parameters and Cardiac Autonomic Activity of Obese and Normal Weight School Children in Puducherry. Indian J. Physiol. Pharmacol. 2016, 60, 247–254. [Google Scholar]
- Qi, Z.; Ding, S. Obesity-associated sympathetic overactivity in children and adolescents: The role of catecholamine resistance in lipid metabolism. J. Pediatr. Endocrinol. Metab. 2016, 29, 113–125. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Task Force Report. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G.; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol. Assess 2003, 7, iii-173. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi, version 2.4; The Jamovi Group: Sydney, Australia, 2023. Available online: https://www.jamovi.org/ (accessed on 1 August 2023).
- Farinatti, P.; Neto, S.R.M.; Dias, I.; Cunha, F.A.; Bouskela, E.; Kraemer-Aguiar, L.G. Short-Term Resistance Training Attenuates Cardiac Autonomic Dysfunction in Obese Adolescents. Pediatr. Exerc. Sci. 2016, 28, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Freitas, I.M.; Miranda, J.A.; Mira, P.A.; Lanna, C.M.; Lima, J.R.; Laterza, M.C. Cardiac autonomic dysfunction in obese normotensive children and adolescents. Rev. Paul. Pediatr. 2014, 32, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Guízar, J.M.; Ahuatzin, R.; Amador, N.; Sánchez, G.; Romer, G. Heart autonomic function in overweight adolescents. Indian Pediatr. 2005, 42, 464–469. [Google Scholar]
- Kaufman, C.L.; Kaiser, D.R.; Steinberger, J.; Kelly, A.S.; Dengel, D.R. Relationships of cardiac autonomic function with metabolic abnormalities in childhood obesity. Obesity 2007, 15, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Riva, P.; Rabbia, F.; Molini, V.; Ferrero, G.B.; Cerutti, F.; Carra, R.; Veglio, F. Heart rate variability in childhood obesity. Clin. Auton. Res. 2001, 11, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A.; Trevizan, P.F.; Scodeler, N.F. Heart rate variability, blood lipids and physical capacity of obese and non-obese children. Arq. Bras. Cardiol. 2009, 93, 239–246. [Google Scholar] [CrossRef]
- Rabbia, F.; Silke, B.; Conterno, A.; Grosso, T.; De Vito, B.; Rabbone, I.; Chiandussi, L.; Veglio, F. Assessment of cardiac autonomic modulation during adolescent obesity. Obes. Res. 2003, 11, 541–548. [Google Scholar] [CrossRef]
- Rabbone, I.; Bobbio, A.; Rabbia, F.; Bertello, M.C.; Ignaccoldo, M.G.; Saglio, E.; Morello, F.; Veglio, F.; Pacini, G.; Cerutti, F. Early cardiovascular autonomic dysfunction, beta cell function and insulin resistance in obese adolescents. Acta Biomed. 2009, 80, 29–35. [Google Scholar] [PubMed]
- Riva, P.; Martini, G.; Rabbia, F.; Milan, A.; Paglieri, C.; Chiandussi, L.; Veglio, F. Obesity and autonomic function in adolescence. Clin. Exp. Hypertens. 2001, 23, 57–67. [Google Scholar] [CrossRef]
- Rodríguez-Colón, S.M.; Bixler, E.O.; Li, X.; Vgontzas, A.N.; Liao, D. Obesity is associated with impaired cardiac autonomic modulation in children. Int. J. Pediatr. Obes. 2011, 6, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Taşçılar, M.E.; Yokuşoğlu, M.; Boyraz, M.; Baysan, O.; Köz, C.; Dündaröz, R. Cardiac autonomic functions in obese children. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 60–64. [Google Scholar] [CrossRef]
- Vanderlei, L.C.; Pastre, C.M.; Freitas Júnior, I.F.; Godoy, M.F. Analysis of cardiac autonomic modulation in obese and eutrophic children. Clinics 2010, 65, 789–792. [Google Scholar] [CrossRef]
- Henning, R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: A review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504–529. [Google Scholar]
- Valensi, P. Autonomic nervous system activity changes in patients with hypertension and overweight: Role and therapeutic implications. Cardiovasc. Diabetol. 2021, 20, 170. [Google Scholar] [CrossRef]
- Freeman, R.; Weiss, S.T.; Roberts, M.; Zbikowski, S.M.; Sparrow, D. The relationship between heart rate variability and measures of body habitus. Clin. Auton. Res. 1995, 5, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Indumathy, J.; Pal, G.K.; Pal, P.; Ananthanarayanan, P.H.; Parija, S.C.; Balachander, J.; Dutta, T.K. Association of sympathovagal imbalance with obesity indices, and abnormal metabolic biomarkers and cardiovascular parameters. Obes. Res. Clin. Pract. 2015, 9, 55–66. [Google Scholar] [CrossRef]
- Mohanta, S.K.; Peng, L.; Li, Y.; Lu, S.; Sun, T.; Carnevale, L.; Perrotta, M.; Ma, Z.; Forstera, B.; Stanic, K.; et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 2022, 605, 152–159. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, T.; Morelli, D.; Creagh, A.; Liu, Z.; Yang, J.; Liu, F.; Zhang, Y.T.; Clifton, D.A. Uncertainties in the Analysis of Heart Rate Variability: A Systematic Review. IEEE Rev. Biomed. Eng. 2023, 17, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Zahorska-Markiewicz, B.; Kuagowska, E.; Kucio, C.; Klin, M. Heart rate variability in obesity. Int. J. Obes. Relat. Metab. Disord. 1993, 17, 21–23. [Google Scholar]
- Rossi, M.; Marti, G.; Ricordi, L.; Fornasari, G.; Finardi, G.; Fratino, P.; Bernardi, L. Cardiac autonomic dysfunction in obese subjects. Clin. Sci. 1989, 76, 567–572. [Google Scholar] [CrossRef]
- Laederach-Hofmann, K.; Mussgay, L.; Ruddel, H. Autonomic cardiovascular regulation in obesity. J. Endocrinol. 2000, 164, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Salinas, A.; Molina-Sotomayor, E.; Cano-Montoya, J.; Gonzalez-Jurado, J.A. Is Active Lifestyle Related to Autonomic Nervous System Function and Lipid Profile in People with Overweight? A Study Pilot. Sustainability 2021, 13, 2439. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef]
- Nagai, N.; Moritani, T. Effect of physical activity on autonomic nervous system function in lean and obese children. Int. J. Obes. 2004, 28, 27–33. [Google Scholar] [CrossRef]
- Hayano, J.; Yamada, A.; Mukai, S.; Sakakibara, Y.; Yamada, M.; Ohte, N.; Hashimoto, T.; Fujinami, T.; Takata, K. Severity of coronary atherosclerosis correlates with the respiratory component of heart rate variability. Am. Heart J. 1991, 121, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Gidron, Y.; Kupper, N.; Kwaijtaal, M.; Winter, J.; Denollet, J. Vagus-brain communication in atherosclerosis-related inflammation: A neuroimmunomodulation perspective of CAD. Atherosclerosis 2007, 195, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, S.; Finn, S.; Hoyer, D.; Guenther, A.; Witte, O.W.; Schultze, T.; Schwab, M. Association Between Systemic Inflammation, Carotid Arteriosclerosis, and Autonomic Dysfunction. Transl. Stroke Res. 2020, 11, 50–59. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Licenziati, M.R.; Acampora, C.; Salvatore, V.; Auriemma, L.; Romano, M.L.; Panico, S.; Rubba, P.; Trevisan, M. Increased Carotid Intima-Media Thickness and Stiffness in Obese Children. Diabetes Care 2004, 27, 2506–2508. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Kiess, W.; de Sousa, G.; Stoffel-Wagner, B.; Wunsch, R. Intima media thickness in childhood obesity: Relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism 2006, 55, 113–118. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Yu, C.W.; Sung, R.Y.; Qiao, M.; Leung, S.S.; Lam, C.W.; Metreweli, C.; Celermajer, D.S. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Eyre, E.L.; Duncan, M.J.; Birch, S.L.; Fisher, J.P. The influence of age and weight status on cardiac autonomic control in healthy chil-dren: A review. Auton. Neurosci. 2014, 186, 8–21. [Google Scholar] [CrossRef]
- Li, J.; Simon, G.; Castro, M.R.; Kumar, V.; Steinbach, M.S.; Caraballo, P.J. Association of BMI, comorbidities and all-cause mortality by using a baseline mortality risk model. PLoS ONE 2021, 16, e0253696. [Google Scholar] [CrossRef]
- El-Ayash, H.; Puyau, M.; Bacha, F. Hyperglycemia: A determinant of cardiac autonomic dysfunction in youth with obesity across the spectrum of glycemic regulation. Pediatr. Obes. 2023, 18, e13063. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Juarascio, A.; Manasse, S.; Minassian, A.; Risbrough, V.; Afari, N. Heart rate variability and emotion regulation among individuals with obesity and loss of control eating. Physiol. Behav. 2019, 199, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


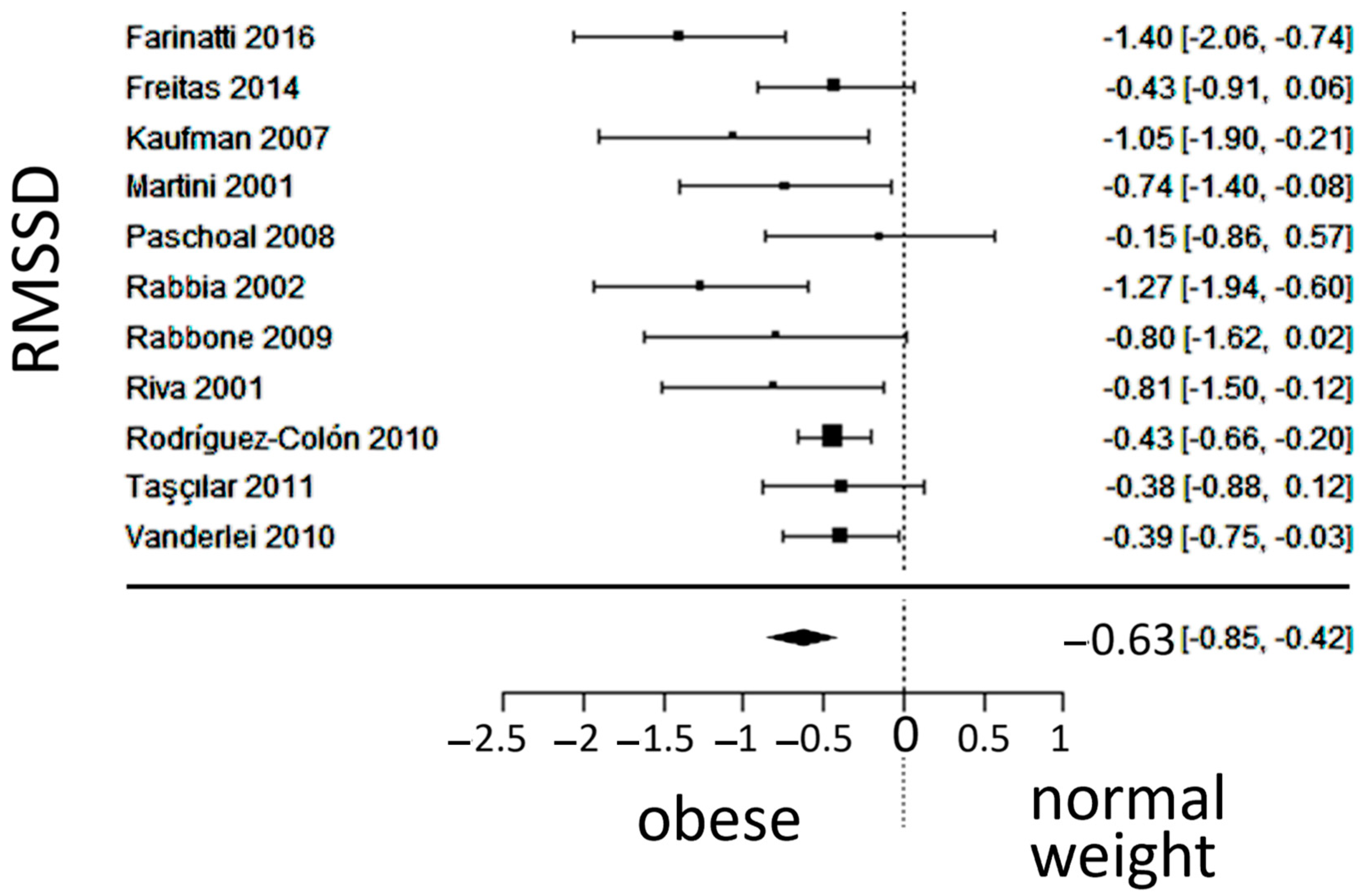
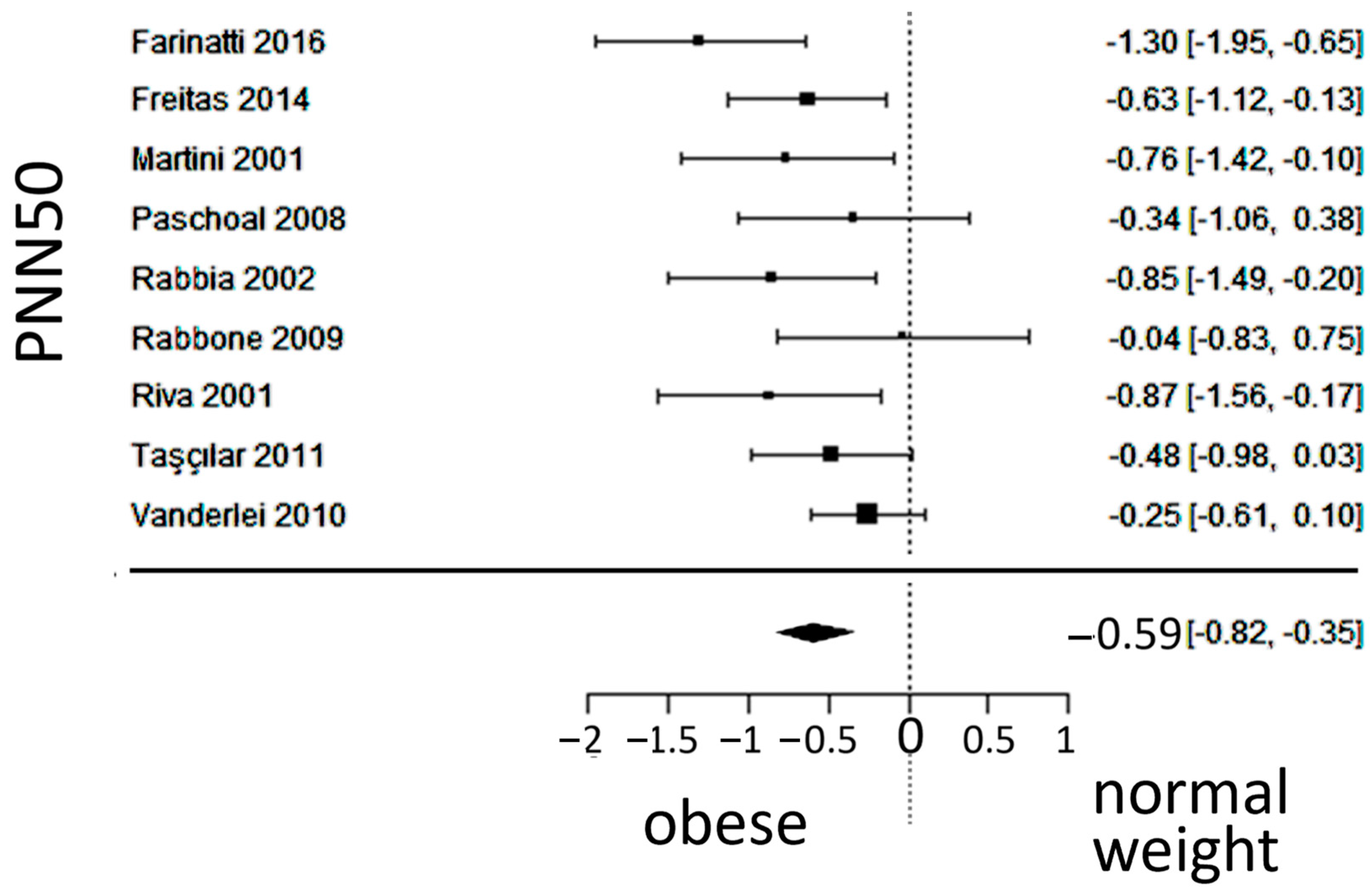
| Reference Number | [19] | [20] | [21] | [22] | [23] | [24] | [25] | [26] | [27] | [28] | [29] | [30] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Selection 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Exposure 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Baseline 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Comparability 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Outcome 6 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| F/U duration | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 |
| F/U adequacy | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 | N/A 7 |
| Score | 5 | 5 | 5 | 5 | 6 | 5 | 6 | 6 | 6 | 5 | 6 | 5 |
| Normal-Weight (n = 680) | Obese (n = 422) | |
|---|---|---|
| Age, years | 9.9 ± 2.5 | 11.6 ± 2.5 |
| Male sex, % | 53.7 | 48.5 |
| BMI, kg/m2 | 17.07 ± 2.36 | 28.79 ± 4.18 |
| SBP, mmHg | 109 ± 11 | 121 ± 12 |
| DBP, mmHg | 65 ± 8 | 74 ± 11 |
| TC, mg/dL | 152.5 ± 26.4 | 163.3 ± 29.5 |
| HDL, mg/dL | 46.4 ± 11.5 | 47.0 ± 9.5 |
| LDL, mg/dL | 95.4 ± 19.8 | 91.6 ± 22.4 |
| TGs mg/dL | 82.9 ± 32.1 | 95.1 ± 33.4 |
| FPG, mmol/L | 84.8 ± 4.7 | 87.8 ± 5.6 |
| Variable | k | τ | τ2 (SE) | I2 | H2 | d.f. | Q | p |
|---|---|---|---|---|---|---|---|---|
| SDNN | 12 | 0.128 | 0.0165 (0.0329) | 20.34% | 1.255 | 11 | 12.405 | 0.334 |
| LF/HF | 12 | 0.243 | 0.0591 (0.0569) | 47.54% | 1.906 | 11 | 20.272 | 0.042 1 |
| LF | 7 | 0.544 | 0.2956 (0.2279) | 75.62% | 4.102 | 6 | 23.420 | <0.001 1 |
| HF | 7 | 0 | 0 (0.0532) | 0% | 1 | 6 | 4.649 | 0.590 |
| RMSSD | 11 | 0.229 | 0.0523 (0.0571) | 43.79% | 1.779 | 10 | 17.077 | 0.073 |
| PNN50 | 9 | 0.199 | 0.0397 (0.063) | 31.53% | 1.460 | 8 | 11.767 | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, G.E.; Balomenou, F.; Sakellariou, X.M.; Tassopoulos, C.; Nikas, D.N.; Giapros, V.; Kolettis, T.M. Autonomic Function in Obese Children and Adolescents: Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1854. https://doi.org/10.3390/jcm13071854
Papadopoulos GE, Balomenou F, Sakellariou XM, Tassopoulos C, Nikas DN, Giapros V, Kolettis TM. Autonomic Function in Obese Children and Adolescents: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(7):1854. https://doi.org/10.3390/jcm13071854
Chicago/Turabian StylePapadopoulos, Georgios E., Foteini Balomenou, Xenofon M. Sakellariou, Christos Tassopoulos, Dimitrios N. Nikas, Vasileios Giapros, and Theofilos M. Kolettis. 2024. "Autonomic Function in Obese Children and Adolescents: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 7: 1854. https://doi.org/10.3390/jcm13071854
APA StylePapadopoulos, G. E., Balomenou, F., Sakellariou, X. M., Tassopoulos, C., Nikas, D. N., Giapros, V., & Kolettis, T. M. (2024). Autonomic Function in Obese Children and Adolescents: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(7), 1854. https://doi.org/10.3390/jcm13071854








