Feasibility of Fluid Responsiveness Assessment in Patients at Risk for Increased Intracranial Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Approval and Patient Consent
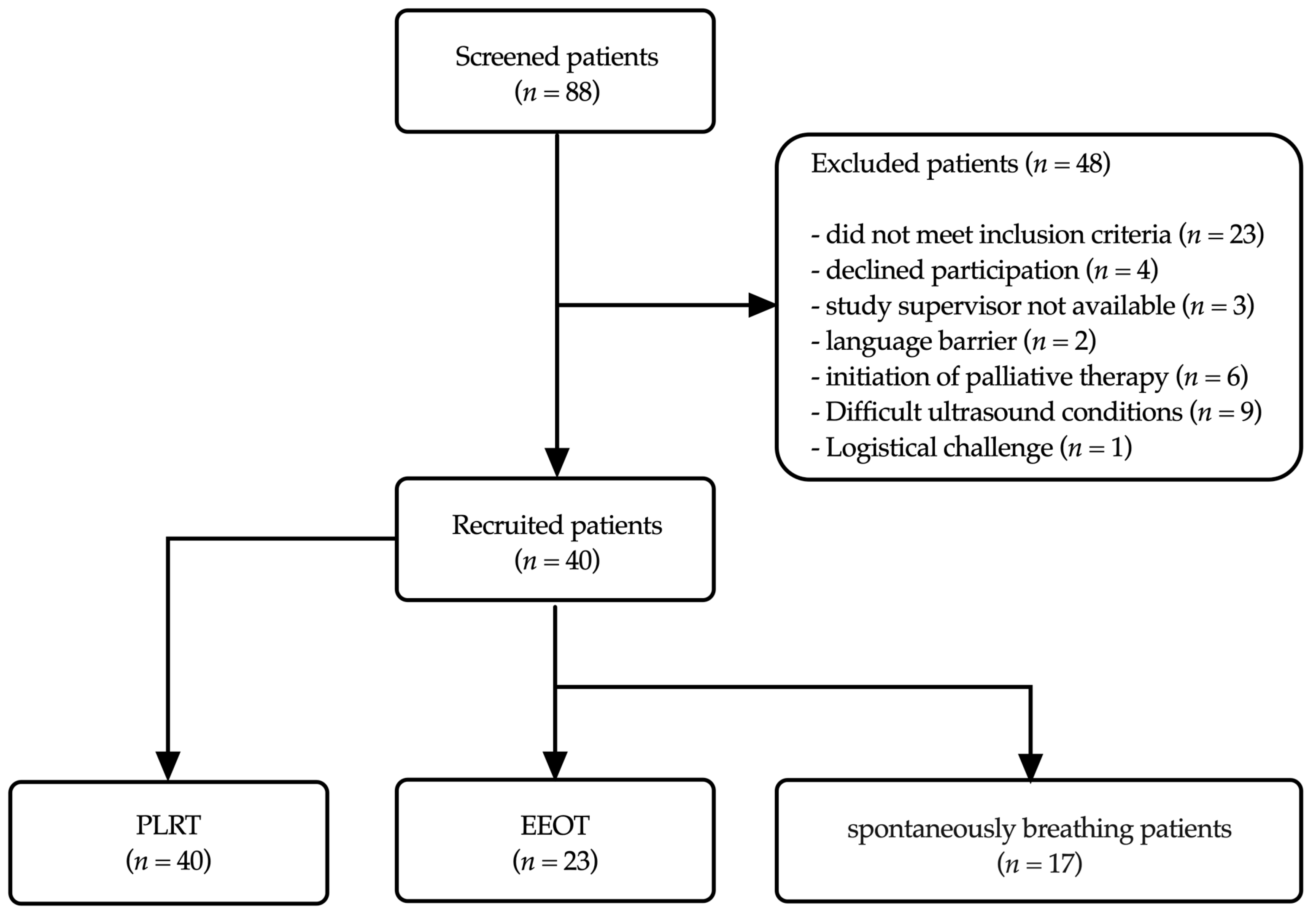
2.3. Patient Selection
2.4. Measurements and Monitoring
2.4.1. ICP Monitoring Techniques
2.4.2. Arterial Line Placement and Monitoring
2.4.3. Cerebral Perfusion Pressure Measurement
2.4.4. Mechanical Ventilation
2.5. Protocols for the Assessment of Fluid Responsiveness
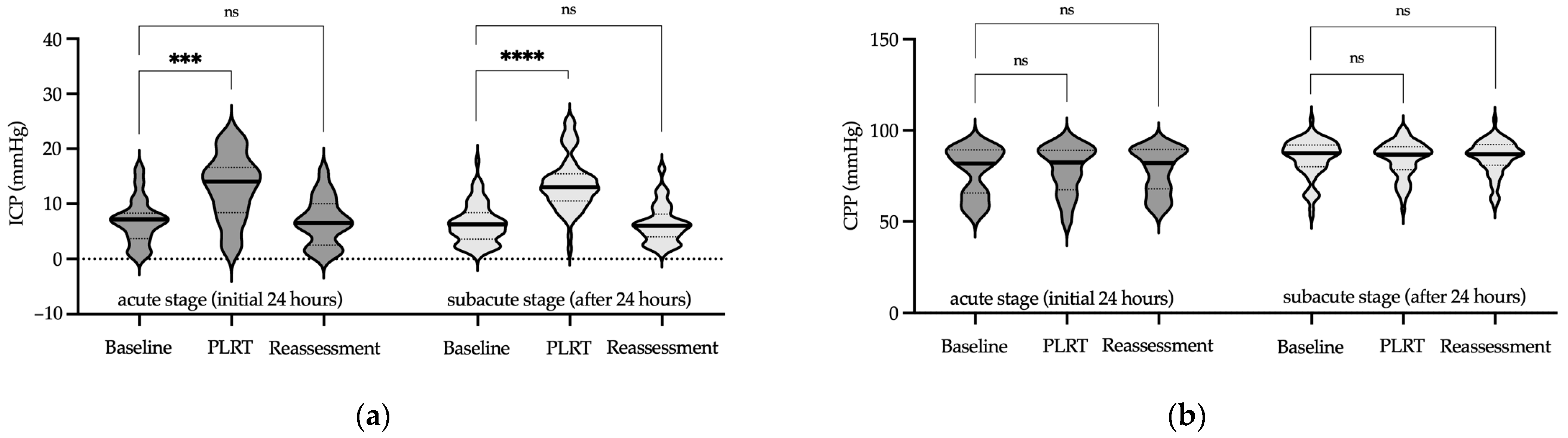
2.5.1. Passive Leg Raise Test (PLRT)
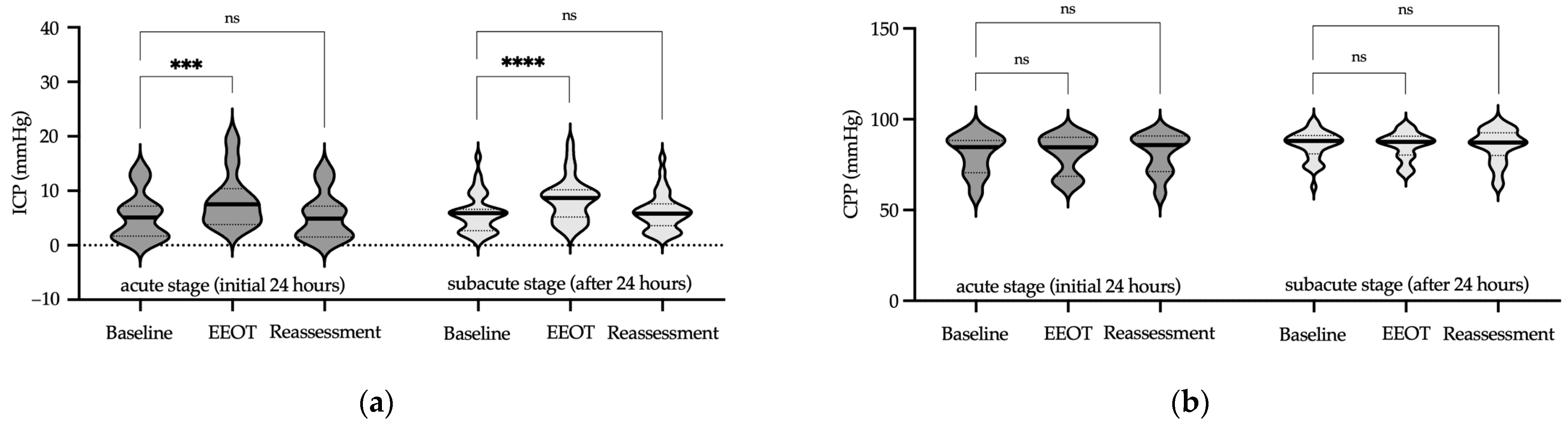
2.5.2. End-Expiratory Occlusion Test (EEOT)
2.5.3. Reason for Disparity in Measurement Numbers between PLRT and EEOT
2.5.4. Sedation during Tests
2.6. Statistical Analysis
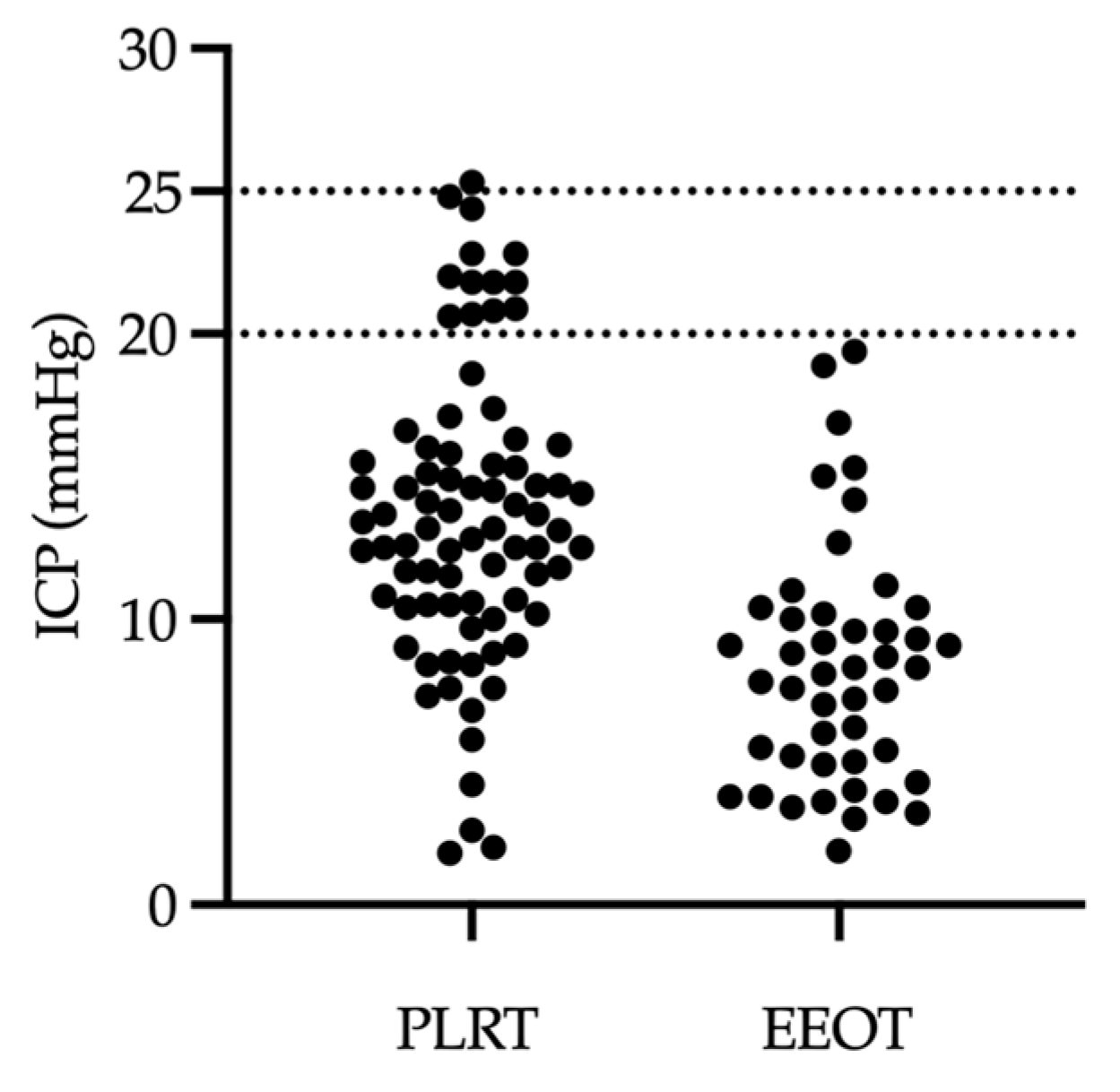
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schizodimos, T.; Soulountsi, V.; Iasonidou, C.; Kapravelos, N. An overview of management of intracranial hypertension in the intensive care unit. J. Anesthesia 2020, 34, 741–757. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care. Neurocrit. Care 2014, 21, 1–26. [Google Scholar] [CrossRef]
- Deem, S.; Diringer, M.; Livesay, S.; Treggiari, M.M. Hemodynamic Management in the Prevention and Treatment of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2023, 39, 81–90. [Google Scholar] [CrossRef]
- Dias, C.; de Castro, A.; Gaio, R.; Silva, R.; Pereira, E.; Monteiro, E. Lung Injury Risk in Traumatic Brain Injury Managed with Optimal Cerebral Perfusion Pressure Guided-Therapy. J. Crit. Care Med. 2023, 9, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Aries, M.J.H.; Czosnyka, M.; Budohoski, K.P.; Steiner, L.A.; Lavinio, A.; Kolias, A.G.M.; Hutchinson, P.J.P.; Brady, K.M.; Menon, D.K.; Pickard, J.D.F.; et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury*. Crit. Care Med. 2012, 40, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, C.; Raj, R.; Zeiler, F.A.; Thelin, E.P. Current state of high-fidelity multimodal monitoring in traumatic brain injury. Acta Neurochir. 2022, 164, 3091–3100. [Google Scholar] [CrossRef]
- Martini, R.P.; Deem, S.; Brown, M.; Souter, M.J.; Yanez, N.D.; Daniel, S.; Treggiari, M.M. The association between fluid balance and outcomes after subarachnoid hemorrhage. Neurocrit. Care 2012, 17, 191–198. [Google Scholar] [CrossRef]
- Heifets, B.D.; Tanaka, P.; Burbridge, M.A. Fluid management concepts for severe neurological illness. Curr. Opin. Anaesthesiol. 2018, 31, 526–531. [Google Scholar] [CrossRef]
- Oddo, M.; Poole, D.; Helbok, R.; Meyfroidt, G.; Stocchetti, N.; Bouzat, P.; Cecconi, M.; Geeraerts, T.; Martin-Loeches, I.; Quintard, H.; et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensiv. Care Med. 2018, 44, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Cavallazzi, R. Does the Central Venous Pressure Predict Fluid Responsiveness? An Updated Meta-Analysis and a Plea for Some Common Sense*. Crit. Care Med. 2013, 41, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Jones, G.M.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocrit. Care 2020, 32, 647–666. [Google Scholar] [CrossRef]
- La Via, L.; Vasile, F.; Perna, F.; Zawadka, M. Prediction of fluid responsiveness in critical care: Current evidence and future perspective. Trends Anaesth. Crit. Care 2024, 54, 101316. [Google Scholar] [CrossRef]
- Monnet, X.; Teboul, J.-L. Passive leg raising. Intensiv. Care Med. 2008, 34, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Teboul, J.-L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensiv. Care Med. Exp. 2015, 3, A587. [Google Scholar] [CrossRef]
- Georges, D.; de Courson, H.; Lanchon, R.; Sesay, M.; Nouette-Gaulain, K.; Biais, M. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: An echocardiographic study. Crit. Care 2018, 22, 32. [Google Scholar] [CrossRef]
- Monnet, X.; Osman, D.; Ridel, C.; Lamia, B.; Richard, C.; Teboul, J.-L. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit. Care Med. 2009, 37, 951–956. [Google Scholar] [CrossRef]
- Gavelli, F.; Teboul, J.-L.; Monnet, X. The end-expiratory occlusion test: Please, let me hold your breath! Crit. Care 2019, 23, 274. [Google Scholar] [CrossRef]
- Bauer, M.; Basic, D.; Riedmann, M.; Muench, E.; Schuerer, L.; Thomé, C.; Freyschlag, C.F. Passive leg raising in brain injury patients within the neurointensive care unit. A prospective trial. Anaesthesiol. Intensiv. Ther. 2021, 53, 200–206. [Google Scholar] [CrossRef]
- Messina, A.; Calabrò, L.; Pugliese, L.; Lulja, A.; Sopuch, A.; Rosalba, D.; Morenghi, E.; Hernandez, G.; Monnet, X.; Cecconi, M. Fluid challenge in critically ill patients receiving haemodynamic monitoring: A systematic review and comparison of two decades. Crit. Care 2022, 26, 186. [Google Scholar] [CrossRef]
- La Via, L.; Astuto, M.; Dezio, V.; Muscarà, L.; Palella, S.; Zawadka, M.; Vignon, P.; Sanfilippo, F. Agreement between subcostal and transhepatic longitudinal imaging of the inferior vena cava for the evaluation of fluid responsiveness: A systematic review. J. Crit. Care 2022, 71, 154108. [Google Scholar] [CrossRef]
- Stocchetti, N.; Carbonara, M.; Citerio, G.; Ercole, A.; Skrifvars, M.B.; Smielewski, P.; Zoerle, T.; Menon, D.K. Severe traumatic brain injury: Targeted management in the intensive care unit. Lancet Neurol. 2017, 16, 452–464. [Google Scholar] [CrossRef]
- Rossi, S.; Picetti, E.; Zoerle, T.; Carbonara, M.; Zanier, E.R.; Stocchetti, N. Fluid Management in Acute Brain Injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 74. [Google Scholar] [CrossRef]
- Battisti-Charbonney, A.; Fisher, J.; Duffin, J. The cerebrovascular response to carbon dioxide in humans. J. Physiol. 2011, 589, 3039–3048. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, D.; Jia, Y.; Tian, Y.; Wang, Y.; Wei, Y.; Zhang, J.; Jiang, R. Analysis of the association of fluid balance and short-term outcome in traumatic brain injury. J. Neurol. Sci. 2016, 364, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Gress, D.R. Monitoring of Volume Status After Subarachnoid Hemorrhage. Neurocrit. Care 2011, 15, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Pelz, J.O.; Fischer, M.-M.; Bungert-Kahl, P.; Lindner, D.; Fricke, C.; Michalski, D. Fluid Balance Variations During the Early Phase of Large Hemispheric Stroke Are Associated with Patients’ Functional Outcome. Front. Neurol. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Uryga, A.; Giardina, A.; Ciliberti, P.; Battaglini, D.; Patroniti, N.; Czosnyka, M.; Monnet, X.; Cecconi, M.; Robba, C. The effect of passive leg raising test on intracranial pressure and cerebral autoregulation in brain injured patients: A physiological observational study. Crit. Care 2024, 28, 23. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Singhal, V. Intracranial pressure monitoring. J. Neuroanaesth. Crit. Care 2015, 02, 193–203. [Google Scholar] [CrossRef]



| Patient characteristics | |
| Age (years) | 65 (56–73) |
| Gender (male/female) | 27/13 (67.5%/32.5%) |
| Body mass index (kg/m2) | 25.5 (23.8–28.2) |
| Referring diagnosis | |
| Subarachnoid hemorrhage (SAH) | 12 (30%) |
| - Non-traumatic SAH | 9 (22.5%) |
| - Traumatic SAH | 3 (7.5%) |
| Intracerebral hemorrhage (ICH) | 9 (22.5%) |
| - Non-traumatic ICH | 6 (15%) |
| - Traumatic ICH | 3 (7.5%) |
| Subarachnoid and intracerebral hemorrhage | 5 (12.5%) |
| Subarachnoid hemorrhage and subdural hematoma | 3 (7.5%) |
| Intraventricular hemorrhage | 3 (7.5%) |
| Secondary acute hydrocephalus * | 5 (12.5%) |
| Secondary (postoperative) hemorrhage | 2 (5%) |
| Traumatic subdural hematoma | 1 (2.5%) |
| Comorbidities | |
| Arterial hypertension | 16 (40%) |
| Atrial fibrillation | 6 (15%) |
| Diabetes mellitus type 2 | 3 (7.5%) |
| COPD | 4 (10%) |
| Chronic kidney disease | 1 (2.5%) |
| Coronary heart disease | 4 (10%) |
| Smoking | 2 (5%) |
| Clinical course and treatment | |
| GCS | 7 (3–14) |
| Days in ICU | 15 (11–20) |
| Antibiotic therapy | 30 (75%) |
| Catecholamine therapy | 22 (55%) |
| Pneumonia | 18 (45%) |
| Respiratory parameters (of ventilated patients) | |
| Tidal volume (ml/kg) | 7 (6–7) |
| PEEP (cmH2O) | 5 (5–5) |
| Driving pressure (cmH2O) | 8.5 (7–10) |
| Respiratory rate (min−1) | 14 (14–15) |
| Dynamic compliance (ml/cmH2O) | 64 (55–72) |
| Fraction of inspired oxygen | 0.35 (0.35–0.40) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivkovic, A.R.; Kjaev, A.; Schönenberger, S.; Krieg, S.M.; Weigand, M.A.; Neumann, J.-O. Feasibility of Fluid Responsiveness Assessment in Patients at Risk for Increased Intracranial Pressure. J. Clin. Med. 2024, 13, 1786. https://doi.org/10.3390/jcm13061786
Zivkovic AR, Kjaev A, Schönenberger S, Krieg SM, Weigand MA, Neumann J-O. Feasibility of Fluid Responsiveness Assessment in Patients at Risk for Increased Intracranial Pressure. Journal of Clinical Medicine. 2024; 13(6):1786. https://doi.org/10.3390/jcm13061786
Chicago/Turabian StyleZivkovic, Aleksandar R., Aleko Kjaev, Silvia Schönenberger, Sandro M. Krieg, Markus A. Weigand, and Jan-Oliver Neumann. 2024. "Feasibility of Fluid Responsiveness Assessment in Patients at Risk for Increased Intracranial Pressure" Journal of Clinical Medicine 13, no. 6: 1786. https://doi.org/10.3390/jcm13061786
APA StyleZivkovic, A. R., Kjaev, A., Schönenberger, S., Krieg, S. M., Weigand, M. A., & Neumann, J.-O. (2024). Feasibility of Fluid Responsiveness Assessment in Patients at Risk for Increased Intracranial Pressure. Journal of Clinical Medicine, 13(6), 1786. https://doi.org/10.3390/jcm13061786







