Recipient Survival among Living Donor vs. Deceased Donor Liver Transplants for Acute Liver Failure in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design and Patient Population
2.3. Study Outcome
2.4. Statistical Methods
3. Results
3.1. Characteristics of Pediatric LDLT vs. DDLT Recipients with ALF
3.2. Survival Analysis
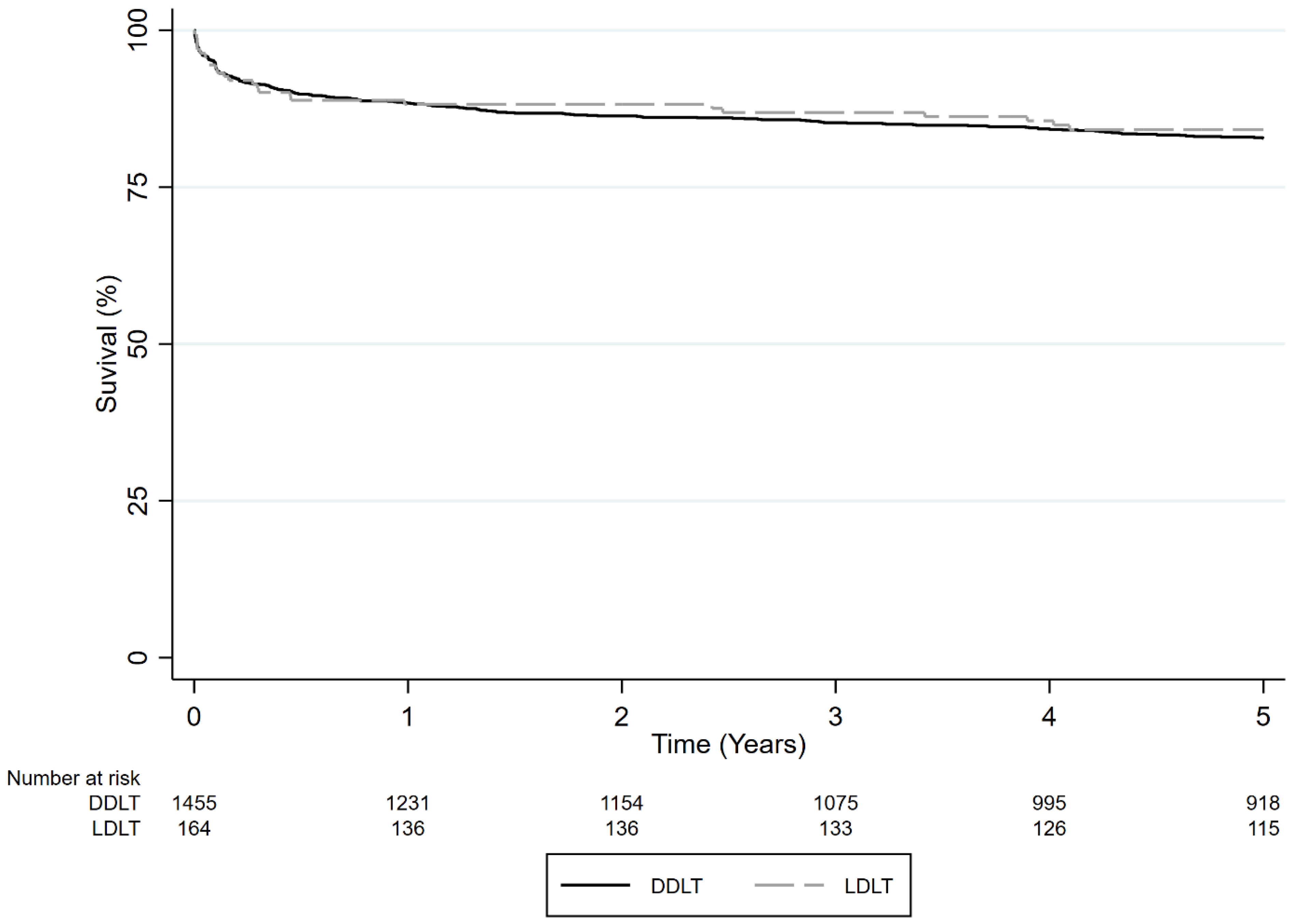
3.2.1. Survival Comparison of All ALF Patients Who Received LDLT vs. DDLT
3.2.2. Survival of Pediatric ALF Patients Who Received LDLT vs. DDLT
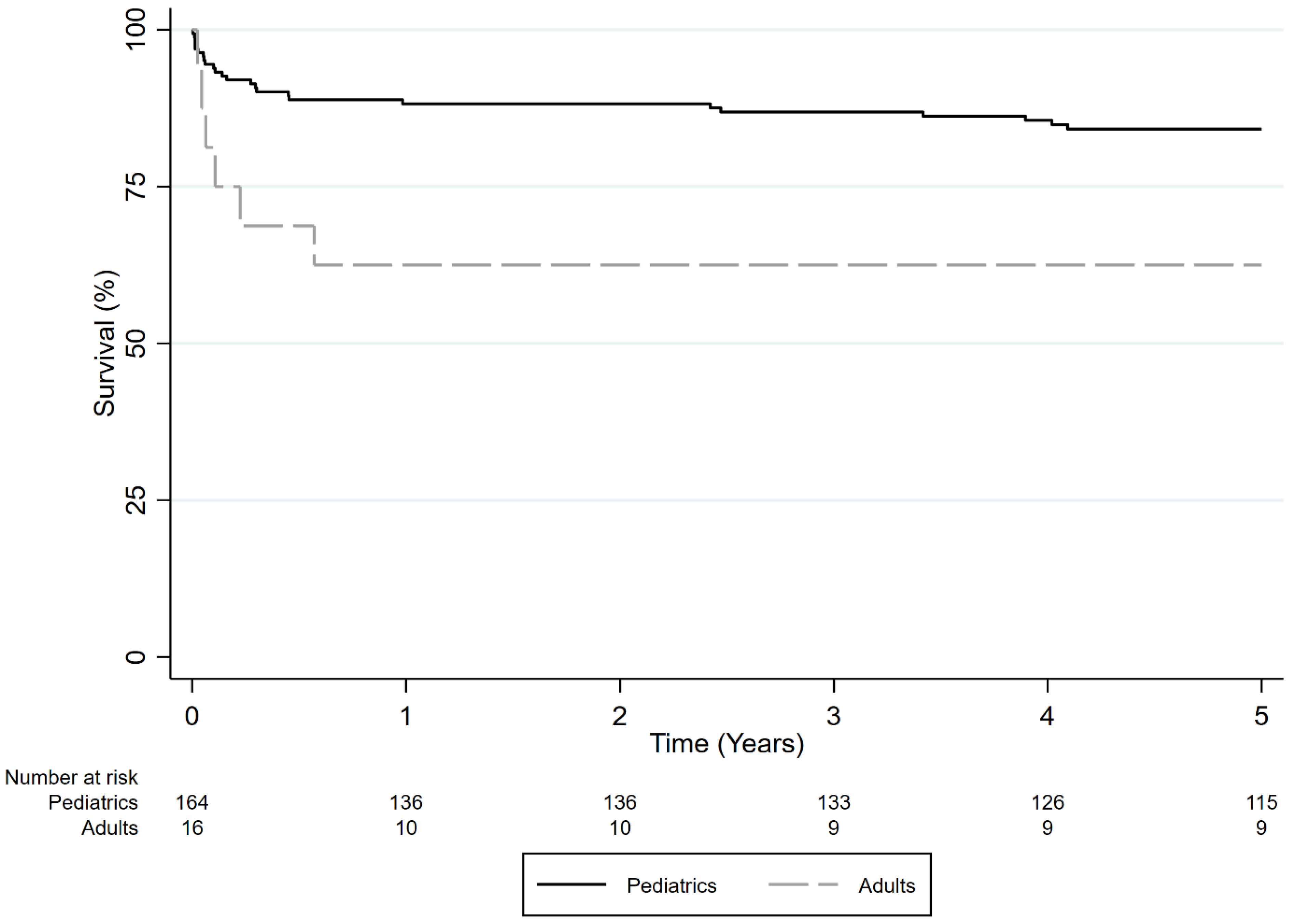
3.2.3. Survival Comparison of Adult vs. Pediatric Patients Who Received LDLT for ALF
3.2.4. Survival of Adult vs. Pediatric Patients Who Received DDLT for ALF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALF | Acute liver failure |
| DDLT | Deceased donor liver transplantation |
| DILI | Drug-induced liver injury |
| LT | Liver transplantation |
| LDLT | Living donor liver transplant |
| OPTN | Organ Procurement and Transplantation Network |
| UNOS | United Network for Organ Sharing |
| US | United States |
References
- Shingina, A.; Ziogas, I.A.; Vutien, P.; Uleryk, E.; Shah, P.S.; Renner, E.; Bhat, M.; Tinmouth, J.; Kim, J. Adult-to-adult living donor liver transplantation in acute liver failure. Transplant. Rev. 2022, 36, 100691. [Google Scholar] [CrossRef]
- Wendon, J.; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Nevens, F.; Samuel, D.; Simpson, K.J.; Yaron, I.; Bernardi, M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef]
- Özden, I.; Yavru, H.A.; Durmaz, Ö.; Orhun, G.; Salmaslıoğlu, A.; Güllüoğlu, M.; Alper, A.; İbiş, C.; Serin, K.R.; Önal, Z.; et al. Complementary Roles of Cadaveric and Living Donor Liver Transplantation in Acute Liver Failure. J. Gastrointest. Surg. 2021, 25, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Abu-Gazala, S.; Olthoff, K.M. Status of Adult Living Donor Liver Transplantation in the United States: Results from the Adult-To-Adult Living Donor Liver Transplantation Cohort Study. Gastroenterol. Clin. N. Am. 2018, 47, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Fuchinoue, W.; Tanaka, K.; Takasaki, K.; Hashimoto, E.; Kawai, T.; Nakajma, I.; Nakagawa, Y.; Fujta, S.; Akamatsu, M.; Kitajima, K.; et al. Living-related liver transplantation for fulminant hepatic failure. Transplant. Proc. 1997, 29, 424–427. [Google Scholar] [CrossRef]
- Wong, N.Z.; Schaubel, D.E.; Reddy, K.R.; Bittermann, T. Transplant center experience influences spontaneous survival and waitlist mortality in acute liver failure: An analysis of the UNOS database. Am. J. Transplant. 2021, 21, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Campsen, J.; Blei, A.T.; Emond, J.C.; Everhart, J.E.; Freise, C.E.; Lok, A.S.; Saab, S.; Wisniewski, K.A.; Trotter, J.F. The Adult-to-Adult Living Donor Liver Transplantation Cohort Study Group. Outcomes of Living Donor Liver Transplantationfor Acute Liver Failure: The Adult-to-Adult LivingDonor Liver Transplantation Cohort Study. Liver Transplant. 2008, 14, 767–768. [Google Scholar] [CrossRef]
- Miller, C.M.; Quintini, C.; Dhawan, A.; Durand, F.; Heimbach, J.K.; Kim-Schluger, H.L.; Kyrana, E.; Lee, S.-G.; Lerut, J.; Lo, C.-M.; et al. The international liver transplantation society living donor liver transplant recipient guideline. Transplantation 2017, 101, 938–944. [Google Scholar] [CrossRef]
- Tran, L.; Humar, A. Expanding living donor liver transplantation in the Western world: Changing the paradigm. Dig. Med. Res. 2020, 3, 52. [Google Scholar] [CrossRef]
- Tran, L.; Humar, A. Current status of adult liver transplantation: Utilization of living donor versus deceased donor graft. Curr. Opin. Organ Transplant. 2021, 26, 133–138. [Google Scholar] [CrossRef]
- Nadalin, S.; Capobianco, I.; Panaro, F.; Di Francesco, F.; Troisi, R.; Sainz-Barriga, M.; Muiesan, P.; Königsrainer, A.; Testa, G. Living donor liver transplantation in Europe. Hepatobiliary Surg. Nutr. 2016, 5, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G. Asian contribution to living donor liver transplantation. J. Gastroenterol. Hepatol. 2006, 21, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Rela, M.; Reddy, M.S. Living donor liver transplant (LDLT) is the way forward in Asia. Hepatol. Int. 2017, 11, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Robson, N.Z.M.H.; Razack, A.H.; Dublin, N. Review paper: Organ transplants: Ethical, social, and religious issues in a multicultural society. Asia-Pac. J. Public Health/Asia-Pac. Acad. Consort. Public Health 2010, 22, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, G.; Kota, V.; Rela, M. Liver transplantation in India. Liver Transplant. 2016, 22, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Rela, M.; Rammohan, A. Why are there so many liver transplants from living donors in Asia and so few in Europe and the US? J. Hepatol. 2021, 75, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Polson, J.; Lee, W.M. AASLD position paper: The management of acute liver failure. Hepatology 2005, 41, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Nair, K.; Thillai, M.; Manikandan, K.; Sethi, P.; Madhusrinivasan, D.; Johns, S.M.; Binoj, S.T.; Mohammed, Z.; Ramachandran, N.M.; et al. Liver Transplant in Acute Liver Failure—Looking Back Over 10 Years. J. Clin. Exp. Hepatol. 2020, 10, 322–328. [Google Scholar] [CrossRef]
- Reuben, A.; Tillman, H.; Fontana, R.J.; Davern, T.; McGuire, B.; Stravitz, R.T.; Durkalski, V.; Larson, A.M.; Liou, I.; Fix, O.; et al. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann. Intern. Med. 2016, 164, 724–732. [Google Scholar] [CrossRef]
- Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Noreen, S.M.; Robinson, A.M.; Miller, E.; Snyder, J.J.; Israni, A.K.; et al. OPTN/SRTR 2017 Annual Data Report: Liver. Am. J. Transplantation Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2019, 19, 184–283. [Google Scholar] [CrossRef]
- Maluf, D.G.; Stravitz, R.T.; Cotterell, A.H.; Posner, M.P.; Nakatsuka, M.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Ham, J.M.; Marcos, A.; et al. Adult living donor versus deceased donor liver transplantation: A 6-year single center experience. Am. J. Transplant. 2005, 5, 149–156. [Google Scholar] [CrossRef]
- Pomposelli, J.; Verbesey, J.; Simpson, M.; Lewis, W.; Gordon, F.; Khettry, U.; Wald, C.; Ata, S.; Morin, D.; Garrigan, K.; et al. Improved survival after live donor adult liver transplantation (LDALT) using right lobe grafts: Program experience and lessons learned. Am. J. Transplant. 2006, 6, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Ganesh, S.; Jorgensen, D.; Tevar, A.; Ganoza, A.; Molinari, M.; Hughes, C. Adult Living Donor Versus Deceased Donor Liver Transplant (LDLT Versus DDLT) at a Single Center: Time to Change Our Paradigm for Liver Transplant. Ann. Surg. 2019, 270, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Lim, Y.S.; Han, S.; Lee, H.C.; Hwang, S.; Lee, S.G. Predicting survival after living and deceased donor liver transplantation in adult patients with acute liver failure. J. Gastroenterol. 2012, 47, 1115–1124. [Google Scholar] [CrossRef]
- Park, S.J.; Lim, Y.-S.; Hwang, S.; Heo, N.Y.; Lee, H.C.; Suh, D.J.; Yu, E.; Lee, S.G. Emergency adult-to-adult living-donor liver transplantation for acute liver failure in a hepatitis B virus endemic area. Hepatology 2010, 51, 903–911. [Google Scholar] [CrossRef]
- Mousa, O.Y.; Nguyen, J.H.; Ma, Y.; Rawal, B.; Musto, K.R.; Dougherty, M.K.; Shalev, J.A.; Harnois, D.M. Evolving Role of Liver Transplantation in Elderly Recipients. Liver Transplant. 2019, 25, 1363–1374. [Google Scholar] [CrossRef]
- Giorgakis, E.M.; Ivanics, T.; Khorsandi, S.E.; Wallace, D.M.; Burdine, L.; Jassem, W.; Mathur, A.K.; Heaton, N. Disparities in the Use of Older Donation after Circulatory Death Liver Allografts in the United States Versus the United Kingdom. Transplantation 2022, 106, e358–e367. [Google Scholar] [CrossRef] [PubMed]
- Haugen, C.E.; Holscher, C.M.; Luo, X.; Bowring, M.G.; Orandi, B.J.; Thomas, A.G.; Garonzik-Wang, J.; Massie, A.B.; Philosophe, B.; McAdams-DeMarco, M.; et al. Assessment of Trends in Transplantation of Liver Grafts from Older Donors and Outcomes in Recipients of Liver Grafts from Older Donors, 2003–2016. JAMA Surg. 2019, 154, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Urrunaga, N.H.; Rachakonda, V.P.; Magder, L.S.; Mindikoglu, A.L. Outcomes of living versus deceased donor liver transplantation for acute liver failure in the United States. Transplant. Proc. 2014, 46, 219–224. [Google Scholar] [CrossRef]
- Sars, C.; Tranäng, M.; Ericzon, B.G.; Berglund, E. Liver transplantation for acute liver failure—A 30-year single center experience. Scand. J. Gastroenterol. 2018, 53, 876–882. [Google Scholar] [CrossRef]
- Sierra, L.; Barba, R.; Ferrigno, B.; Goyes, D.; Diaz, W.; Patwardhan, V.R.; Saberi, B.; Bonder, A. Living-Donor Liver Transplant and Improved Post-Transplant Survival in Patients with Primary Sclerosing Cholangitis. J. Clin. Med. 2023, 12, 2807. [Google Scholar] [CrossRef] [PubMed]
- Yoeli, D.; Choudhury, R.A.; Moore, H.B.; Jackson, W.E.; Nydam, T.L.; Wachs, M.E.; Pomfret, E.A.; Adams, M.A. Living Donor Liver Transplant Center Volume Influences Waiting List Survival Among Children Listed for Liver Transplantation. Transplantation 2022, 106, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Yoeli, D.; Goss, M.; Galván, N.T.N.; Desai, M.S.; Miloh, T.A.; Rana, A. Trends in pediatric liver transplant donors and deceased donor circumstance of death in the United States, 2002-2015. Pediatr. Transplant. 2018, 22, e13156. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Pallister, Z.; Halazun, K.; Cotton, R.; Guiteau, J.; Nalty, C.C.; O’mahony, C.A.; Goss, J.A. Pediatric liver transplant center volume and the likelihood of transplantation. Pediatrics 2015, 136, e99–e107. [Google Scholar] [CrossRef] [PubMed]
- Ascher Bartlett, J.M.; Yanni, G.; Kwon, Y.; Emamaullee, J. Pediatric acute liver failure: Reexamining key clinical features, current management, and research prospects. Liver Transplant. 2022, 28, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Firl, D.J.; Sasaki, K.; McVey, J.; Hupertz, V.; Radhakrishnan, K.; Fujiki, M.; Eghtesad, B.; Miller, C.M.; Quintini, C.; Hashimoto, K. Improved Survival Following Living Donor Liver Transplantation for Pediatric Acute Liver Failure: Analysis of 20 Years of US National Registry Data. Liver Transplant. 2019, 25, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Mogul, D.B.; Lee, J.; Purnell, T.S.; Massie, A.B.; Ishaque, T.; Segev, D.L.; Bridges, J.F.P. Barriers to access in pediatric living-donor liver transplantation. Pediatr. Transplant. 2019, 23, e13513. [Google Scholar] [CrossRef] [PubMed]
- Nobel, Y.R.; Forde, K.A.; Wood, L.; Cartiera, K.; Munoz-Abraham, A.S.; Yoo, P.S.; Abt, P.L.; Goldberg, D.S. Racial and ethnic disparities in access to and utilization of living donor liver transplants. Liver Transpl. 2015, 21, 904–913. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Kim, H.B.; Vakili, K. Pediatric liver transplantation. Semin. Pediatr. Surg. 2017, 26, 217–223. [Google Scholar] [CrossRef]
- Mogul, D.B.; Luo, X.; Bowring, M.G.; Chow, E.K.; Massie, A.B.; Schwarz, K.B.; Cameron, A.M.; Bridges, J.F.; Segev, D.L. Fifteen-Year Trends in Pediatric Liver Transplants: Split, Whole Deceased, and Living Donor Grafts. J. Pediatr. 2018, 196, 148–153.e2. [Google Scholar] [CrossRef] [PubMed]


| Patient Characteristics (n = 1619) | LDLT (n = 164, 10.1%) | DDLT (n = 1455, 89.9%) | p-Value |
|---|---|---|---|
| Age | 3 (1–6) | 4 (1–12) | <0.001 |
| Gender, female, n (%) | 62 (37.8%) | 668 (45.9%) | 0.04 |
| Blood type | 0.26 | ||
| O | 96 (58.5%) | 754 (51.8%) | |
| A | 46 (28.0%) | 474 (32.5%) | |
| B | 20 (12.2%) | 182 (12.5%) | |
| AB | 2 (1.2%) | 45 (3.1%) | |
| Race | 0.02 | ||
| White | 95 (57.9%) | 664 (45.6%) | |
| African American/Black | 19 (11.6%) | 254 (17.5%) | |
| Hispanic | 41 (25.0%) | 387 (26.6%) | |
| Asian | 7 (4.3%) | 84 (5.8%) | |
| Other | 2 (1.1%) | 66 (4.5%) | |
| Etiology | 0.11 | ||
| Hepatitis A | 1 (0.1%) | 1 (0.6%) | |
| Hepatitis B | 0 (0.0%) | 2 (0.1%) | |
| DILI | 4 (2.4%) | 62 (4.3%) | |
| Wilson disease | 5 (3.1%) | 87 (6.0%) | |
| Biliary atresia | 10 (6.1%) | 110 (7.6%) | |
| Other | 64 (39.0%) | 458 (31.5%) | |
| Unknown | 80 (48.8%) | 735 (50.5%) | |
| Sodium (mmol/L) | 140.3 (138–141) | 140.3 (138–142) | 0.40 |
| Creatinine (mg/dL) | 0.4 (0.2–0.7) | 0.4 (0.3–0.9) | 0.01 |
| Bilirubin (mg/dL) | 17.8 (10.3–23.1) | 15.7 (7.3–22.7) | 0.07 |
| INR | 3 (2–4.3) | 2.6 (1.7–3.8) | 0.007 |
| Dialysis | 10 (6.1%) | 182 (12.5%) | 0.02 |
| Ascites | 57 (31.7%) | 569 (39.1%) | <0.001 |
| Hepatic encephalopathy | 51 (31.1%) | 3918 (81.9%) | 0.04 |
| Wait time (days) | 3.5 (2–9) | 5 (2–13) | 0.01 |
| Donor | |||
| Donor age (years) | 32 (26–37) | 16 (3–27) | <0.001 |
| Donor gender, female | 95 (57.9%) | 603 (41.4%) | <0.001 |
| Blood type | 0.19 | ||
| O | 122 (74.3%) | 1042 (71.6%) | |
| A | 26 (15.8%) | 307 (21.1%) | |
| B | 15 (9.1%) | 89 (6.1%) | |
| AB | 1 (0.6%) | 17 (1.1%) | |
| BMI | 24.4 (22.1–27.0) | 20.7 (17.0–24.3) | <0.001 |
| Cold ischemia time (hours) | 2.0 (1.0–4.7) | 7 (5.9–9) | <0.001 |
| Patient Characteristics (n = 3340) | LDLT (n = 16, 0.5%) | DDLT (n = 3324, 99.5%) |
|---|---|---|
| Age | 34.5 (21–49.5) | 40 (29–53) |
| Female gender | 14 (87.5%) | 2258 (67.9%) |
| Blood type | ||
| O | 9 (56.2%) | 1553 (46.7%) |
| A | 4 (25.0%) | 1147 (34.5%) |
| B | 3 (18.7%) | 475 (14.2%) |
| AB | 0 (0.0%) | 149 (4.4%) |
| Race | ||
| White | 10 (62.5%) | 1895 (57.0%) |
| African American/Black | 3 (18.7%) | 687 (20.6%) |
| Hispanic | 2 (12.5%) | 383 (11.5%) |
| Asian | 1 (6.2%) | 287 (8.6%) |
| Other | 0 (0.0%) | 72 (2.1%) |
| Etiology | ||
| Hepatitis A | 1 (6.2%) | 58 (1.7%) |
| Hepatitis B | 0 (0.0%) | 259 (7.7%) |
| DILI | 3 (18.7%) | 753 (22.6%) |
| Wilson disease | 1 (6.2%) | 222 (6.6%) |
| Biliary atresia | 0 (0.0%) | 0 (0.0%) |
| Other | 6 (37.5%) | 1435 (43.1%) |
| Unknown | 5 (31.2%) | 597 (17.9%) |
| Sodium (mmol/L) | 140.3 (137–141) | 140.3 (137–143) |
| Creatinine (mg/dL) | 1.0 (0.7–2.0) | 1.2 (0.8–2.4) |
| Bilirubin (mg/dL) | 5.2 (2.9–17.8) | 19.3 (9.2–27.2) |
| INR | 2.2 (1.3–4.6) | 3 (2.2–4.3) |
| Dialysis | 2 (12.5%) | 758 (22.8%) |
| Ascites | 6 (37.5%) | 1877 (56.4%) |
| Hepatic encephalopathy | 14 (87.5%) | 3075 (92.5%) |
| Wait time (days) | 3.5 (2–117) | 2 (2–4) |
| Donor characteristics | ||
| Donor age (years) | 45 (33.5–50) | 36 (22–50) |
| Donor gender, female | 8 (50.0%) | 1368 (41.1%) |
| Blood type | ||
| O | 11 (68.7%) | 2483 (74.7%) |
| A | 4 (25.0%) | 680 (20.4%) |
| B | 1 (6.2%) | 143 (4.3%) |
| AB | 0 (0.0%) | 18 (0.5%) |
| BMI | 24.8 (22.6–27.5) | 25.1 (22.3–28.2) |
| Cold ischemia time (hours) | 1.7 (1–4.8) | 6.2 (5–7.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moughames, E.; Gurakar, M.; Khan, A.; Alsaqa, M.; Ozturk, N.B.; Bonder, A.; Gurakar, A.; Saberi, B. Recipient Survival among Living Donor vs. Deceased Donor Liver Transplants for Acute Liver Failure in the United States. J. Clin. Med. 2024, 13, 1729. https://doi.org/10.3390/jcm13061729
Moughames E, Gurakar M, Khan A, Alsaqa M, Ozturk NB, Bonder A, Gurakar A, Saberi B. Recipient Survival among Living Donor vs. Deceased Donor Liver Transplants for Acute Liver Failure in the United States. Journal of Clinical Medicine. 2024; 13(6):1729. https://doi.org/10.3390/jcm13061729
Chicago/Turabian StyleMoughames, Eric, Merve Gurakar, Amir Khan, Marwan Alsaqa, N. Begum Ozturk, Alan Bonder, Ahmet Gurakar, and Behnam Saberi. 2024. "Recipient Survival among Living Donor vs. Deceased Donor Liver Transplants for Acute Liver Failure in the United States" Journal of Clinical Medicine 13, no. 6: 1729. https://doi.org/10.3390/jcm13061729
APA StyleMoughames, E., Gurakar, M., Khan, A., Alsaqa, M., Ozturk, N. B., Bonder, A., Gurakar, A., & Saberi, B. (2024). Recipient Survival among Living Donor vs. Deceased Donor Liver Transplants for Acute Liver Failure in the United States. Journal of Clinical Medicine, 13(6), 1729. https://doi.org/10.3390/jcm13061729





