Early Breast Cancer: Could Combined LOCalizerTM and Ultrasound Localization Replace the Metallic Wire? A Multicentric Study
Abstract
1. Introduction
2. Patient and Methods
2.1. Study Design
2.2. Study Setting and Study Population
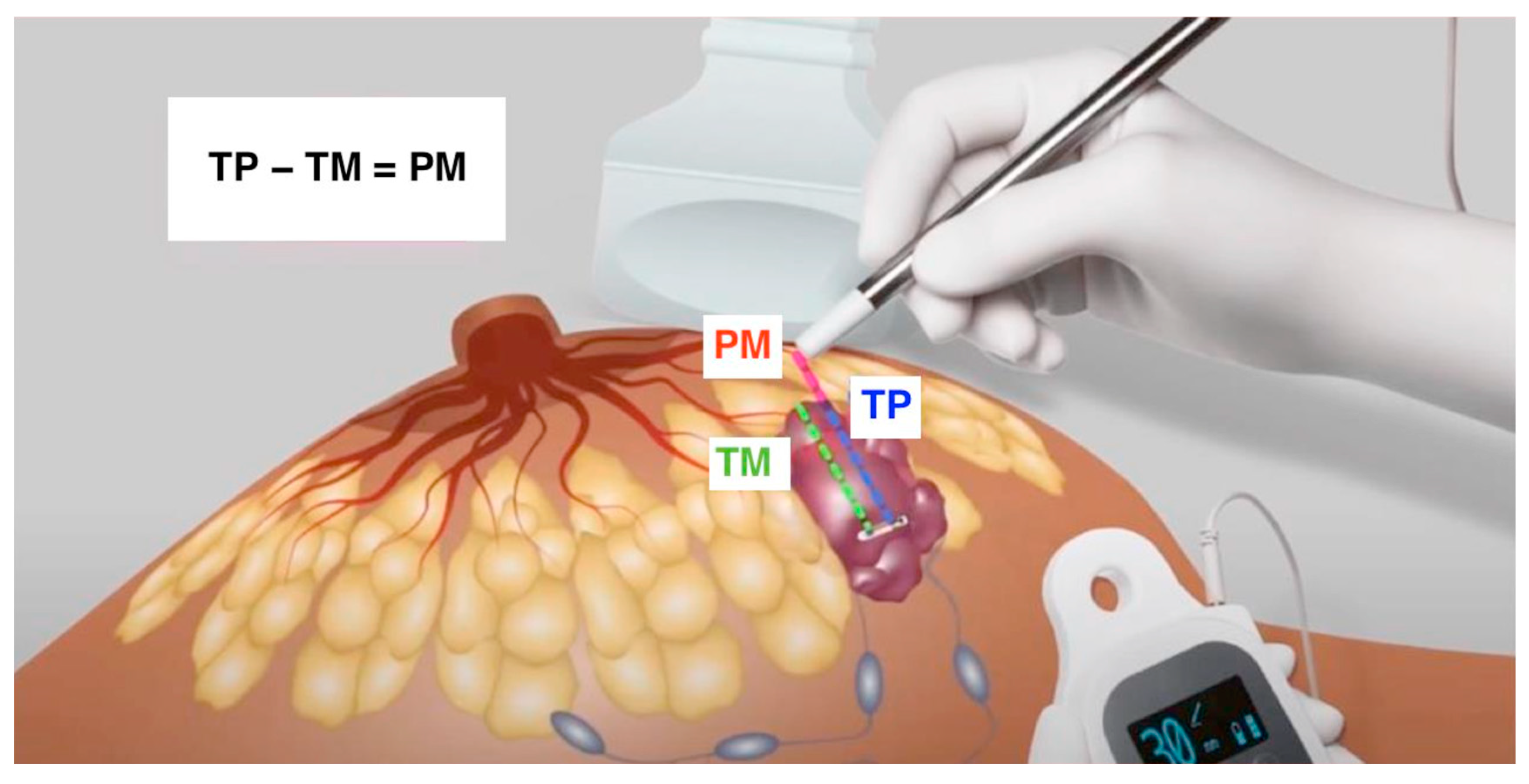
2.3. Combined Technique: LOCalizeTM and US
2.4. Wire-Guided Localization
2.5. Outcome Measures
2.6. Study Endpoints
2.7. Statistical Analysis
3. Results
3.1. Primary Outcome
3.2. Secondary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Ruggiero, R.; Pirozzi, R.; Gualtieri, G.; Terracciano, G.; Parisi, S.; Nesta, G.; Gazeneo, L.; Lanza Volpe, M.; Rinaldi, S.; Docimo, L. Overview on surgical management of papillary thyroid microcarcinoma. G. Chir. 2019, 40, 81–87. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Ünal, G.K.; Aslan, H.S.; Değirmencioğlu, S.; Aykota, M.R. Ultrasound-guided wire localization biopsy in non-palpable breast lesions: Predictive factors for malignancy. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1320–1327. [Google Scholar] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Panel Members. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Tardioli, S.; Ballesio, L.; Gigli, S.; DIPastena, F.; D’Orazi, V.; Giraldi, G.; Monti, M.; Amabile, M.I.; Pasta, V. Wire-guided Localization in Non-palpable Breast Cancer: Results from Monocentric Experience. Anticancer Res. 2016, 36, 2423–2427. [Google Scholar] [PubMed]
- Webster, A.J.; Kelly, B.N.; McGugin, C.; Coopey, S.B.; Smith, B.L.; Gadd, M.A.; Specht, M.C. Comparison of Wireless Localization Alternatives with Wire Localization for Nonpalpable Breast Lesions. J. Am. Coll. Surg. 2022, 234, 1091–1099. [Google Scholar] [CrossRef]
- Parisi, S.; Gambardella, C.; Ruggiero, R.; Tolone, S.; Lucido, F.S.; Docimo, L. Radiofrequency Identification—RFID using LOCalizer-Tag in Non-palpable Breast Lump. Indian J. Surg. 2022, 85, 934–938. [Google Scholar] [CrossRef]
- Parisi, S.; Gambardella, C.; Conzo, G.; Ruggiero, R.; Tolone, S.; Lucido, F.S.; Iovino, F.; Fisone, F.; Brusciano, L.; Parmeggiani, D.; et al. Advanced Localization Technique for Non-Palpable Breast Cancer: Radiofrequency alone VS Combined Technique with Ultrasound. J. Clin. Med. 2023, 12, 5076. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The new TNM-based staging of breast cancer. Virchows Arch. 2018, 472, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Norman, G. Likert scales, levels of measurement and the “laws” of statistics. Adv. Health Sci. Educ. 2010, 15, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Kinner, S.; Herbrik, M.; Maderwald, S.; Umutlu, L.; Nassenstein, K. Preoperative MR-guided wire localization for suspicious breast lesions: Comparison of manual and automated software calculated targeting. Eur. J. Radiol. 2014, 83, e80–e83. [Google Scholar] [CrossRef]
- Petrillo, A.; Di Giacomo, R.; Esposito, E.; Vallone, P.; Setola, S.V.; Raso, M.M.; Granata, V.; Barretta, M.L.; Siani, C.; Rinaldo, C.; et al. Preoperative localisation of nonpalpable breast lesions using magnetic markers in a tertiary cancer centre. Eur. Radiol. Exp. 2022, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Heindl, F.; Schulz-Wendtland, R.; Jud, S.; Erber, R.; Hack, C.C.; Preuss, C.; Behrens, A.; Pöschke, P.; Emons, J. Evaluation of a Wireless Localization System for Nonpalpable Breast Lesions—Feasibility and Cost-effectiveness in Everyday Clinical Routine. In Vivo 2022, 36, 2342–2349. [Google Scholar] [CrossRef]
- Moy, L.; Newell, M.S.; Mahoney, M.C.; Bailey, L.; Barke, L.D.; Carkaci, S.; D’Orsi, C.; Goyal, S.; Haffty, B.G.; Harvey, J.A.; et al. ACR Appropriateness Criteria stage I breast cancer: Initial workup and surveillance for local recurrence and distant metastases in asymptomatic women. J. Am. Coll. Radiol. 2014, 11, 1160–1168. [Google Scholar] [CrossRef]
- Hayes, M.K. Update on Preoperative Breast Localization. Radiol. Clin. N. Am. 2017, 55, 591–603. [Google Scholar] [CrossRef]
- McGugin, C.; Spivey, T.; Coopey, S.; Smith, B.; Kelly, B.; Gadd, M.; Hughes, K.; Dontchos, B.; Specht, M. Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Cancer Res. Treat. 2019, 177, 735–739. [Google Scholar] [CrossRef]
- Malter, W.; Eichler, C.; Hanstein, B.; Mallmann, P.; Holtschmidt, J. First Reported Use of Radiofrequency Identification (RFID) Technique for Targeted Excision of Suspicious Axillary Lymph Nodes in Early Stage Breast Cancer—Evaluation of Feasibility and Review of Current Recommendations. In Vivo 2020, 34, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Law, W.; Cao, X.; Wright, F.C.; Slodkowska, E.; Look Hong, N.; Curpen, B. Adequacy of invasive and in situ breast carcinoma margins in radioactive seed and wire-guided localization lumpectomies. Breast J. 2021, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, A.; den Dekker, B.M.; van Dalen, T.; Jongen, L.; van der Schaaf, M.C.; Alic, L.; Ten Haken, B.; Pijnappel, R.M.; Dassen, A.E. Radiofrequency localization of nonpalpable breast cancer in a multicentre prospective cohort study: Feasibility, clinical acceptability, and safety. Breast Cancer Res. Treat. 2023, 201, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.R.; Gilman, L.; Specht, M.; D’Alessandro, H.A.; Miles, R.C.; Lehman, C.D. Retrospective Review of Preoperative Radiofrequency Tag Localization of Breast Lesions in 848 Patients. AJR Am. J. Roentgenol. 2021, 217, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Veyssiere, H.; Dressaire, M.; Pete, R.; Pinard, C.; Molnar, I.; Abrial, C.; Ginzac, A.; Durando, X.; Tekath, M. RFID trial: Localization of non-palpable breast lesions using radiofrequency identification tags or wire. BMC Cancer 2023, 23, 679. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Kühn, T.; Masannat, Y.; Rubio, I.; de Boniface, J.; Ditsch, N.; Karadeniz Cakmak, G.; Karakatsanis, A.; Dave, R.; Hahn, M.; et al. Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers 2023, 15, 1173. [Google Scholar] [CrossRef]
| Group A 68 Patients | Group B 98 Patients | p | |
|---|---|---|---|
| Age (years) ° | 56.8 ± 9.4 | 57.8 ± 12.4 | p = 0.840 |
| BMI ° | 29.6 ± 5.9 | 26.6 ± 5.8 | p = 0.220 |
| Staging | |||
| pT1a | 29 (42.6%) | 39 (39.8%) | p = 0.680 |
| pT1b | 23 (33.8%) | 33 (33.7%) | p = 0.980 |
| pT1c | 16 (23.6%) | 26 (26.5%) | p = 0.980 |
| Breast Side | |||
| Right | 37 (54.4%) | 50 (51.0%) | p = 0.630 |
| Left | 31 (45.6%) | 48 (49.0%) | p = 0.810 |
| Breast region | |||
| Upper Lateral Quadrant | 31 (45.6%) | 38 (38.8%) | p = 0.100 |
| Upper Medial Quadrant | 15 (22.1%) | 34 (34.8%) | p = 0.004 * |
| Lower Lateral Quadrant | 13 (19.1%) | 13 (13.2%) | p = 0.250 |
| Lower Medial Quadrant | 9 (13.2%) | 13 (13.2%) | p = 1.000 |
| Group A 68 Patients | Group B 98 Patients | p | |
|---|---|---|---|
| Oncologic radicality at definitive pathological exam ° | 68 (100%) | 92 (93.9%) | p = 0.004 * |
| Operative time (minutes) ° | 14.8 ± 7.0 | 13.9 ± 5.1 | p = 0.720 |
| Specimen volume (cm3) ° | 14.4 ± 6.9 | 20.6 ± 6.5 | p = 0.003 * |
| Specimen weight (g) ° | 19.2 ± 4.8 | 24.0 ± 5.1 | p = 0.002 * |
| Patients’ Satisfaction | |||
| -anxiety | 8.6 ± 0.5 | 7.8 ± 0.9 | p = 0.001 * |
| -discomfort | 5.1 ± 1.1 | 6.5 ± 1.4 | p = 0.001 * |
| -pain | 5.5 ± 1.2 | 7.0 ± 1.4 | p = 0.001 * |
| -fear for dislocation | 6.4 ± 1.4 | 6.5 ± 1.2 | p = 0.890 |
| -cosmetic results | 2.8 ± 1.1 | 6.9 ± 0.9 | p = 0.001 * |
| (Likert) ° | −8.6 ± 0.8 | 7.0 ± 0.7 | p = 0.001 * |
| Clinicians’ Satisfaction (Likert) ° | 9.7 | 7.8 | 0.003 * |
| Mortality | 0 | 0 | p = 1.00 |
| Recurrence | 0 | 0 | p = 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, S.; Gambardella, C.; Santoriello, A.; Ruggiero, R.; Iovino, F.; Fisone, F.; Mongardini, F.M.; Lucido, F.S.; Tolone, S.; Docimo, L. Early Breast Cancer: Could Combined LOCalizerTM and Ultrasound Localization Replace the Metallic Wire? A Multicentric Study. J. Clin. Med. 2024, 13, 1713. https://doi.org/10.3390/jcm13061713
Parisi S, Gambardella C, Santoriello A, Ruggiero R, Iovino F, Fisone F, Mongardini FM, Lucido FS, Tolone S, Docimo L. Early Breast Cancer: Could Combined LOCalizerTM and Ultrasound Localization Replace the Metallic Wire? A Multicentric Study. Journal of Clinical Medicine. 2024; 13(6):1713. https://doi.org/10.3390/jcm13061713
Chicago/Turabian StyleParisi, Simona, Claudio Gambardella, Antonio Santoriello, Roberto Ruggiero, Francesco Iovino, Francesca Fisone, Federico Maria Mongardini, Francesco Saverio Lucido, Salvatore Tolone, and Ludovico Docimo. 2024. "Early Breast Cancer: Could Combined LOCalizerTM and Ultrasound Localization Replace the Metallic Wire? A Multicentric Study" Journal of Clinical Medicine 13, no. 6: 1713. https://doi.org/10.3390/jcm13061713
APA StyleParisi, S., Gambardella, C., Santoriello, A., Ruggiero, R., Iovino, F., Fisone, F., Mongardini, F. M., Lucido, F. S., Tolone, S., & Docimo, L. (2024). Early Breast Cancer: Could Combined LOCalizerTM and Ultrasound Localization Replace the Metallic Wire? A Multicentric Study. Journal of Clinical Medicine, 13(6), 1713. https://doi.org/10.3390/jcm13061713





