Intraoperative Extravascular Ultrasound in the Identification of Flow-Limiting Dissections after Balloon Angioplasty in the Femoropopliteal Segment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Study Devices
2.3. Study Procedures
2.4. Intraoperative Extravascular Ultrasound
2.5. Endpoints and Definitions
2.6. Statistical Analysis
3. Results
3.1. Patient and Lesion Characteristics
3.2. Procedural Characteristics
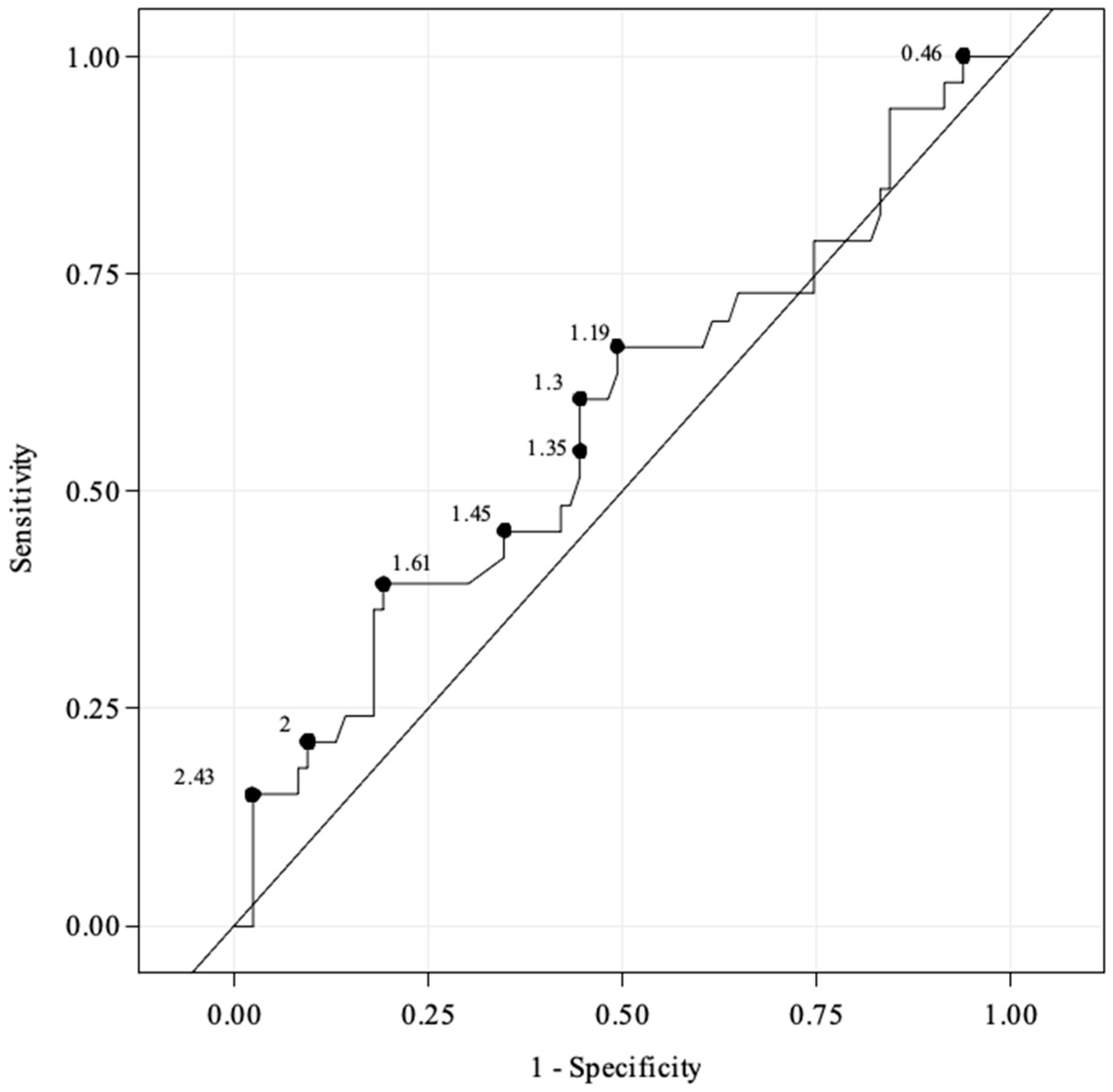
3.3. Diagnostic Performance of EVUS Added to Angiography for Detection of FLD
3.4. Efficacy and Safety Outcomes of the Combination Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, D.N.; Armstrong, E.J.; Aronow, H.D.; Gigliotti, O.S.; Jaff, M.R.; Klein, A.J.; Parikh, S.A.; Prasad, A.; Rosenfield, K.; Shishehbor, M.H.; et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions. Catheter. Cardiovasc. Interv. 2018, 92, 124–140. [Google Scholar] [CrossRef]
- Micari, A.; Brodmann, M.; Keirse, K.; Peeters, P.; Tepe, G.; Frost, M.; Wang, H.; Zeller, T.; the IN.PACT Global Study Investigators. Drug-Coated Balloon Treatment of Femoropopliteal Lesions for Patients with Intermittent Claudication and Ischemic Rest Pain: 2-Year Results from the IN.PACT Global Study. JACC Cardiovasc. Interv. 2018, 11, 945–953. [Google Scholar] [CrossRef]
- Thieme, M.; Von Bilderling, P.; Paetzel, C.; Karnabatidis, D.; Perez Delgado, J.; Lichtenberg, M.; Lutonix Global SFA Registry Investigators. The 24-Month Results of the Lutonix Global SFA Registry: Worldwide Experience with Lutonix Drug-Coated Balloon. JACC Cardiovasc. Interv. 2017, 10, 1682–1690. [Google Scholar] [CrossRef]
- Schroë, H.; Holden, A.H.; Goueffic, Y.; Jansen, S.J.; Peeters, P.; Keirse, K.; Ito, W.; Vermassen, F.; Micari, A.; Blessing, E.; et al. Stellarex drug-coated balloon for treatment of femoropopliteal arterial disease-the ILLUMENATE Global Study: 12-Month results from a prospective, multicenter, single-arm study. Catheter. Cardiovasc. Interv. 2018, 91, 497–504. [Google Scholar] [CrossRef]
- Allan, R.B.; Wise, N.C.; Wong, Y.T.; Delaney, C.L. Comparison of Angiographic Dissection Classification Systems in the Femoropopliteal Arteries Using IVUS Validation and Reliability Testing. J. Endovasc. Ther. 2022, 29, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Cranny, G.; Burch, J.; Aguiar-Ibanez, R.; Craig, D.; Wright, K.; Berry, E.; Gough, M.; Kleijnen, J.; Westwood, M. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol. Assess. 2007, 11, iii-184. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- ISO 14155:2020; Clinical Investigation of Medical Devices for Human Subjects Good Clinical Practice. ISO: Geneva, Switzerland, 2020.
- Ranke, C.; Creutzig, A.; Alexander, K. Duplex scanning of the peripheral arteries: Correlation of the peak velocity ratio with angiographic diameter reduction. Ultrasound Med. Biol. 1992, 18, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Macharzina, R.R.; Schmid, S.F.; Beschorner, U.; Noory, E.; Rastan, A.; Vach, W.; Schwarzwälder, U.; Sixt, S.; Bürgelin, K.; Neumann, F.-J.; et al. Duplex ultrasound assessment of native stenoses in the superficial femoral and popliteal arteries: A comparative study examining the influence of multisegment lesions. J. Endovasc. Ther. 2015, 22, 254–260. [Google Scholar] [CrossRef]
- Kawarada, O.; Hozawa, K.; Zen, K.; Huang, H.-L.; Kim, S.H.; Choi, D.; Park, K.; Kato, K.; Kato, T.; Tsubakimoto, Y.; et al. Peak systolic velocity ratio derived from quantitative vessel analysis for restenosis after femoropopliteal intervention: A multidisciplinary review from Endovascular Asia. Cardiovasc Interv Ther. 2020, 35, 52–61. [Google Scholar] [CrossRef]
- Van der Lugt, A.; Gussenhoven, E.J.; Mali, W.P.; Reekers, J.; Seelen, J.; Tielbeek, A.; Pieterman, H. Effect of balloon angioplasty in femoropopliteal arteries assessed by intravascular ultrasound. Eur. J. Vasc. Endovasc. Surg. 1997, 13, 549–556. [Google Scholar] [CrossRef][Green Version]
- Rogers, J.H.; Lasala, J.M. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J. Invasive Cardiol. 2004, 16, 493–499. [Google Scholar]
- Kobayashi, N.; Hirano, K.; Yamawaki, M.; Araki, M.; Sakai, T.; Sakamoto, Y.; Mori, S.; Tsutsumi, M.; Honda, Y.; Ito, Y. Simple classification and clinical outcomes of angiographic dissection after balloon angioplasty for femoropopliteal disease. J. Vasc. Surg. 2018, 67, 1151–1158. [Google Scholar] [CrossRef]
- Giusca, S.; Lichtenberg, M.; Hagstotz, S. Comparison of ante-versus retrograde access for the endovascular treatment of long and calcified, de novo femoropopliteal occlusive lesions. Heart Vessels 2020, 35, 346–359. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Mosarla, R.C.; Rosenfield, K.; Kohi, M.; Lichtenberg, M.; Meissner, M.; Varcoe, R.; Holden, A.; Jaff, M.R.; Chalyan, D.; et al. Appropriate Use of Intravascular Ultrasound During Arterial and Venous Lower Extremity Interventions. JACC Cardiovasc. Interv. 2022, 15, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Shammas, N.W.; Torey, J.T.; Shammas, W.J. Dissections in Peripheral Vascular Interventions: A Proposed Classification Using Intravascular Ultrasound. J. Invasive Cardiol. 2018, 30, 145–146. [Google Scholar] [PubMed]
- Voûte, M.T.; Stathis, A.; Schneider, P.A.; Thomas, S.D.; Brodmann, M.; Armstrong, E.J.; Holden, A.; Varcoe, R.L. Delphi Consensus Study toward a Comprehensive Classification System for Angioplasty-Induced Femoropopliteal Dissection: The DISFORM Study. JACC Cardiovasc. Interv. 2021, 14, 2391–2401. [Google Scholar] [CrossRef]
- Ascher, E.; Marks, N.A.; Hingorani, A.P.; Schutzer, R.W.; Nahata, S. Duplex-guided balloon angioplasty and subintimal dissection of infrapopliteal arteries: Early results with a new approach to avoid radiation exposure and contrast material. J. Vasc. Surg. 2005, 42, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Ascher, E.; Marks, N.A.; Hingorani, A.P.; Schutzer, R.W.; Mutyala, M. Duplex-guided endovascular treatment for occlusive and stenotic lesions of the femoral-popliteal arterial segment: A comparative study in the first 253 cases. J. Vasc. Surg. 2006, 44, 1230–1237. [Google Scholar] [CrossRef]
- Ascher, E.; Hingorani, A.P.; Marks, N.; Puggioni, A.; Shiferson, A.; Tran, V.; Jacob, T. Predictive factors of femoropopliteal patency after suboptimal duplex-guided balloon angioplasty and stenting: Is recoil a bad sign? Vascular 2008, 16, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Fazzini, S.; Pennetta, F.F.; Turriziani, V.; Vona, S.; Marchetti, A.A.; Ippoliti, A. Extravascular Ultrasound (EVUS) to Assess the Results of Peripheral Endovascular Procedures. Diagnostics 2023, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Bosiers, M.; Scheinert, D.; Hendriks, J.M.H.; Wissgott, C.; Peeters, P.; Zeller, T.; Brodmann, M.; Staffa, R.; TOBA Investigators. Results from the Tack Optimized Balloon Angioplasty (TOBA) study demonstrate the benefits of minimal metal implants for dissection repair after angioplasty. J. Vasc. Surg. 2016, 64, 109–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Description | Total (n = 150) |
|---|---|
| Age (yrs.) | 68.7 ± 9.8 |
| Male | 102 (68.0%) |
| Female | 48 (32.0%) |
| Hypertension | 120 (80.0%) |
| Ex- or current smoker | 120 (80.0%) |
| Hyperlipidaemia | 111 (74.0%) |
| History Peripheral Arterial Occlusive Disease | 89 (59.3%) |
| Previous peripheral interventions/surgeries | 57 (38.0%) |
| Coronary artery disease | 46 (30.7%) |
| Diabetes | 45 (30.0%) |
| Insulin-dependent | 21 (14.0%) |
| Non-insulin-dependent | 24 (16.0%) |
| Renal disease (insufficiency) | 27 (18.0%) |
| Cerebrovascular disease | 22 (14.7%) |
| Cancer | 21 (14.0%) |
| Rutherford clinical category (n = 148) | |
| 2 | 26 (17.6%) |
| 3 | 104 (70.3%) |
| 4 | 18 (12.2%) |
| Ankle–brachial index of the target limb | 0.67 ± 0.16 |
| Description | Total (n = 167) |
|---|---|
| Target lesion length (mm) | 103.3 ± 73.4 |
| Length of occluded section (mm) | 89.6 ± 73.5 |
| Reference vessel diameter (mm) | 5.2 ± 0.7 |
| Diameter stenosis (mm) | 89.0± 11.1 |
| Target lesion location | |
| PPA | 6 (3.6%) |
| SFA | 142 (85.0%) |
| SFA and PPA | 19 (11.4%) |
| Target lesion type | |
| De novo stenotic lesion | 101 (60.5%) |
| Restenosis | 5 (3.0%) |
| Occlusion | 58 (34.7%) |
| Re-occlusion | 3 (1.8%) |
| Calcification scoring | |
| PACSS Grade 0 | 40 (24.0%) |
| PACSS Grade 1 | 36 (21.6%) |
| PACSS Grade 2 | 45 (26.9%) |
| PACSS Grade 3 | 22 (13.2%) |
| PACSS Grade 4 | 24 (14.4%) |
| TASC classification | |
| TASC A | 48 (28.7%) |
| TASC B | 66 (39.5%) |
| TASC C | 40 (24.0%) |
| TASC D | 13 (7.8%) |
| Description | Total (n = 167) |
|---|---|
| Number of PTA balloons used | 164 (98.2%) |
| 1 | 146 (87.4%) |
| 2 | 10 (6.0%) |
| 3 | 6 (3.6%) |
| 4 | 0 (0.0%) |
| 5 | 1 (0.6%) |
| 6 | 0 (0.0%) |
| 7 | 1 (0.6%) |
| Number of DCBs used | 167 (100%) |
| 1 | 104 (62.3%) |
| 2 | 40 (24.0%) |
| 3 | 22 (13.2%) |
| 4 | 1 (0.6%) |
| Number of stents used | 59 (35.3%) |
| 0 | 108 (64.7%) |
| 1 | 46 (27.5%) |
| 2 | 9 (5.4%) |
| 3 | 4 (2.4%) |
| Length of stent (n= 76, mm) | 98.1 ± 45.3 |
| Post dilatation with POBA | 70 (41.9%) |
| Number of POBA used | |
| 1 | 66 (94.3) |
| 2 | 4 (5.7%) |
| Target lesion length (n = 59 mm) for lesions treated with at least one stent | 129.1 ± 77.1 |
| Description | Total (n = 150) |
|---|---|
| Procedure duration (min) | 92.9 ± 42.5 |
| Puncture site | |
| Femoral | 149 (99.3%) |
| Pedal | 1 (0.7%) |
| Direction | |
| Antegrade | 59 (39.3%) |
| Crossover | 82 (54.7%) |
| Retrograde | 9 (6.0%) |
| Approach | |
| Sub-intimal | 9 (6.0%) |
| Intraluminal | 141 (94.0%) |
| Access sheath (French) | |
| 4 | 12 (8.0%) |
| 5 | 28 (18.7%) |
| 6 | 100 (66.7%) |
| 7 | 9 (6.0%) |
| 8 | 1 (0.7%) |
| Residual stenosis post-DCB | 22.4 ± 21.8 |
| Dissection post-DCB (total) * | 75 (44.9%) |
| Cat. A | 24 (32.0%) |
| Cat. B | 30 (40.0%) |
| Cat. C | 9 (12.0%) |
| Cat. D | 11 (14.7%) |
| Cat. E | 0 (0.0%) |
| Cat. F | 1 (1.3%) |
| Flow-limiting dissection post-DCB | 26 (34.7%) |
| Post-procedural residual stenosis (%) | 10.2 ± 10.4 |
| Stenting rate | 59 (35.3%) |
| DCB technical success ** | 103 (95.4%) |
| Stent technical success | 58 (98.3%) |
| Procedural success | 143 (95.3%) |
| Before EVUS | ||
|---|---|---|
| Angiography independent review ‘FLD’ | Angiography independent review ‘No FLD’ | |
| Investigator reported upon ‘Angiography’: ‘FLD’ | 13 (11%) | 7 (6%) |
| Investigator reported upon ‘Angiography’: ‘No FLD’ | 22 (18%) | 79 (65%) |
| Sensitivity 37% [21.5–55.1] | Specificity 92% [83.9–96.7] | |
| After EVUS | ||
| Angiography independent review ‘FLD’ | Angiography independent review ‘No FLD’ | |
| Investigator reported upon ‘Angiography + EVUS’: ‘FLD’ | 10 (8%) | 6 (5%) |
| Investigator reported upon ‘Angiography + EVUS’: ‘No FLD’ | 25 (21%) | 80 (66%) |
| Sensitivity 29% [14.6–46.3] | Specificity 93% [85.4–97.4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwipatayi, B.P.; Dodd, J.; Hanna, J.; Gouëffic, Y.; Brodmann, M.; Guerra, M.; Schmidt, A.; Loewe, C.; Grözinger, G.; Korosoglou, G.; et al. Intraoperative Extravascular Ultrasound in the Identification of Flow-Limiting Dissections after Balloon Angioplasty in the Femoropopliteal Segment. J. Clin. Med. 2024, 13, 1635. https://doi.org/10.3390/jcm13061635
Mwipatayi BP, Dodd J, Hanna J, Gouëffic Y, Brodmann M, Guerra M, Schmidt A, Loewe C, Grözinger G, Korosoglou G, et al. Intraoperative Extravascular Ultrasound in the Identification of Flow-Limiting Dissections after Balloon Angioplasty in the Femoropopliteal Segment. Journal of Clinical Medicine. 2024; 13(6):1635. https://doi.org/10.3390/jcm13061635
Chicago/Turabian StyleMwipatayi, Bibombe Patrice, James Dodd, Joseph Hanna, Yann Gouëffic, Marianne Brodmann, Mercedes Guerra, Andrej Schmidt, Christian Loewe, Gerd Grözinger, Grigorios Korosoglou, and et al. 2024. "Intraoperative Extravascular Ultrasound in the Identification of Flow-Limiting Dissections after Balloon Angioplasty in the Femoropopliteal Segment" Journal of Clinical Medicine 13, no. 6: 1635. https://doi.org/10.3390/jcm13061635
APA StyleMwipatayi, B. P., Dodd, J., Hanna, J., Gouëffic, Y., Brodmann, M., Guerra, M., Schmidt, A., Loewe, C., Grözinger, G., Korosoglou, G., Lichtenberg, M., & Deloose, K. (2024). Intraoperative Extravascular Ultrasound in the Identification of Flow-Limiting Dissections after Balloon Angioplasty in the Femoropopliteal Segment. Journal of Clinical Medicine, 13(6), 1635. https://doi.org/10.3390/jcm13061635






