Cognitive Impairment in People Living with HIV and the Impact of Mood: Results from a Cross-Sectional Study
Abstract
1. Background
2. Methods
2.1. Design of the Study
2.2. Inclusion/Exclusion Criteria
2.3. Assessment
- ○
- MoCA [64,65]: It evaluates different cognitive domains: visuospatial and executive abilities, naming, memory, attention, language, abstraction, and spatial and temporal orientation. The first is assessed through a series of exercises, including drawing a line to sequence alternating digits and letters, a cube shape, and a clock showing 10 min past 11. Verbal fluency is tested by identifying the names of three animals. The screening test evaluates short-term and delayed memories by requiring subjects to repeat five words spoken by the examiner immediately and then again after a delay, respectively. Attention is tested through the repetition of a series of numbers forward and backwards, identification of the letter “A” in a sequence of letters, tapping the hand on the table, and a subtraction exercise. Language is assessed by repeating two sentences and producing a list of words starting with the letter “F” within one minute. Abstraction is evaluated by explaining what two pairs of words have in common. Lastly, spatial and temporal orientation are tested by asking the patient about the date and location of the visit. It takes approximately 10 min to administer the MoCA. The test’s total score ranges from 0 (maximum impairment) to 30 (absence of impairment) and is adjusted for education level: an additional point [72] is attributed if the patient has 12 years or less of education. Cognitive impairment is indicated by scores below 26, whereas a normal condition is suggested by scores above 26.
- ○
- Patient Health Questionnaire—two items (PHQ-2) [73,74]: It is a brief screening tool, used to evaluate depressive symptoms and anhedonia over the past two weeks. It consists of two questions, which are the first two items of the PHQ-9. The first question is, “Over the last two weeks, how often have you been bothered by any of the following problems?”. The two items under evaluation are “little interest or pleasure in doing things” and “feeling down, depressed, or hopeless”. Four response options are available, and these are “not at all”, “several days”, “more than half the days”, and “nearly every day”, scored as 0, 1, 2, and 3, respectively. As such, the PHQ-2 score ranges from 0 (no depressive symptoms) to 6 (daily depressive symptoms). A score of 3 or more indicates a depressive mood, with a sensitivity of 83% and a specificity of 90% [73], unlike the PHQ-9 score ≥ 10, whose sensitivity and specificity are 88% [75].
- ○
- Activities of Daily Living (ADLs) [76,77]: The Katz ADL scale is used to indicate a person’s functional status. It evaluates autonomy in common daily routine activities, oriented towards taking care of one’s own body and enabling basic survival and well-being. These activities include personal hygiene, getting dressed, using the toilet, walking, sitting, standing, lying down, climbing stairs, continence, and eating. The scale is scored by assigning 1 if the patient can perform the activity and 0 if he/she cannot. It ranges from 0, indicating complete dependence, to 6, indicating complete autonomy. A score of 3 or 4 indicates moderate impairment, while a score of 2 or less indicates severe functional impairment.
- ○
- Instrumental Activities of Daily Living (IADLs) [76,77]: This assessment evaluates one’s ability to perform daily instrumental activities to support their life at home and in the community. These activities require more complex interactions than those used in ADLs (activities of daily living). Some examples include using the telephone, managing finances, shopping, food preparation, housekeeping, laundry, transportation, and being responsible for taking one’s medications. The score ranges from 0 (complete dependence) to 8 (complete autonomy). A score of 4 or 5 indicates moderate dependence, while a score of 3 or less indicates severe functional impairment.
- ○
- Immunovirological assessment: CD4+ lymphocyte count (present and nadir, which means the lowest measured value), CD4+/CD8+ lymphocytes ratio, HIV RNA load levels (present and zenith, which means the highest measured value).
- ○
- Haemoglobin (Hb), white blood cells (WBCs), and platelet (PLT) count, prothrombin time–international normalised ratio (PT–INR), albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, low-density lipoprotein (LDL), ferritin, vitamin B12, folate, thyroid-stimulating hormone (TSH), C-reactive protein (CRP).
2.4. Statistical Analysis
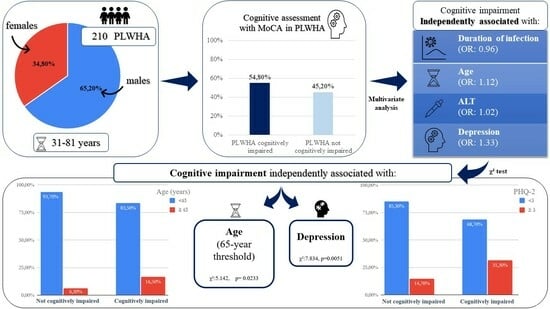
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 13 July 2023).
- “Le Nuove Diagnosi di HIV e i Casi di Aids in Italia nel 2022, i Dati dell’Istituto Superiore di Sanità”. Available online: https://www.salute.gov.it/portale/hiv/homeHIV.jsp (accessed on 16 November 2023).
- Guaraldi, G.; Milic, J.; Mussini, C. Aging with HIV. Curr. HIV/AIDS Rep. 2019, 16, 475–481. [Google Scholar] [CrossRef]
- Lu, D.Y.; Wu, H.Y.; Yarla, N.S.; Xu, B.; Ding, J.; Lu, T.R. HAART in HIV/AIDS Treatments: Future Trends. Infect. Disord. Drug Targets 2018, 18, 15–22. [Google Scholar] [CrossRef]
- Dionne, B. Key Principles of Antiretroviral Pharmacology. Infect. Dis. Clin. N. Am. 2019, 33, 787–805. [Google Scholar] [CrossRef]
- Tasca, K.I.; Calvi, S.A.; Souza, L.d.R. Immunovirological parameters and cytokines in HIV infection. Rev. Soc. Bras. Med. Trop. 2012, 45, 663–669. [Google Scholar] [CrossRef]
- Kouanfack, C.; Unal, G.; Schaeffer, L.; Kfutwah, A.; Aghokeng, A.; Mougnutou, R.; Tchemgui-Noumsi, N.; Alessandri-Gradt, E.; Delaporte, E.; Vray, M.; et al. Comparative Immunovirological and Clinical Responses to Antiretroviral Therapy Between HIV-1 Group O and HIV-1 Group M Infected Patients. Clin. Infect. Dis. 2020, 70, 1471–1477. [Google Scholar] [CrossRef]
- Park, W.B.; Choe, P.G.; Jo, J.H.; Kim, S.-H.; Bang, J.H.; Bin Kim, H.; Kim, N.J.; Oh, M.-D.; Choe, K.W. Immune reconstitution inflammatory syndrome in the first year after HAART: Influence on long-term clinical outcome. AIDS 2006, 20, 2390–2392. [Google Scholar] [CrossRef]
- Pau, A.K.; George, J.M. Antiretroviral therapy: Current drugs. Infect. Dis. Clin. N. Am. 2014, 28, 371–402. [Google Scholar] [CrossRef]
- Poorolajal, J.; Hooshmand, E.; Mahjub, H.; Esmailnasab, N.; Jenabi, E. Survival rate of AIDS disease and mortality in HIV-infected patients: A meta-analysis. Public Health 2016, 139, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Couzigou, C.; Semaille, C.; Le Strat, Y.; Pinget, R.; Pillonel, J.; Lot, F.; Cazein, F.; Vittecoq, D.; Desenclos, J.-C.; The Aids Survival Study Group. Differential improvement in survival among patients with AIDS after the introduction of HAART. AIDS Care 2007, 19, 523–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thapa, S.; Shrestha, U. Immune Reconstitution Inflammatory Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Geocze, L.; Mucci, S.; De Marco, M.A.; Nogueira-Martins, L.A.; Citero, V.d.A. Quality of life and adherence to HAART in HIV-infected patients. Rev. Saude Publica 2010, 44, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Marzetti, E.; Canevelli, M.; Guaraldi, G. Geriatric syndromes: How to treat. Virulence 2017, 8, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Ito, H.; Suzuki, T.; Araki, A.; Hosoi, T.; Sawabe, M. Reviewing the definition of “elderly”. Geriatr. Gerontol. Int. 2006, 6, 149–158. [Google Scholar] [CrossRef]
- Ghosn, J.; Taiwo, B.; Seedat, S.; Autran, B.; Katlama, C. HIV. Lancet 2018, 392, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Wagle, A.; Goerlich, E.; Post, W.S.; Woldu, B.; Wu, K.C.; Hays, A.G. HIV and Global Cardiovascular Health. Curr. Cardiol. Rep. 2022, 24, 1149–1157. [Google Scholar] [CrossRef]
- Salis, F.; Palimodde, A.; Demelas, G.; Scionis, M.I.; Mandas, A. Frailty and comorbidity burden in Atrial Fibrillation. Front. Public Health 2023, 11, 1134453. [Google Scholar] [CrossRef]
- Guaraldi, G.; Milic, J. Why Am I Getting Fat? Exploring Immune-Metabolic Pathways to Central Fat Accumulation in Persons With HIV. J. Infect. Dis. 2020, 221, 343–345. [Google Scholar] [CrossRef]
- Hruz, P.W. HIV and endocrine disorders. Endocrinol. Metab. Clin. N. Am. 2014, 43, xvii–xviii. [Google Scholar] [CrossRef]
- Back, D.; Marzolini, C. The challenge of HIV treatment in an era of polypharmacy. J. Int. AIDS Soc. 2020, 23, e25449. [Google Scholar] [CrossRef]
- Salis, F.; Palimodde, A.; Rundeddu, S.; Mandas, A. STOPP/START Anti-aggregation and Anticoagulation Alerts in Atrial Fibrillation. Curr. Vasc. Pharmacol. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, J.; Serrano-Villar, S.; Vivancos, M.J.; Rubio, R.; Moreno, S.; HIV-associated comorbidities Study Group. Management of Comorbidities in Treated HIV Infection: A Long Way to Go: HIV, comorbidities and aging. Int. J. Antimicrob. Agents 2022, 59, 106493. [Google Scholar] [CrossRef] [PubMed]
- Loddo, S.; Salis, F.; Rundeddu, S.; Serchisu, L.; Peralta, M.M.; Mandas, A. Nutritional Status and Potentially Inappropriate Medications in Elderly. J. Clin. Med. 2022, 11, 3465. [Google Scholar] [CrossRef]
- Wei, J.; Hou, J.; Su, B.; Jiang, T.; Guo, C.; Wang, W.; Zhang, Y.; Chang, B.; Wu, H.; Zhang, T. The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 581346. [Google Scholar] [CrossRef]
- Ellis, R.; Langford, D.; Masliah, E. HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat. Rev. Neurosci. 2007, 8, 33–44. [Google Scholar] [CrossRef]
- Cysique, L.A.; Brew, B.J. Comorbid depression and apathy in HIV-associated neurocognitive disorders in the era of chronic HIV infection. Handb. Clin. Neurol. 2019, 165, 71–82. [Google Scholar] [CrossRef]
- Glass, J.D.; Wesselingh, S.L. Microglia in HIV-associated neurological diseases. Microsc. Res. Tech. 2001, 54, 95–105. [Google Scholar] [CrossRef]
- Spies, G.; Ahmed-Leitao, F.; Fennema-Notestine, C.; Cherner, M.; Seedat, S. Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. J. Neurovirol. 2016, 22, 149–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eggers, C.; Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; German Association of Neuro-AIDS und Neuro-Infectiology (DGNANI). HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Elbirt, D.; Mahlab-Guri, K.; Bezalel-Rosenberg, S.; Gill, H.; Attali, M.; Asher, I. HIV-associated neurocognitive disorders (HAND). Isr. Med. Assoc. J. 2015, 17, 54–59. [Google Scholar] [PubMed]
- Grant, I.; Franklin, D.R.; Deutsch, R.; Woods, S.P.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.; Collier, A.C.; et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014, 82, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013.
- Ances, B.M.; Ellis, R.J. Dementia and neurocognitive disorders due to HIV-1 infection. Semin. Neurol. 2007, 27, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Carbonell, D.; Ye, F.; Ramanath, N.; Garcia-Mesa, Y.; Knapp, P.E.; Hauser, K.F.; Karn, J. Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog. 2019, 15, e1008249. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. HIV-related neurotoxicity. Brain Pathol. 1991, 1, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Burdo, T.H. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: Observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J. Neuroimmune Pharmacol. 2012, 7, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Borjabad, A.; Brooks, A.I.; Volsky, D.J. Gene expression profiles of HIV-1-infected glia and brain: Toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders. J. Neuroimmune Pharmacol. 2010, 5, 44–62. [Google Scholar] [CrossRef]
- Spurgat, M.S.; Tang, S.J. Single-Cell RNA-Sequencing: Astrocyte and Microglial Heterogeneity in Health and Disease. Cells 2022, 11, 2021. [Google Scholar] [CrossRef]
- Uzasci, L.; Nath, A.; Cotter, R. Oxidative stress and the HIV-infected brain proteome. J. Neuroimmune Pharmacol. 2013, 8, 1167–1180. [Google Scholar] [CrossRef]
- Buckley, S.; Byrnes, S.; Cochrane, C.; Roche, M.; Estes, J.D.; Selemidis, S.; Angelovich, T.A.; Churchill, M.J. The role of oxidative stress in HIV-associated neurocognitive disorders. Brain Behav. Immun. Health 2021, 13, 100235. [Google Scholar] [CrossRef]
- Sabri, F.; Titanji, K.; De Milito, A.; Chiodi, F. Astrocyte activation and apoptosis: Their roles in the neuropathology of HIV infection. Brain Pathol. 2003, 13, 84–94. [Google Scholar] [CrossRef]
- Kumar, A.; Abbas, W.; Herbein, G. HIV-1 latency in monocytes/macrophages. Viruses 2014, 6, 1837–1860. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Paolicelli, R.C. Microglia-Mediated Synapse Loss in Alzheimer’s Disease. J. Neurosci. 2018, 38, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Song, C.; Lv, S.; Chang, L.; Hua, W.; Weng, W.; Wu, H.; Dai, L. Role of microglia in HIV-1 infection. AIDS Res. Ther. 2023, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Milanini, B.; Samboju, V.; Cobigo, Y.; Paul, R.; Javandel, S.; Hellmuth, J.; Allen, I.; Miller, B.; Valcour, V. Longitudinal brain atrophy patterns and neuropsychological performance in older adults with HIV-associated neurocognitive disorder compared with early Alzheimer’s disease. Neurobiol. Aging 2019, 82, 69–76. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Salis, F.; Costaggiu, D.; Mandas, A. Mini-Mental State Examination: Optimal Cut-off Levels for Mild and Severe Cognitive Impairment. Geriatrics 2023, 8, 12. [Google Scholar] [CrossRef]
- Skinner, S.; Adewale, A.J.; DeBlock, L.; Gill, M.J.; Power, C. Neurocognitive screening tools in HIV/AIDS: Comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009, 10, 246–252. [Google Scholar] [CrossRef]
- Kumar, S.; Himanshu, D.; Tandon, R.; Atam, V.; Sawlani, K.K.; Verma, S.K. Prevalence of HIV Associated Neurocognitive Disorder using Modified Mini Mental State Examination and its Correlation with CD4 Counts and Anti-retroviral Therapy. J. Assoc. Physicians India 2019, 67, 47–51. [Google Scholar]
- Franco-Marina, F.; García-González, J.J.; Wagner-Echeagaray, F.; Gallo, J.; Ugalde, O.; Sánchez-García, S.; Espinel-Bermúdez, C.; Juárez-Cedillo, T.; Rodríguez, M.V.; García-Peña, C. The Mini-mental State Examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int. Psychogeriatr. 2010, 22, 72–81. [Google Scholar] [CrossRef]
- Ganasen, K.A.; Fincham, D.; Smit, J.; Seedat, S.; Stein, D. Utility of the HIV Dementia Scale (HDS) in identifying HIV dementia in a South African sample. J. Neurol. Sci. 2008, 269, 62–64. [Google Scholar] [CrossRef]
- Bottiggi, K.A.; Chang, J.J.; Schmitt, F.A.; Avison, M.J.; Mootoor, Y.; Nath, A.; Berger, J.R. The HIV Dementia Scale: Predictive power in mild dementia and HAART. J. Neurol. Sci. 2007, 260, 11–15. [Google Scholar] [CrossRef]
- Rosca, E.C.; Tadger, P.; Cornea, A.; Tudor, R.; Oancea, C.; Simu, M. International HIV Dementia Scale for HIV-Associated Neurocognitive Disorders: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1124. [Google Scholar] [CrossRef]
- Molinaro, M.B.; Sacktor, N.; Nakigozi, G.M.; Anok, A.B.; Batte, J.M.; Kisakye, A.M.; Myanja, R.B.; Nakasujja, N.M.; Robertson, K.R.; Gray, R.H.M.; et al. Utility of the International HIV Dementia Scale for HIV-Associated Neurocognitive Disorder. J. Acquir. Immune Defic. Syndr. 2020, 83, 278–283. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Y.; Long, J.; Feng, Q.; Wang, R.; Su, L.; Zhao, T.; Wei, B. Diagnostic accuracy of the International HIV Dementia Scale and HIV Dementia Scale: A meta-analysis. Exp. Ther. Med. 2012, 4, 665–668. [Google Scholar] [CrossRef][Green Version]
- Haddow, L.J.; Floyd, S.; Copas, A.; Gilson, R.J. A systematic review of the screening accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PLoS ONE. 2013, 8, e61826. [Google Scholar] [CrossRef]
- López, E.; Steiner, A.J.; Smith, K.; Thaler, N.S.; Hardy, D.J.; Levine, A.J.; Al-Kharafi, H.T.; Yamakawa, C.; Goodkin, K. Diagnostic utility of the HIV dementia scale and the international HIV dementia scale in screening for HIV-associated neurocognitive disorders among Spanish-speaking adults. Appl. Neuropsychol. Adult. 2017, 24, 512–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milanini, B.; Paul, R.; Bahemana, E.; Adamu, Y.; Kiweewa, F.; Langat, R.; Owuoth, J.; Allen, E.; Polyak, C.; Ake, J.; et al. Limitations of the International HIV Dementia Scale in the current era. AIDS 2018, 32, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Belfiori, M.; Salis, F.; Demelas, G.; Mandas, A. Association between Depressive Mood, Antidepressant Therapy and Neuropsychological Performances: Results from a Cross-Sectional Study on Elderly Patients. Brain Sci. 2024, 14, 54. [Google Scholar] [CrossRef]
- Costaggiu, D.; Pinna, E.; Serchisu, L.; Barcellona, D.; Piano, P.; Ortu, F.; Marongiu, F.; Mandas, A. The Repeatable Battery for the Assessment of Neuropsychological Status as a screening strategy for HIV-Associated Neurocognitive Disorders. AIDS Care 2021, 33, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Rosca, E.C.; Albarqouni, L.; Simu, M. Montreal Cognitive Assessment (MoCA) for HIV-Associated Neurocognitive Disorders. Neuropsychol. Rev. 2019, 29, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, M.-J.; Mayo, N.; Fellows, L.K.; Lebedeva, E.; Higgins, J.; Overton, E.T.; Ances, B.M.; Koski, L. A better screening tool for HIV-associated neurocognitive disorders: Is it what clinicians need? AIDS 2015, 29, 895–902. [Google Scholar] [CrossRef]
- Ayele, B.A.; Amogne, W.; Gemechu, L. HIV-associated neurocognitive disorder and HIV-associated myelopathy in a patient with a preserved CD4, but high viral load-a rarely reported phenomenon: A case report and literature review. BMC Infect. Dis. 2020, 20, 574. [Google Scholar] [CrossRef]
- Le, L.T.; Price, R.W.; Gisslén, M.; Zetterberg, H.; Emu, B.; Fabre, R.; Christian, P.; Andersen, S.; Spudich, S.; Vassallo, M. Correlation between CD4/CD8 ratio and neurocognitive performance during early HIV infection. HIV Med. 2023, 24, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.M.S.; Sunada, N.M.O.; Casseb, J. T-lymphocyte activation markers in patients with HIV-1-associated neurocognitive disorder. J. Neurovirol. 2022, 28, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.; Tang, B.; Vaida, F.; Mcclernon, D.; Deutsch, R.; Cherner, M.; Cookson, D.; Crescini, M.; Grant, I.; Ellis, R.J.; et al. Low-Level HIV RNA in Cerebrospinal Fluid and Neurocognitive Performance: A Longitudinal Cohort Study. J. Acquir. Immune Defic. Syndr. 2021, 87, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Amusan, P.; Power, C.; Gill, M.J.; Gomez, D.; Johnson, E.; Rubin, L.H.; Fujiwara, E. Lifetime antiretroviral exposure and neurocognitive impairment in HIV. J. Neurovirol. 2020, 26, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Gelman, B.B.; Lisinicchia, J.G.B.; Morgello, S.; Masliah, E.; Commins, D.; Achim, C.L.; Fox, H.S.; Kolson, D.L.; Grant, I.; Singer, E.; et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J. Acquir. Immune Defic. Syndr. 2013, 62, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med. Care 2003, 41, 1284–1292. [Google Scholar] [CrossRef]
- Maurer, D.M. Screening for depression. Am. Fam. Physician 2012, 85, 139–144. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Pashmdarfard, M.; Azad, A. Assessment tools to evaluate Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) in older adults: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 33. [Google Scholar] [CrossRef]
- Hyejin, L.; Bumjo, O.; Sunyoung, K.; Kiheon, L. ADL/IADL dependencies and unmet healthcare needs in older persons: A nationwide survey. Arch. Gerontol. Geriatr. 2021, 96, 104458. [Google Scholar] [CrossRef]
- Goodkin, K.; Miller, E.N.; Cox, C.; Reynolds, S.; Becker, J.T.; Martin, E.; A Selnes, O.; Ostrow, D.G.; Sacktor, N.C. Effect of ageing on neurocognitive function by stage of HIV infection: Evidence from the Multicenter AIDS Cohort Study. Lancet HIV 2017, 4, e411–e422. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, D.; Underwood, J.; Bagkeris, E.; Boffito, M.; Post, F.; Mallon, P.; Vera, J.; Williams, I.; Anderson, J.; Johnson, M.; et al. Depression, lifestyle factors and cognitive function in people living with HIV and comparable HIV-negative controls. HIV Med. 2019, 20, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Molsberry, S.A.; Lecci, F.; Kingsley, L.; Junker, B.; Reynolds, S.; Goodkin, K.; Levine, A.J.; Martin, E.; Miller, E.N.; Munro, C.A.; et al. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. AIDS 2015, 29, 713–721. [Google Scholar] [CrossRef]
- Wendelken, L.A.; Jahanshad, N.; Rosen, H.J.; Busovaca, E.B.; Allen, I.; Coppola, G.; Adams, C.B.; Rankin, K.P.; Milanini, B.; Clifford, K.B.; et al. ApoE ε4 Is Associated with Cognition, Brain Integrity, and Atrophy in HIV over Age 60. J. Acquir. Immune Defic. Syndr. 2016, 73, 426–432. [Google Scholar] [CrossRef]
- Schroecksnadel, K.; Sarcletti, M.; Winkler, C.; Mumelter, B.; Weiss, G.; Fuchs, D.; Kemmler, G.; Zangerle, R. Quality of life and immune activation in patients with HIV-infection. Brain Behav. Immun. 2008, 22, 881–889. [Google Scholar] [CrossRef]
- Everall, I.P.; Salaria, S.; Atkinson, J.H.; Young, C.; Corbeil, J.; Grant, I.; Masliah, E.; Hnrc, F.T. Diminished somatostatin gene expression in individuals with HIV and major depressive disorder. Neurology 2006, 67, 1867–1869. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R., Jr.; Deutsch, R.; Letendre, S.; Ellis, R.J.; Casaletto, K.; Marquine, M.J.; Woods, S.P.; Vaida, F.; Atkinson, J.H.; et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: The longitudinal CHARTER study. Clin. Infect. Dis. 2015, 60, 473–480. [Google Scholar] [CrossRef]
- Vance, D.E. The cognitive consequences of stigma, social withdrawal, and depression in adults aging with HIV. J. Psychosoc. Nurs. Ment. Health Serv. 2013, 51, 18–20. [Google Scholar] [CrossRef] [PubMed]
- McMahan, C.; Dietrich, D.K.; Horne, E.F.; Kelly, E.; Geannopoulos, K.; Julnes, P.S.S.; Ham, L.; Santamaria, U.; Lau, C.-Y.; Wu, T.; et al. Neurocognitive Dysfunction With Neuronal Injury in People With HIV on Long-Duration Antiretroviral Therapy. Neurology, 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Variable | Minimum | Maximum | Median | IQR |
|---|---|---|---|---|
| Age (years) | 30 | 81 | 58 | 52–61 |
| Years of HIV infection | 0 | 40 | 25 | 13–31 |
| MoCA (adjusted for ages of school) | 9 | 30 | 25 | 23–27 |
| PHQ-2 | 0 | 6 | 1 | 0–2 |
| ADLs | 3 | 6 | 6 | 6–6 |
| IADLs | 3 | 8 | 8 | 8–8 |
| CD4+ T-cells (cells/μL) | 79 | 1978 | 769 | 540–1003 |
| CD4+/CD8+ T-cell ratio | 0.09 | 11.12 | 1.04 | 0.66–1.53 |
| CD4+ T-cell nadir (cells/μL) | 3 | 804 | 254 | 118–392 |
| HIV RNA (copies/μL) | 0 | 215 | 0 | 0–0 |
| HIV RNA zenith (copies/μL) | 20 | 17,270,926 | 58,420 | 17,100–161,000 |
| Hb (g/dL) | 8.7 | 19.5 | 14.6 | 13.3–15.7 |
| WBCs (×103/μL) | 2.27 | 12.72 | 6.16 | 5.11–7.28 |
| PLTs (×103/μL) | 52 | 451 | 224 | 177–264 |
| PT (INR) | 0.86 | 6.61 | 0.95 | 0.93–0.98 |
| Albumin (g/dL) | 3.45 | 5.09 | 4.3 | 4.07–4.48 |
| AST (U/L) | 9 | 196 | 21 | 18–27 |
| ALT (U/L) | 9 | 143 | 20 | 14–29 |
| Creatinine (mg/dL) | 0.73 | 6.02 | 1.05 | 0.93–1.19 |
| LDL (mg/dL) | 16 | 216 | 111 | 88–130 |
| Ferritin (μg/L) | 4.4 | 1133.2 | 105.8 | 59–178.7 |
| Vitamin B12 (ng/mL) | 237 | 1225 | 495.5 | 406–598 |
| Folate (ng/mL) | 1.7 | 24 | 9.5 | 7–13.4 |
| TSH (μU/L) | 0.05 | 16.57 | 1.59 | 1.1–2.12 |
| CRP (mg/L) | 0 | 65.3 | 1.1 | 0.4–4 |
| Variable | % |
|---|---|
| Nucleoside reverse transcriptase inhibitor (NRTI) | 82.4 |
| Nonnucleoside reverse transcriptase inhibitor (NNRTI) | 35.7 |
| Nucleotide reverse transcriptase inhibitor (NtRTI) | 71.4 |
| Protease inhibitor (PI) | 15.7 |
| Integrase inhibitor (INI) | 66.7 |
| CCR5 inhibitor | 1.4 |
| Variable * | Standard Error | OR | 95%CI | p |
|---|---|---|---|---|
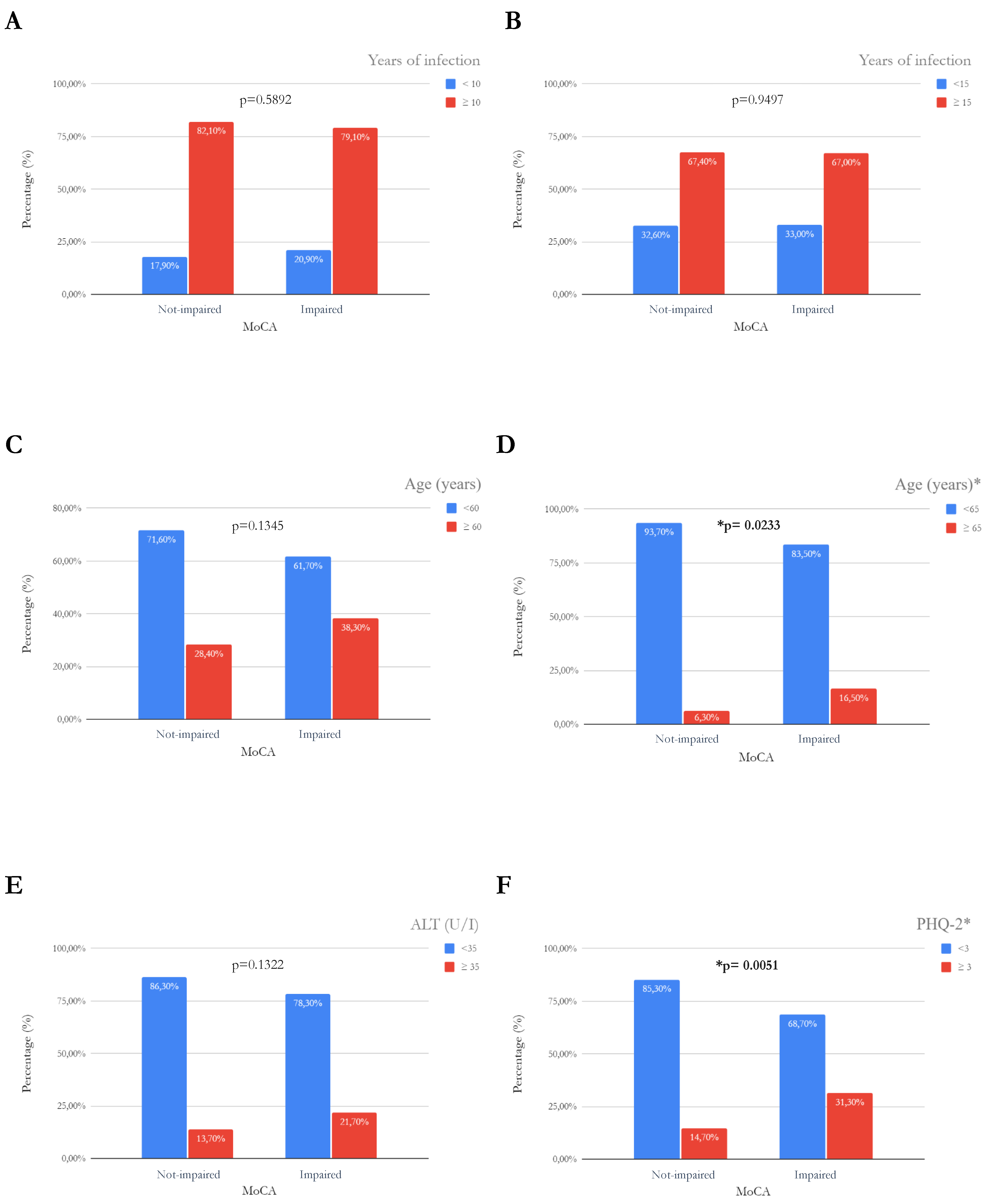
| Years of infection | 0.017 | 0.96 | 0.93–0.99 | 0.0099 |
| Age (years) | 0.023 | 1.12 | 1.07–1.17 | <0.0001 |
| ALT (U/L) | 0.009 | 1.02 | 1.01–1.04 | 0.0262 |
| PHQ-2 | 0.094 | 1.33 | 1.11–1.60 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salis, F.; Belfiori, M.; Bellisai, A.; Bernardini, E.; Murtas, M.; Piras, R.; Serreli, S.; Ortu, F.; Piano, P.; Del Giacco, S.; et al. Cognitive Impairment in People Living with HIV and the Impact of Mood: Results from a Cross-Sectional Study. J. Clin. Med. 2024, 13, 1631. https://doi.org/10.3390/jcm13061631
Salis F, Belfiori M, Bellisai A, Bernardini E, Murtas M, Piras R, Serreli S, Ortu F, Piano P, Del Giacco S, et al. Cognitive Impairment in People Living with HIV and the Impact of Mood: Results from a Cross-Sectional Study. Journal of Clinical Medicine. 2024; 13(6):1631. https://doi.org/10.3390/jcm13061631
Chicago/Turabian StyleSalis, Francesco, Maristella Belfiori, Alice Bellisai, Eleonora Bernardini, Michele Murtas, Rossella Piras, Silvia Serreli, Francesco Ortu, Paola Piano, Stefano Del Giacco, and et al. 2024. "Cognitive Impairment in People Living with HIV and the Impact of Mood: Results from a Cross-Sectional Study" Journal of Clinical Medicine 13, no. 6: 1631. https://doi.org/10.3390/jcm13061631
APA StyleSalis, F., Belfiori, M., Bellisai, A., Bernardini, E., Murtas, M., Piras, R., Serreli, S., Ortu, F., Piano, P., Del Giacco, S., & Mandas, A. (2024). Cognitive Impairment in People Living with HIV and the Impact of Mood: Results from a Cross-Sectional Study. Journal of Clinical Medicine, 13(6), 1631. https://doi.org/10.3390/jcm13061631