Sustained Disease Control in DME Patients upon Treatment Cessation with Brolucizumab
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garweg, J.G.; Stefanickova, J.; Hoyng, C.; Schmelter, T.; Niesen, T.; Sowade, O.; Sivaprasad, S. Vision-Related Quality of Life in Patients with Diabetic Macular Edema Treated with Intravitreal Aflibercept: The AQUA Study. Ophthalmol. Retin. 2019, 3, 567–575. [Google Scholar] [CrossRef]
- Jansen, M.E.; Krambeer, C.J.; Kermany, D.S.; Waters, J.N.; Tie, W.; Bahadorani, S.; Singer, J.; Comstock, J.M.; Wannamaker, K.W.; Singer, M.A.; et al. Appointment Compliance in Patients with Diabetic Macular Edema and Exudative Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 186–190. [Google Scholar] [CrossRef]
- Bressler, S.B.; Odia, I.; Glassman, A.R.; Danis, R.P.; Grover, S.; Hampton, G.R.; Jampol, L.M.; Maguire, M.G.; Melia, M. Changes in Diabetic Retinopathy Severity When Treating Diabetic Macular Edema with Ranibizumab: DRCR.net Protocol I 5-Year Report. Retina 2018, 38, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.E.; Jo, J.; Kim, Y.J.; Lee, J. Factors Affecting Intensive Aflibercept Treatment Response in Diabetic Macular Edema: A Real-World Study. J. Diabetes Res. 2023, 2023, 1485059. [Google Scholar] [CrossRef] [PubMed]
- Kiss, S.; Chandwani, H.S.; Cole, A.L.; Patel, V.D.; Lunacsek, O.E.; Dugel, P.U. Comorbidity and health care visit burden in working-age commercially insured patients with diabetic macular edema. Clin. Ophthalmol. 2016, 10, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, C.; Helms, M.; Böhringer, D.; Agostini, H.T.; Stahl, A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin. Ophthalmol. 2018, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Štefanickova, J.; Hoyng, C.; Niesen, T.; Schmelter, T.; Leal, S.; Sivaprasad, S.; Schmidt-Erfurth, U.; Wedrich, A.; Ali, F.; et al. Dosing Regimens of Intravitreal Aflibercept for Diabetic Macular Edema Beyond the First Year: VIOLET, a Prospective Randomized Trial. Adv. Ther. 2022, 39, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Ngah, N.F.; Muhamad, N.A.; Mohamed, S.O.; Aziz, R.A.A.; Ma’Amor, N.H.; Tarmidzi, N.A.A.; Hashim, H.; Ishak, H.; Muda, W.N.W.; Muda, R.; et al. Guidelines from an expert panel for the management of diabetic macular edema in the Malaysian population. Int. J. Ophthalmol. 2023, 16, 712–720. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Lang, G.E.; Holz, F.G.; Schlingemann, R.O.; Lanzetta, P.; Massin, P.; Gerstner, O.; Bouazza, A.S.; Shen, H.; Osborne, A.; et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: The RESTORE extension study. Ophthalmology 2014, 121, 1045–1053. [Google Scholar] [CrossRef]
- Bakri, S.J.; Delyfer, M.N.; Grauslund, J.; Andersen, S.; Karcher, H. Real-World Persistence and Treatment Interval in Patients with Diabetic Macular Edema Treated with Anti-Vascular Endothelial Growth Factors in the USA. Ophthalmol. Ther. 2023, 12, 2465–2477. [Google Scholar] [CrossRef]
- Vargas-Peirano, M.; Verdejo, C.; Vergara-Merino, L.; Loézar, C.; Hoehmann, M.; Pérez-Bracchiglione, J. Intravitreal antivascular endothelial growth factor in diabetic macular oedema: Scoping review of clinical practice guidelines recommendations. Br. J. Ophthalmol. 2023, 107, 313–319. [Google Scholar] [CrossRef]
- Relton, S.D.; Chi, G.C.; Lotery, A.J.; West, R.M.; McKibbin, M. Associations with Non-Persistence with Intra-Vitreal Therapy for Neovascular Age-Related Macular Degeneration at 24 Months. Ophthalmologica 2023, 246, 90–98. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef]
- Rosenberg, D.; Deonarain, D.M.; Gould, J.; Sothivannan, A.; Phillips, M.R.; Sarohia, G.S.; Sivaprasad, S.; Wykoff, C.C.; Cheung, C.M.G.; Sarraf, D.; et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: A systematic review and meta-analysis. Eye 2023, 37, 6–16. [Google Scholar] [CrossRef]
- Matonti, F.; Korobelnik, J.F.; Dot, C.; Gualino, V.; Soler, V.; Mrejen, S.; Delyfer, M.-N.; Baillif, S.; Streho, M.; Gascon, P.; et al. Comparative Effectiveness of Intravitreal Anti-Vascular Endothelial Growth Factor Therapies for Managing Neovascular Age-Related Macular Degeneration: A Meta-Analysis. J. Clin. Med. 2022, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Chau, T.T.H. Five-year outcome of aflibercept intravitreal injection in naïve patients with neovascular age-related macular degeneration using a modified treat-and-extend regimen: Results from a prospective observational study. Taiwan J. Ophthalmol. 2023, 13, 219–224. [Google Scholar] [PubMed]
- Mitchell, P.; Holz, F.G.; Hykin, P.; Midena, E.; Souied, E.; Allmeier, H.; Lambrou, G.; Schmelter, T.; Wolf, S.; on behalf of the ARIES Study Investigators. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: The ARIES Study: A Randomized Clinical Trial. Retina 2021, 41, 1911–1920. [Google Scholar] [CrossRef]
- Menke, M.N.; Zinkernagel, M.S.; Ebneter, A.; Wolf, S. Functional and anatomical outcome of eyes with neovascular age-related macular degeneration treated with intravitreal ranibizumab following an exit strategy regimen. Br. J. Ophthalmol. 2014, 98, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.C.; Park, K.H.; Park, S.J.; Joo, K.; Woo, S.J. Discontinuation of treatment and retreatment of neovascular age-related macular degeneration in the real-world: Bundang AMD cohort study report 5. Front. Med. 2023, 10, 1204026. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Traine, P.G.; Garweg, R.A.; Wons, J.; Gerhardt, C.; Pfister, I.B. Continued anti-VEGF treatment does not prevent recurrences in eyes with stable neovascular age-related macular degeneration using a treat-and-extend regimen: A retrospective case series. Eye 2022, 36, 862–868. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef] [PubMed]
- Apuzzo, A.; Gravina, S.; Panozzo, G.; Lattanzio, R.; Cicinelli, M.V.; Bandello, F. From diagnosis to prognosis: A paradigm shift for multimodal imaging in assessing diabetic macular edema. Eur. J. Ophthalmol. 2023, 34, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Garweg, J.G.; Regillo, C.; Souied, E.; Wolf, S.; Dhoot, D.S.; Agostini, H.T.; Chang, A.; Laude, A.; Wachtlin, J.; et al. KESTREL and KITE Phase 3 studies: 100-week results with brolucizumab in patients with diabetic macular edema. Am. J. Ophthalmol. 2023, 260, 70–83. [Google Scholar] [CrossRef]
- Brown, D.M.; Nguyen, Q.D.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Schlottmann, P.G.; Rundle, A.C.; Zhang, J.; Rubio, R.G.; et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013, 120, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.J.; Ayala, A.; Bressler, N.M.; Browning, D.; Flaxel, C.J.; Glassman, A.R.; Jampol, L.M.; Stone, T.W. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015, 122, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.B.; Glassman, A.R.; Almukhtar, T.; Bressler, N.M.; Ferris, F.L.; Googe, J.M.; Gupta, S.K.; Jampol, L.M.; Melia, M.; Wells, J.A. Five-Year Outcomes of Ranibizumab with Prompt or Deferred Laser Versus Laser or Triamcinolone Plus Deferred Ranibizumab for Diabetic Macular Edema. Am. J. Ophthalmol. 2016, 164, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.R.; Wells, J.A., 3rd; Josic, K.; Maguire, M.G.; Antoszyk, A.N.; Baker, C.; Beaulieu, W.T.; Elman, M.J.; Jampol, L.M.; Sun, J.K.; et al. Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study). Ophthalmology 2020, 127, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Korobelnik, J.F.; Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Midena, E.; Boyer, D.S.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology 2016, 123, 2376–2385. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Le, R.T.; Khurana, R.N.; Brown, D.M.; Ou, W.C.; Wang, R.; Clark, W.L.; Boyer, D.S. Outcomes With As-Needed Aflibercept and Macular Laser Following the Phase III VISTA DME Trial: ENDURANCE 12-Month Extension Study. Am. J. Ophthalmol. 2017, 173, 56–63. [Google Scholar] [CrossRef]
- Schneider, M.; Bjerager, J.; Hodzic-Hadzibegovic, D.; Klefter, O.N.; Subhi, Y.; Hajari, J. Short-term outcomes of treatment switch to faricimab in patients with aflibercept-resistant neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2024. [Google Scholar] [CrossRef]
- Li, G.; Zhu, N.; Ji, A. Comparative efficacy and safety of Faricimab and other anti-VEGF therapy for age-related macular degeneration and diabetic macular edema: A systematic review and meta-analysis of randomized clinical trials. Medicine 2023, 102, e36370. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Roberts, C.L.; Saggau, D.D.; Alliman, K.J. High-Dose Aflibercept for Neovascular AMD and DME in Suboptimal Responders to Standard-Dose Aflibercept. J. Vitr. Dis. 2023, 7, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Boscia, G.; Pozharitskiy, N.; Grassi, M.O.; Borrelli, E.; D’Addario, M.; Alessio, G.; Boscia, F.; Viggiano, P. Choroidal remodeling following different anti-VEGF therapies in neovascular AMD. Sci. Rep. 2024, 14, 1941. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Attiku, Y.; Sadda, S.R. Endpoints of Anti-Vascular Endothelial Growth Factor Clinical Trials for Diabetic Macular Edema. In Diabetic Macular Edema; Saxena, S., Cheung, G., Lai, T.Y., Sadda, S.R., Eds.; Springer: Singapore, 2022; pp. 185–198. [Google Scholar] [CrossRef]
- Yayla, U.; Sevik, M.O.; Karabaş, V.L.; Şahin, Ö.; Özkaya, A.; Yenerel, N.M.; Açıkalın Öncel, B.; Kaplan, F.B.; Önder Tokuç, E.; Kanar, H.S.; et al. Real-World Outcomes of Intravitreal Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema in Türkiye: MARMASIA Study Group Report No. 1. Turk. J. Ophthalmol. 2023, 53, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.; Schiefelbein, J.; Fu, D.J.; Schworm, B.; Sim, D.; Herold, T.; Priglinger, S.; Kortuem, K. Two Year Visual Acuity and Structural Outcomes in Patients with Diabetic Macular Oedema Treated with Intravitreal Aflibercept—A Retrospective Cohort Study. Clin. Ophthalmol. 2020, 14, 533–541. [Google Scholar] [CrossRef]
- Massin, P.; Creuzot-Garcher, C.; Kodjikian, L.; Girmens, J.F.; Delcourt, C.; Fajnkuchen, F.; Glacet-Bernard, A.; Guillausseau, P.J.; Guthux, F.; Blin, P.; et al. Real-World Outcomes after 36-Month Treatment with Ranibizumab 0.5 mg in Patients with Visual Impairment due to Diabetic Macular Edema (BOREAL-DME). Ophthalmic Res. 2021, 64, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, F.; Wachtlin, J.; Kuehlewein, L.; Gamulescu, M.A.; Bertelmann, T.; Feucht, N.; Voegeler, J.; Koch, M.; Liakopoulos, S.; Schmitz-Valckenberg, S.; et al. Intravitreal Ranibizumab Therapy for Diabetic Macular Edema in Routine Practice: Two-Year Real-Life Data from a Non-interventional, Multicenter Study in Germany. Diabetes Ther. 2018, 9, 2271–2289. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Bressler, N.M. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: Recent clinically relevant findings from DRCR.net Protocol T. Curr. Opin. Ophthalmol. 2017, 28, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.R.; Somfai, G.M.; de Smet, M.D.; Donati, G.; Menke, M.N.; Garweg, J.G.; Ceklic, L. The Role of Intravitreal Corticosteroids in the Treatment of DME: Predictive OCT Biomarkers. Int. J. Mol. Sci. 2022, 23, 7585. [Google Scholar] [CrossRef]
- Weiss, M.; Sim, D.A.; Herold, T.; Schumann, R.G.; Liegl, R.; Kern, C.; Kreutzer, T.; Schiefelbein, J.; Rottmann, M.; Priglinger, S.; et al. Compliance and Adherence of Patients with Diabetic Macular Edema to Intravitreal Anti-Vascular Endothelial Growth Factor Therapy in Daily Practice. Retina 2018, 38, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Mitchell, T.C.; Rusakevich, A.M.; Brown, D.M.; Wykoff, C.C. Noncompliance in Prospective Retina Clinical Trials: Analysis of Factors Predicting Loss to Follow-up. Am. J. Ophthalmol. 2020, 210, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Giocanti-Aurégan, A.; García-Layana, A.; Peto, T.; Gentile, B.; Chi, G.C.; Mirt, M.; E Kosmas, C.; Lambert, J.; Lanar, S.; Lewis, H.B.; et al. Drivers of and Barriers to Adherence to Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema Treatment Management Plans: A Multi-National Qualitative Study. Patient Prefer. Adherence 2022, 16, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Velu, S.; Rajalakshmi, R.; Surya, J.; Mohan, V.; Raman, A.; Raman, R. Compliance with follow-up in patients with diabetic macular edema: Eye care center vs. diabetes care center. Indian J. Ophthalmol. 2023, 71, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Nguyen, Q.D.; Brown, D.M.; Basu, K.; Ehrlich, J.S. Outcomes with As-Needed Ranibizumab after Initial Monthly Therapy: Long-Term Outcomes of the Phase III RIDE and RISE Trials. Ophthalmology 2015, 122, 2504–2513.e2501. [Google Scholar] [CrossRef]
- Couturier, A.; Giocanti-Auregan, A.; Massin, P. Treatment switch in diabetic macular edema: Literature review and management algorithm. J. Fr. D’ophtalmologie 2020, 43, 710–717. [Google Scholar] [CrossRef]
- Raman, R.; Giridhar, S.; Verma, L.; Rajendran, A.; Bhend, M.; Goyal, M.; Ramasamy, K.; Rajalakshmi; Padmaja, R.; Natarajan, S.; et al. Diabetic macular edema treatment guidelines in India: All India Ophthalmological Society Diabetic Retinopathy Task Force and Vitreoretinal Society of India consensus statement. Indian J. Ophthalmol. 2021, 69, 3076–3086. [Google Scholar] [CrossRef]
- Comyn, O.; Sivaprasad, S.; Peto, T.; Neveu, M.M.; Holder, G.E.; Xing, W.; Bunce, C.V.; Patel, P.J.; Egan, C.A.; Bainbridge, J.W.; et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). Am. J. Ophthalmol. 2014, 157, 960–970. [Google Scholar] [CrossRef]
- Sinclair, S.H.; Alaniz, R.; Presti, P. Laser treatment of diabetic macular edema: Comparison of ETDRS-level treatment with threshold-level treatment by using high-contrast discriminant central visual field testing. Semin. Ophthalmol. 1999, 14, 214–222. [Google Scholar] [CrossRef]
- Striph, G.G.; Hart, W.M.; Olk, R.J., Jr. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology 1988, 95, 1673–1679. [Google Scholar] [CrossRef]
- Kothari, A.R.; Raman, R.P.; Sharma, T.; Gupta, M.; Laxmi, G. Is there a correlation between structural alterations and retinal sensitivity in morphological patterns of diabetic macular edema? Indian J. Ophthalmol. 2013, 61, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Edington, M.; Sachdev, A.; Morjaria, R.; Chong, V. Structural-Functional Correlation in Patients with Diabetic Macular Edema. Retina 2017, 37, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.C.; Schuchard, R.A. Visual function in patients with choroidal neovascularization resulting from age-related macular degeneration: The importance of looking beyond visual acuity. Optom. Vis. Sci. 2006, 83, 178–189. [Google Scholar] [CrossRef]
- Massin, P.; Bandello, F.; Garweg, J.G.; Hansen, L.L.; Harding, S.P.; Larsen, M.; Mitchell, P.; Sharp, D.; Wolf-Schnurrbusch, U.E.K.; Gekkieva, M.; et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010, 33, 2399–2405. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Marcus, D.M.; Midena, E.; Korobelnik, J.-F.; Saroj, N.; Gibson, A.; Vitti, R.; Berliner, A.J.; Liu, Z.W.; Zeitz, O.; et al. Intravitreal Aflibercept Injection in Eyes with Substantial Vision Loss After Laser Photocoagulation for Diabetic Macular Edema: Subanalysis of the VISTA and VIVID Randomized Clinical Trials. JAMA Ophthalmol. 2017, 135, 107–114. [Google Scholar] [CrossRef]
- Abraham, P.; Yue, H.; Wilson, L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am. J. Ophthalmol. 2010, 150, 315–324.e311. [Google Scholar] [CrossRef] [PubMed]
- Udaondo, P.; Parravano, M.; Vujosevic, S.; Zur, D.; Chakravarthy, U. Update on Current and Future Management for Diabetic Maculopathy. Ophthalmol. Ther. 2022, 11, 489–502. [Google Scholar] [CrossRef] [PubMed]
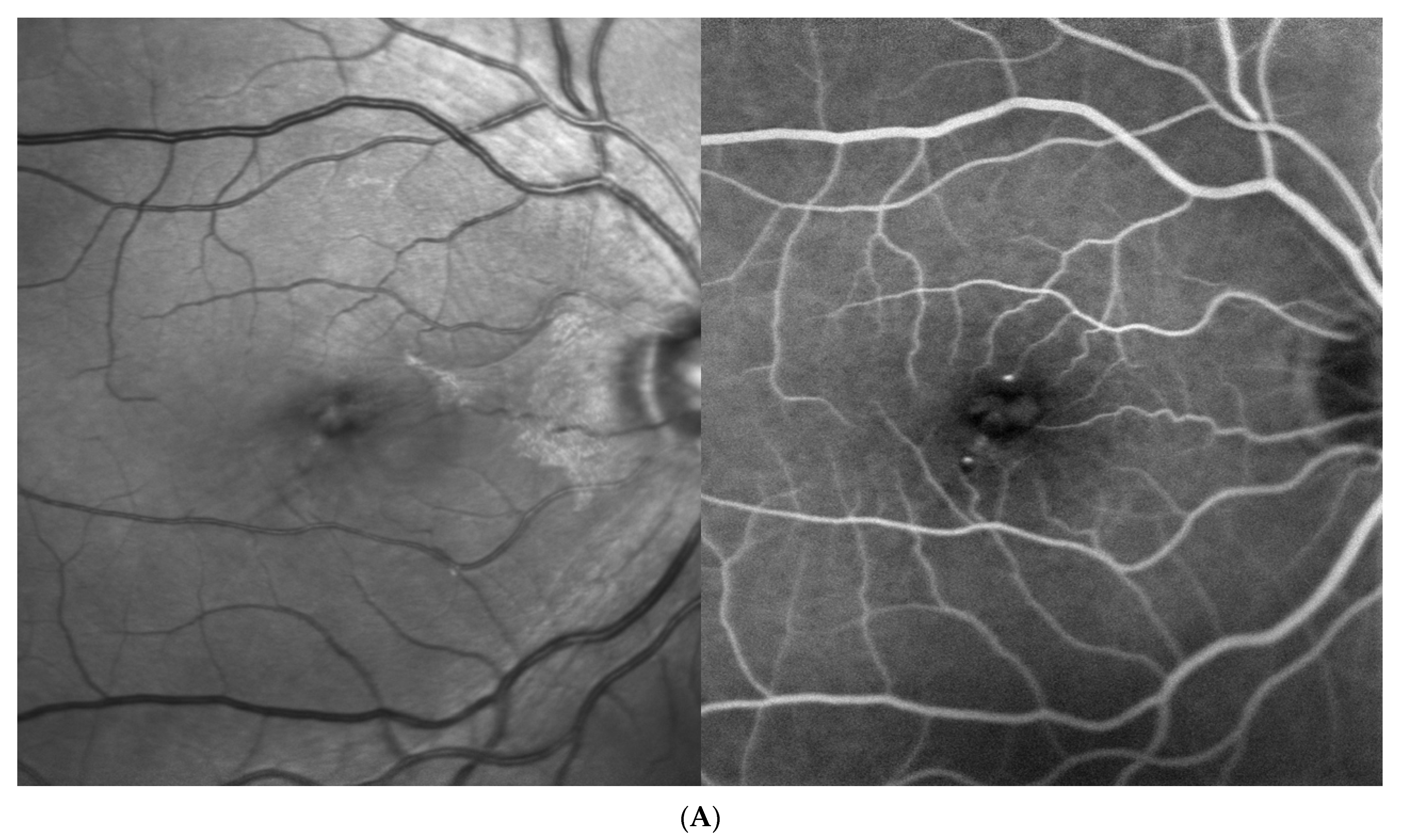
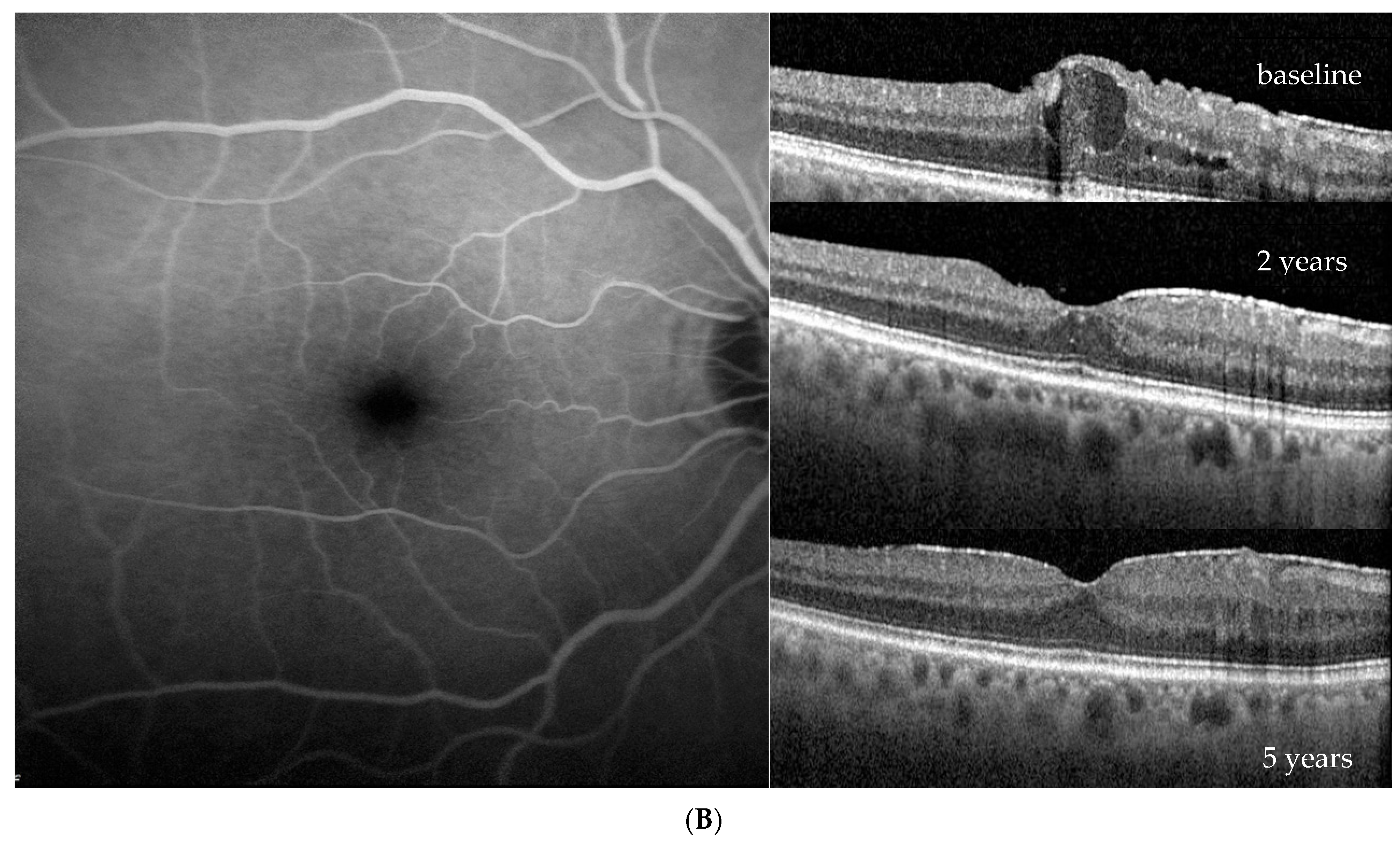
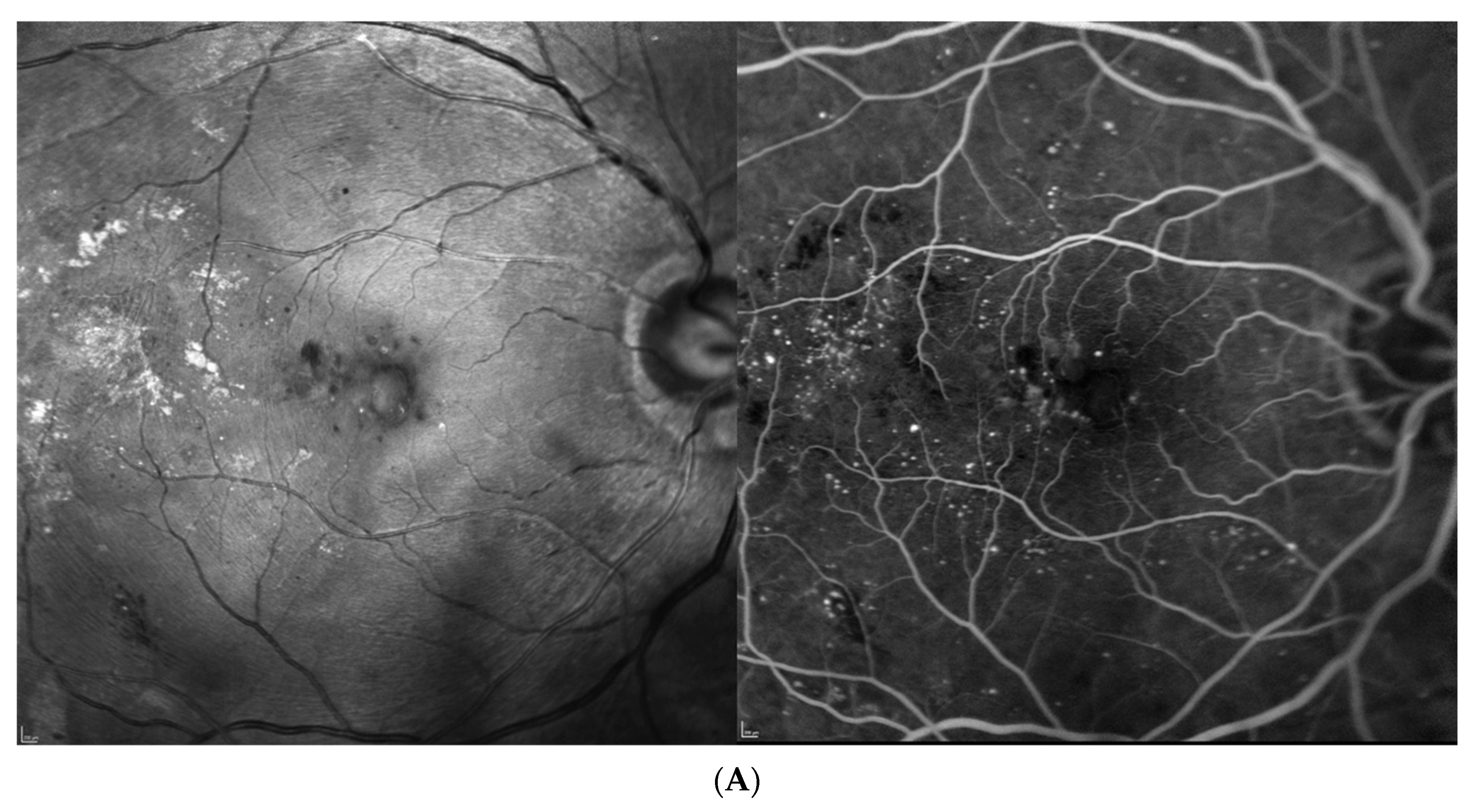
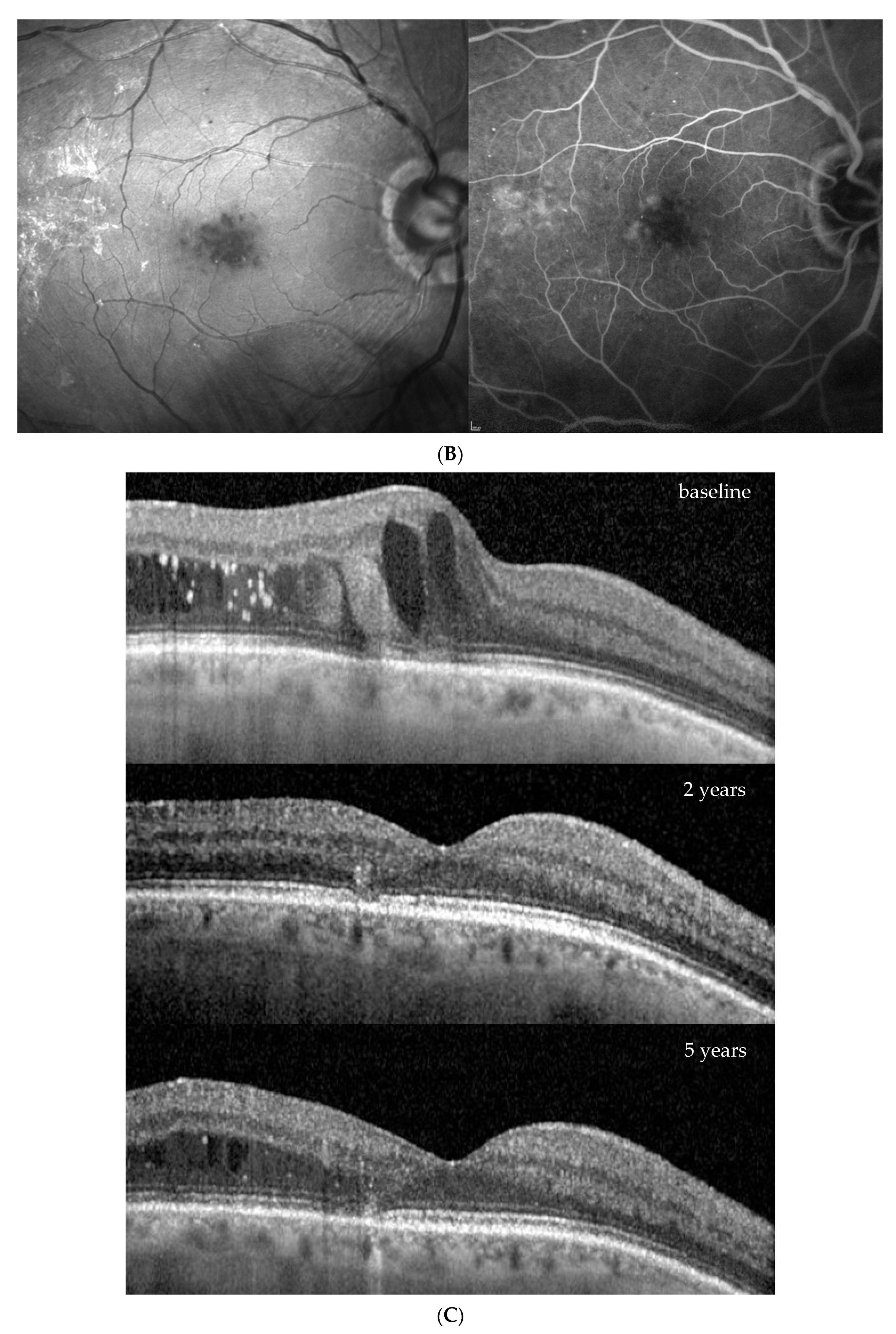
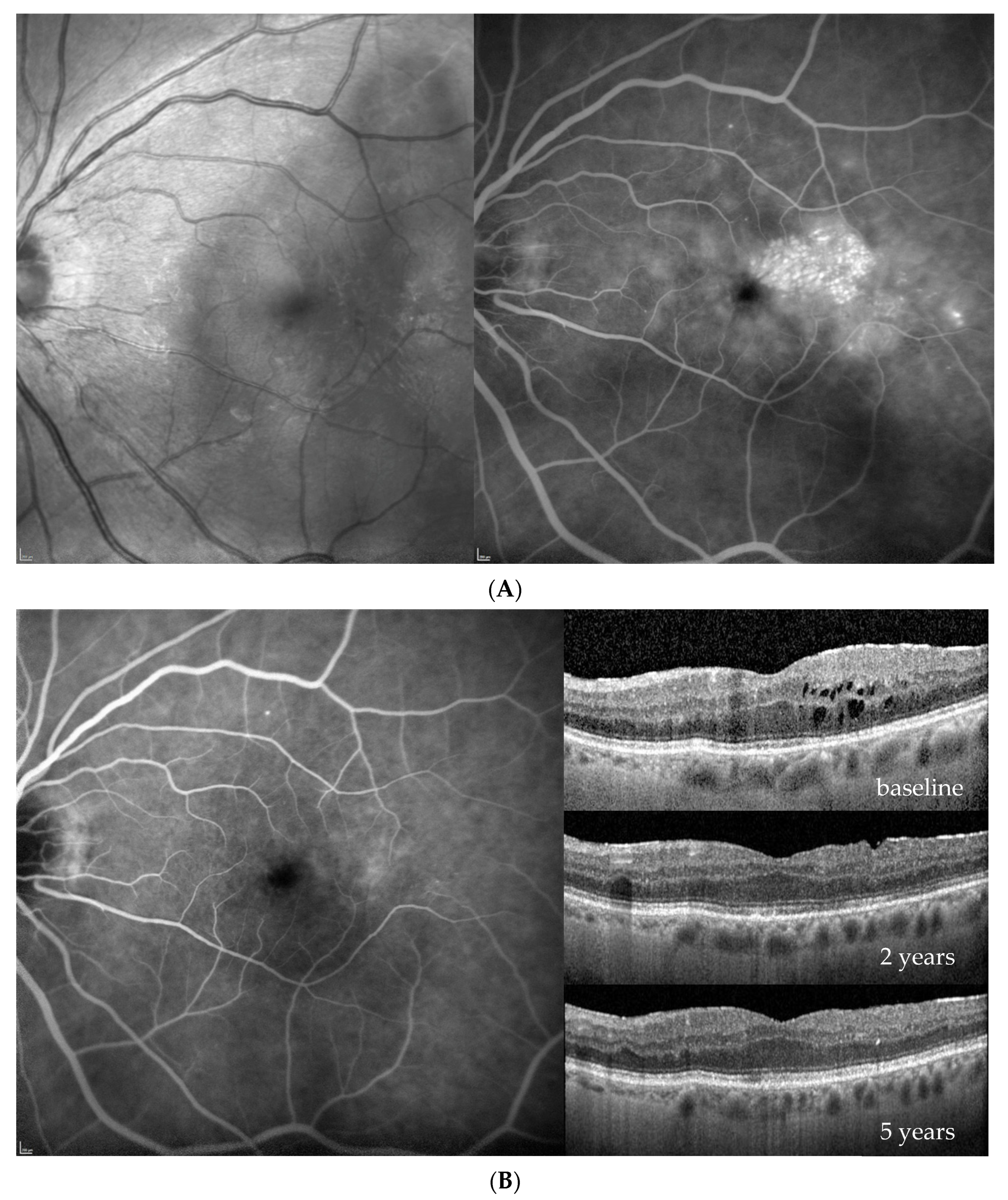
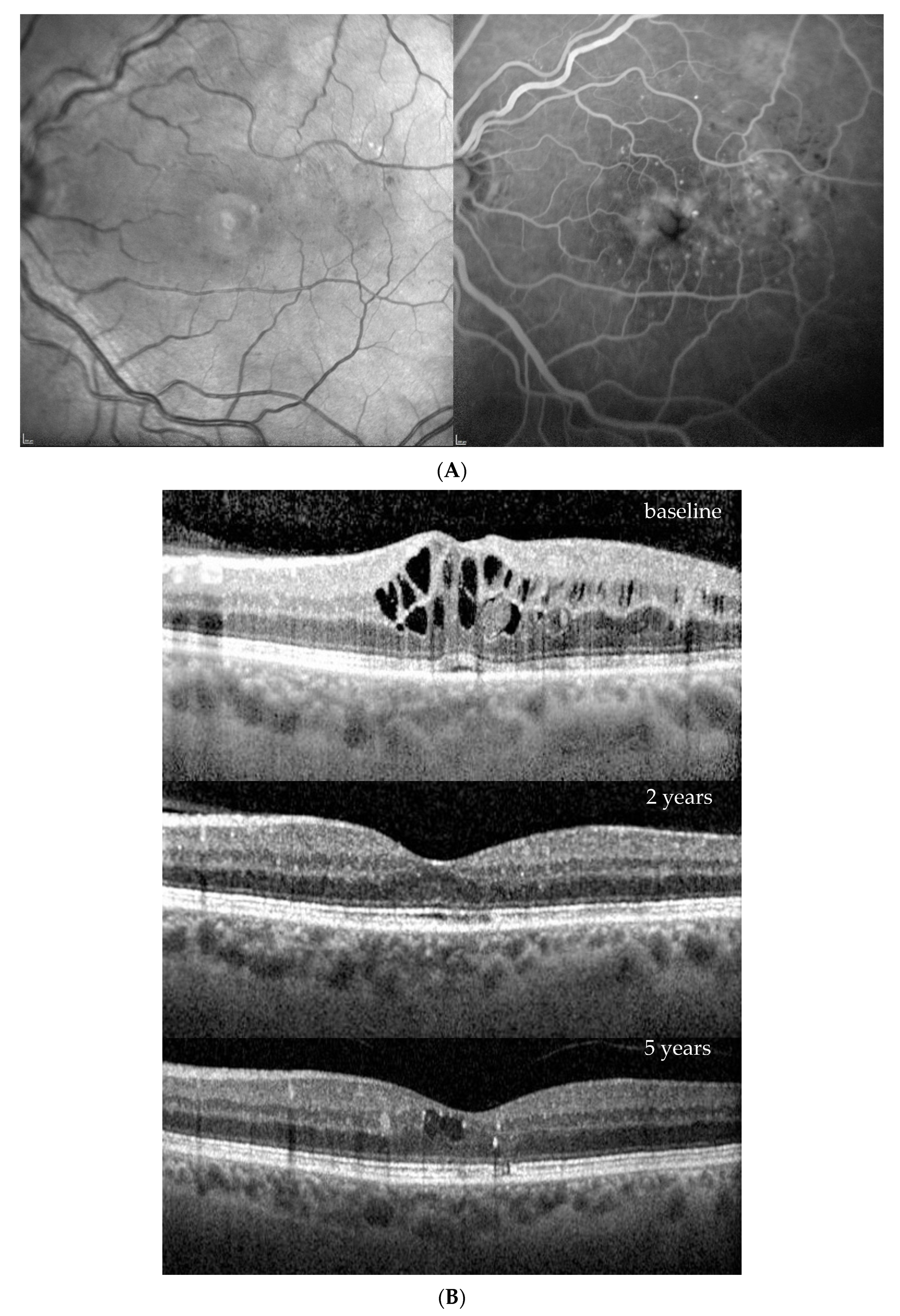
| Baseline (at DME Treatment Initiation) | Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|---|
| Age | years | 74 | 78 | 73 | 66 |
| Gender | m/f | m | m | f | m |
| Body Mass Index | kg/m2 | 29.6 | 29.3 | 35.9 | 24.2 |
| Diabetes type | 2 | 2 | 2 | 2 | |
| Diabetes duration | years | 20 | 13 | 34 | 20 |
| Hb1aC | % | 6.2 | 9 | 8.3 | 9.8 |
| Insulin dependence | no | yes | yes | yes | |
| Hypertension | yes | yes | yes | yes | |
| Dyslipidemia | yes | no | no | no | |
| Sleep apnea | yes | yes | yes | no | |
| Smoking state | unknown | unknown | former smoker | former smoker | |
| Regular sporting activity | hours per day | 0 | 1.5 | 0 | 3 |
| Major cardiovascular events * | number | 1 | 2 | 2 | 0 |
| Visual impairment due to DME in the partner eye | no | no | yes | no | |
| Affected Eye | R | R | L | L | |
|---|---|---|---|---|---|
| BCVA | ETDRS score | 77 | 73 | 69 | 73 |
| Snellen decimal | 0.8 | 0.63 | 0.5 | 0.63 | |
| CST | µm | 409 | 466 | 410 | 470 |
| NPDR severity | mild | moderate | moderate | mild | |
| Epiretinal membrane | yes | no | yes | no | |
| Presence of vitreomacular traction | no | no | no | no | |
| 24 months | |||||
| BCVA | ETDRS score | 90 | 81 | 86 | 80 |
| Snellen decimal | 1.25 | 0.8 | 1.0 | 0.8 | |
| CST | µm | 330 | 274 | 337 | 591 |
| NPDR severity | background | mild | mild | mild | |
| 36 months | |||||
| BCVA | Snellen decimal | 1 | 1 | 1 | 0.8 |
| CST | µm | 345 | 302 | 361 | 365 |
| 48 months | |||||
| BCVA | Snellen decimal | 1.25 | 1.0 | 1.0 | 1.0 |
| CST | µm | 339 | 266 | 344 | 293 |
| 60 months | |||||
| BCVA | Snellen decimal | 1.0 | 1.25 | 1.0 | 0.63 |
| CST | µm | 342 | 263 | 340 | 513 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garweg, J.G.; Steinhauer, S. Sustained Disease Control in DME Patients upon Treatment Cessation with Brolucizumab. J. Clin. Med. 2024, 13, 1534. https://doi.org/10.3390/jcm13061534
Garweg JG, Steinhauer S. Sustained Disease Control in DME Patients upon Treatment Cessation with Brolucizumab. Journal of Clinical Medicine. 2024; 13(6):1534. https://doi.org/10.3390/jcm13061534
Chicago/Turabian StyleGarweg, Justus G., and Sonja Steinhauer. 2024. "Sustained Disease Control in DME Patients upon Treatment Cessation with Brolucizumab" Journal of Clinical Medicine 13, no. 6: 1534. https://doi.org/10.3390/jcm13061534
APA StyleGarweg, J. G., & Steinhauer, S. (2024). Sustained Disease Control in DME Patients upon Treatment Cessation with Brolucizumab. Journal of Clinical Medicine, 13(6), 1534. https://doi.org/10.3390/jcm13061534






