Abstract
The early and accurate stratification of intracranial cerebral artery stenosis (ICAS) is critical to inform treatment management and enhance the prognostic outcomes in patients with cerebrovascular disease (CVD). Digital subtraction angiography (DSA) is an invasive and expensive procedure but is the gold standard for the diagnosis of ICAS. Over recent years, transcranial color-coded Doppler ultrasound (TCCD) has been suggested to be a useful imaging method for accurately diagnosing ICAS. However, the diagnostic accuracy of TCCD in stratifying ICASs among patients with CVD remains unclear. Therefore, this systematic review and meta-analysis aimed at evaluating the diagnostic accuracy of TCCD in the stratification of intracranial steno-occlusions among CVD patients. A total of six databases—Embase, CINAHL, Medline, PubMed, Google Scholar, and Web of Science (core collection)—were searched for studies that assessed the diagnostic accuracy of TCCD in stratifying ICASs. The meta-analysis was performed using Meta-DiSc 1.4. The Quality Assessment of Diagnostic Accuracy Studies tool version 2 (QUADAS-2) assessed the risk of bias. Eighteen studies met all of the eligibility criteria. TCCD exhibited a high pooled diagnostic accuracy in stratifying intracranial steno-occlusions in patients presenting with CVD when compared to DSA as a reference standard (sensitivity = 90%; specificity = 87%; AUC = 97%). Additionally, the ultrasound parameters peak systolic velocity (PSV) and mean flow velocity (MFV) yielded a comparable diagnostic accuracy of “AUC = 0.96”. In conclusion, TCCD could be a noble, safe, and accurate alternative imaging technique to DSA that can provide useful diagnostic information in stratifying intracranial steno-occlusions in patients presenting with CVD. TCCD should be considered in clinical cases where access to DSA is limited.
1. Introduction
Cerebrovascular disease (CVD) is broadly categorized as a group of conditions affecting blood vessels and blood supply to the brain. In order to harmonize the various classifications and nomenclature of CVD, the term “stroke” is the most appropriately used definition for CVD [1]. According to Feigin et al. [2], stroke is the second leading cause of death and the third leading cause of mortality and disability in the world, whereas ischemic stroke is reported to be the most prevalent subtype of stroke, accounting for more than 80% of all stroke cases [3]. Occlusions or severe stenoses of cerebral arteries (intracranial and extracranial) are associated with plaque build-up within arteries in atherosclerotic disease, and constitute a main parameter for ischemic stroke risk estimation [4]. Carotid artery stenoses account for 10–20% of ischemic strokes [5,6,7,8], whereas in the Asian population, between 33% to 67% of stroke cases are due to intracranial cerebral artery stenoses (ICAS) [9,10]. The early and accurate stratification of ICAS is critical to inform treatment management and enhance prognostic outcomes in CVD patients, as selecting patients to undergo surgical revascularization and thrombolysis is informed by the degree of stenosis [11,12].
Digital subtraction angiography (DSA) is the primary imaging modality for evaluating the cerebral arteries in the diagnosis of atherosclerotic stenosis [13,14]. However, DSA is invasive, expensive, and not readily available. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are also used in the assessment of ICAS, but they are expensive and involve the administration of contrast agents. Ultrasonography is a non-invasive, inexpensive, and readily available imaging modality. Duplex carotid ultrasound (DCUS) and transcranial Doppler (TCD) ultrasound are the cornerstone ultrasonography techniques for extracranial and intracranial cerebral arteries assessment, respectively. However, the clinical utility of TCD is limited because it cannot provide anatomical information to allow for precise differentiation between individual vessels, especially in the wake of anatomical variations. Additionally, at a technical level, Doppler angle correction that helps improve the measurement accuracy of blood flow velocities is not possible with TCD. Over recent years, transcranial color-coded Doppler (TCCD) has emerged, and is increasingly becoming available on ultrasound systems and adopted into clinical practice [15]. Contrary to non-imaging TCD, TCCD enables direct visualization of intracranial cerebral arteries through the intact skull by color-coding blood flow velocities [16]. TCCD offers the opportunity for angle correction that enhances the accuracy of blood flow velocity measurements. However, despite the additional technical benefits offered by the TCCD technique, the diagnostic accuracy of TCCD in stratifying ICASs in patients presenting with CVD still remains understudied; hence, further investigation is required. Thus, this study aimed at evaluating the diagnostic accuracy of TCCD in the stratification of intracranial steno-occlusions among patients with CVD, by systematically reviewing and meta-analyzing available literature on the accuracy of TCCD and comparing these results with angiography for the diagnosis of ICAS. It was hypothesized that the TCCD technique has a high diagnostic accuracy in stratifying ICASs.
2. Materials and Methods
The study involved a comprehensive search of six electronic databases: Medline, PubMed, Google Scholar, Web of Science (core collection), Cumulative Index to Nursing and Allied Health Literature (CINAHL Complete via EbscoHost), and Embase. The online library of our institution was used to access the databases, and studies published in the English language from 1990 to 5 October 2023 were retrieved. The database search started on 10 February 2023, and the last search was conducted on 5 October 2023. A rerun of the search was conducted prior to the final analysis. The study followed the preferred reporting items for systematic review and meta-analysis (PRISMA) 2020 guidelines, as informed by Page et al. [17].
2.1. Search Strategy
The search strategy included searching for concepts related to the PICO framework structured research question, where P (study population) = cerebrovascular disease; I (intervention) = novel ultrasonography imaging technique, transcranial color-coded Doppler ultrasonography; C (comparison) = angiography techniques (DSA, CTA, and MRA), and histopathology used as the reference standards; and O (outcome) = diagnostic accuracy indicators (overall accuracy, sensitivity, specificity, diagnostic odds ratio (DOR), area under the receiver operating curve (AUC)). The concepts were searched using the following: (1) MeSH descriptors in Medline and PubMed, Emtree terms in Embase and CINAHL subject headings, and (2) keywords and their related terms (synonyms, hyponyms). Relevant references from the selected studies were retrieved for further evaluation. The Boolean operator “OR” was used to search within each PICO element concept (MeSH or Emtree terms) and the related entry terms or synonyms, whereas “AND” was applied to search across the concepts of the PICO framework elements. The database search strings are attached in Supplementary Table S1.
2.2. Inclusion and Exclusion Criteria
The studies included in this systematic review and meta-analysis were restricted to (1) original studies that were peer-reviewed; (2) those published in the English language; (3) those involving human adults subjects >18 years with suspected cerebrovascular diseases; (4) those assessing the diagnostic accuracy of TCCD technique in the diagnosis of ICAS; (5) those with DSA, CTA, and/or MRA used as the reference standard; (6) those performed in clinical settings; and (7) studies with informed consent and institutional ethical approval obtained prior to data collection.
The exclusion criteria applied to studies that (1) referenced standards other than angiographic imaging techniques (DSA, CTA, MRA) and histopathology; (2) had inadequate information on the diagnostic performance outcome measures; (3) included conference proceedings, posters, case reports, reviews, editorial letters, or commentaries; (4) assessed diagnostic accuracy of non-imaging TCD and other imaging modalities apart from TCCD; (5) were not published in the English language; and (6) involved subjects <18 years who could not give informed consent, and to which CVD is not common.
2.3. Data Extraction
The records retrieved from the database were exported to a collaborative systematic review management software—Rayyan (web application, no public version, available at: http://rayyan.qcri.org)—to allow for a collaborative review process. Two reviewers, S.T.G. and T.V.N., independently screened the titles and abstracts of the retrieved records. This was followed by a full text assessment of screened records for eligibility, and a subsequent quality assessment of the eligible studies undertaken by same reviewers. A third reviewer, M.T.C.Y., acted as the moderator, and was responsible for resolving any disagreements between the two independent reviewers. The relevant data pertaining to the authors’ name, date of publication, subjects’ demographic and clinical information, study aim, study methodology, reference standard against which the index test was compared, and the diagnostic performance indicators were extracted from the eligible studies based on the PRISMA 2020 guidelines and recorded on an Excel spreadsheet.
2.4. Quality Assessment
The Quality Assessment of Diagnostic Accuracy Studies tool version 2 (QUADAS-2) was utilized to assess the risk of bias and the methodological quality. This involved assessing the risk of bias—applicability concerns in the four domains of (1) patient selection, (2) index test, (3) reference standard, and (4) flow and timing. A total of 14 questions were asked, and each correct answer was awarded a single point according to the methodology by Sultan et al. [18]. The risk of bias and applicability in these domains were categorized into high, low, or unclear, and a meta-analysis was performed in studies that exhibited a minimal risk of bias in the assessed four domains.
2.5. Statistical Analysis
Meta-DiSc (version 1.4, the unit of the clinical biostatistics team of Ramón y Cajal Hospital in Madrid, Spain) was used to assess pooled diagnostic performance (sensitivity, specificity, overall accuracy, diagnostic odds ratio, area under the receiver operating curve (AUCROC)) of the eligible studies in the diagnosis and stratification of ICAS. The heterogeneity of the included studies was assessed based on inconsistency (I2), and the DerSimonian–Laird random effects model incorporated in Meta-Disc 1.4 was performed to cater to between-study heterogeneity.
3. Results
3.1. Literature Search
A total of 1183 records were retrieved from the database search, as follows: PubMed (n = 485), Embase (n = 381), CINAHL (n = 85), Medline (n = 13), Web of Science (n = 19), and Google Scholar (n = 200), as shown in Figure 1. The deduplication process identified and removed a total of 67 articles. The remaining 1116 records underwent title and abstract screening, and 1070 articles were excluded. A total of 46 articles were subjected to full text eligibility assessment, and 28 of these articles did not meet the eligibility criteria and were excluded. Finally, a total of eighteen studies met all of the eligibility criteria [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The main reason for rejection was utilization of imaging modalities other than TCCD such as non-imaging TCD, MRA, and CTA. Additionally, studies that reported reliability indices without assessing the accuracy measures, such as sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy, were also excluded [33]. Although Gerriets et al. [34] was included in the systematic review, it was excluded from the meta-analysis, as it did not provide adequate measures of diagnostic accuracy. Furthermore, studies that utilized power motion mode TCD (PMD TCD) were excluded [35,36]. The study by Liu et al. [37] was excluded, as no full article could be retrieved, regardless of the study reporting the diagnostic accuracy of TCCD with DSA as the reference standard.
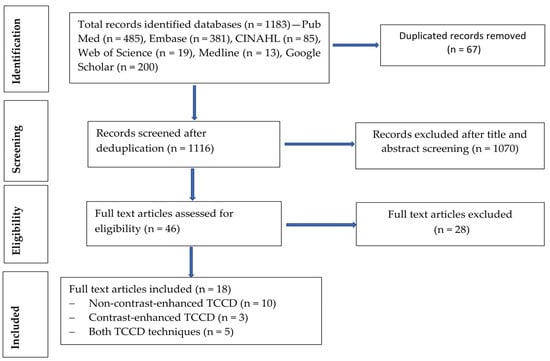
Figure 1.
Study selection process (flow chart diagram).
3.2. Study Characteristics
The eighteen studies comprised fourteen prospective studies [19,20,22,24,26,27,28,29,30,31,32,38,39,40] and four retrospective studies [21,23,25,41] (Table 1 and Table 2). The total number of patients in the included studies who underwent both index and reference tests was 3082, consisting of 2092 males (68%) and 990 females (32%). A total of 13 studies reported the trans-temporal window (TTW) failure rate, and the pooled mean TTW failure rate was 13.5% (Table 3). The period between TCCD and reference standard imaging examination of the included studies varied, ranging from as little as 12 min to as much as within 90 days (Table 2).

Table 1.
Main characteristics of patients in the included studies.

Table 2.
Main study design characteristics of the included studies. The data extracted are shown in this table: (a) study design, (b) type of ultrasound machine and contrast media, (c) TCCD technique, (d), reference method, (e) time of index to reference test.

Table 3.
Diagnostic performance indicators of transcranial color-coded Doppler ultrasound (TCCD) for the detection of steno-occlusive disease in each of the 30 individual analyses.
The included studies were observed to exhibit significant study methodological variability with respect to the (1) TCCD techniques—contrast-enhanced versus non-contrast TCCD, (2) ultrasound diagnostic parameter, (3) assessment site, (4) degree of stenosis at which accuracy was established, and (5) reference standard used (DSA, MRA, or CTA). A total of seven (39%) studies [24,26,28,29,38,39,40] used contrast-enhanced TCCD technique alone whereas nine (50%) studies [19,21,23,25,27,30,31,32,41] used solely non-contrast TCCD technique. A combination of the two techniques was utilized in two studies (11%) [20,22] (Table 3). Our study further observed variations in the ultrasound contrast agents used among the included studies, with Levovist (Schering AG, Berlin Germany) and SonoVue (Branco, Italy) being the commonly used contrast agents. No complications or serious side effects associated with the use of contrast agents were reported in the studies. Additionally, we observed that different types of ultrasound machines were used across the studies, but they all used a phased array transducer with a frequency range of 1–5 MHz for the TCCD examinations (Table 2).
With respect to the site of assessment, a majority of the studies assessed the anterior circulation with a focus on middle cerebral artery (MCA) stenoses—nine (50%) studies [22,29,30,31,32,38,39,40,41]. The other seven (39%) studies assessed both anterior and posterior circulations [19,20,21,23,24,27,28], whereas two (11%) studies focused on posterior circulation, mainly the basilar artery (BA) [25,26].
Peak systolic velocity (PSV) and mean flow velocity (MFV) represented the main TCCD ultrasound diagnostic parameters for stenosis assessment. PSV-based diagnosis of ICAS was reported in 14 out of 30 (47%) analyses of the 18 studies, and MFV in 8 (27%) (Table 3). However, the cutoff values of these two ultrasound diagnostic parameters varied across the different degrees of stenosis and sites of assessment. The PSV cut-off values for the diagnosis of greater than or equal to 50% stenosis in the MCA, excluding specific categories (70–99%), ranged from 140 to 220 cm/s with a pooled mean of 186 ± 34 cm/s, whereas the MFV ranged from 80 to 110 cm/s with a pooled mean of 95 ± 21 cm/s. Additionally, for BA stenoses greater than or equal to 50%, the PSV cut-off values ranged from 120 to 150 cm/s with a pooled mean of 137 ± 15 cm/s, and the corresponding MFV ranged from 65 to 90 cm/s with a pooled mean of 78 ± 18 cm/s. The diagnostic criteria for totally occluded MCAs were mainly absent flow signals in the observed vessel, whilst Doppler signals were observed in anterior cerebral artery (ACA) or contralateral MCA.
3.3. Studies’ Methodological Quality Assessment Based on QUADAS 2 Tool
The results on the methodological quality of the included studies based on QUADAS version 2 are shown in Table 4. There was generally low risk of bias and applicability concerns across the domains of patient selection, index test, reference standard, and flow and timing in the eligible studies.

Table 4.
QUADAS 2 methodological quality assessment results (risk of bias and applicability concerns).
3.4. Diagnostic Accuracy of TCCD Technique for the Detection of Steno-Occlusive Disease
3.4.1. (DSA, MRA, CTA) as Reference Standards
The pooled diagnostic accuracy indicators of the TCCD technique for 30 analyses utilizing either DSA, MRA, CTA, or a combination of these three imaging modalities as reference standards are shown in Figure 2a–d. The pooled sensitivity, specificity, AUC, and DOR were 83% (95% CI: 81–85%), 87% (95% CI: 86–88%), 0.96, and 98 (95% CI: 56–169), respectively.
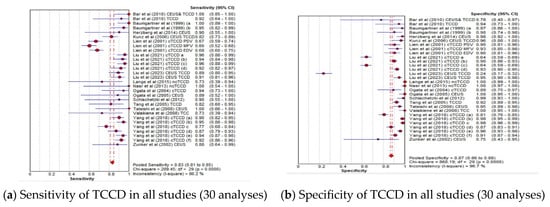
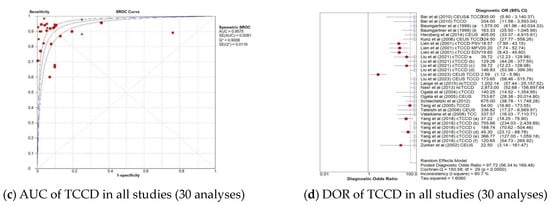
Figure 2.
Diagnostic accuracy indicators of TCCD technique when all studies (30 analyses) using either DSA, MRA, CTA, or any combination of the three imaging modalities as reference standards are considered. (a) Sensitivity of TCCD in all studies (30 analyses), (b) Specificity of TCCD in all studies (30 analyses), (c) AUC of TCCD in all studies (30 analyses), (d) DOR of TCCD in all studies (30 analyses). The position of the red circles corresponds to the diagnostic accuracy indicator value for each individual study, whilst the position of the red diamond shaped box represents the pooled diagnostic accuracy indicator value.
3.4.2. TCCD Compared to Only DSA as Reference Standard
The pooled diagnostic accuracy results for the subgroup analysis in which TCCD was compared to solely DSA as the reference standard are shown in Figure 3a–d. TCCD demonstrated good diagnostic performance and the pooled sensitivity, specificity, AUC, and DOR were 90% (95% CI: 88–91%), 86% (95% CI: 85–87%), 0.97, and 121 (95% CI: 61–169), respectively.
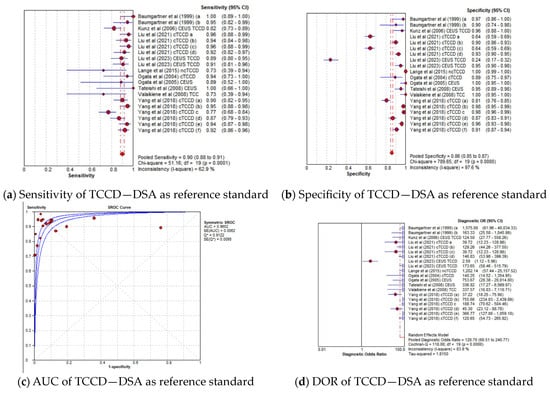
Figure 3.
Diagnostic accuracy indicators of TCCD technique when compared to only DSA as the reference standard. (a) Sensitivity, (b) Specificity, (c) AUC, (d) DOR. The position of the red circles corresponds to the diagnostic accuracy indicator value for each individual study, whilst the position of the red diamond shaped box represents the pooled diagnostic accuracy indicator value.
3.4.3. Diagnostic Accuracy of TCCD Technique According to Stenosis Categories
The pooled sensitivity, specificity, AUC, and DOR of the TCCD technique in stratifying stenoses ≥50% to near occlusion were 91% (95% CI: 89–93%), 88% (95% CI: 87–89%), 0.97, and 148 (95% CI: 84–262), respectively (Figure 4a–d). The corresponding pooled sensitivity, specificity, AUC, and DOR for diagnosing total occlusions were 92% (95% CI: 84–97%), 98% (95% CI: 96–99%), 0.98, and 148 (95% CI: 84–262), respectively (Figure 5a–d). Although the TCCD technique yielded a good and comparable diagnostic accuracy with angiographic techniques (DSA, MRA, and CTA) in stratifying both stenoses greater than or equal to 50% to near occlusion and total occlusion, the diagnosis of total occlusion by TCCD had a higher specificity in the diagnosis of total occlusion (98%) when compared to the diagnosis of stenosis ≥50% to near occlusions (88%). The observed high specificity could probably be explained by the fact that it is easier to exclude the possibility of total occlusion when color or spectral Doppler signals are observed.
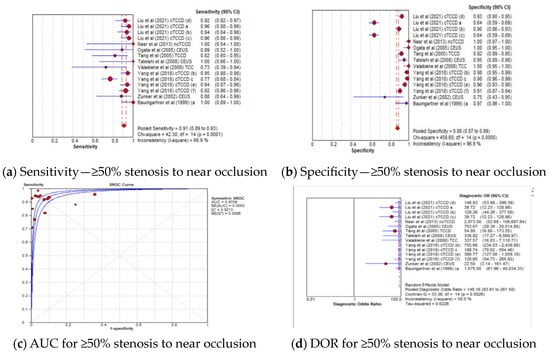
Figure 4.
Diagnostic Accuracy of TCCD Technique for stratifying ≥50% Stenosis to Near Occlusion stenosis. (a) Sensitivity—≥50% stenosis to near occlusion, (b) Specificity—≥50% stenosis to near occlusion, (c) AUC for ≥50% stenosis to near occlusion, (d) DOR for ≥50% stenosis to near occlusion. The position of the red circles corresponds to the diagnostic accuracy indicator value for each individual study, whilst the position of the red diamond shaped box represents the pooled diagnostic accuracy indicator value.
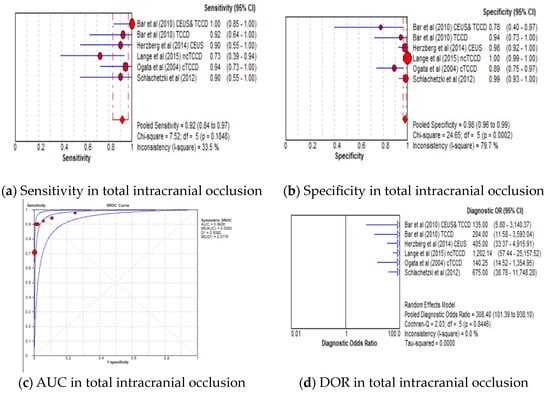
Figure 5.
Diagnostic Accuracy of TCCD Technique for the diagnosis of total intracranial occlusion. (a) Sensitivity—total intracranial occlusion, (b) Specificity—total intracranial occlusion, (c) AUC—total intracranial occlusion, (d) DOR—total intracranial occlusion. The position of the red circles corresponds to the diagnostic accuracy indicator value for each individual study, whilst the position of the red diamond shaped box represents the pooled diagnostic accuracy indicator value.
3.4.4. Diagnostic Accuracy of TCCD Technique According to Ultrasound Parameters
The pooled sensitivity, specificity, AUC, and DOR of utilizing PSV as the diagnostic parameter were 85% (95% CI: 82–87%), 85% (95% CI: 84–87%), 0.96, and 106 (95% CI: 39–288), respectively (Figure 6a–d), whereas the pooled sensitivity, specificity, AUC, and DOR of using MFV as the diagnostic parameter were 84% (95% CI: 81–87%), 87% (95% CI: 85–88%), 0.96, and 79 (95% CI: 39–157), respectively (Figure 6e–h). The cut-off values of the TCCD ultrasound parameters varied for each stenosis category and assessment site, as shown in Table 3. Since both TCCD diagnostic parameters (PSV and MFV) were observed to yield high and comparable diagnostic performance with similar AUC (96%), the two parameters were considered useful in stratifying ICAS among CVD patients.
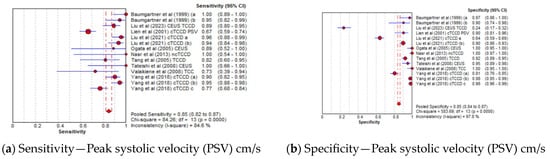
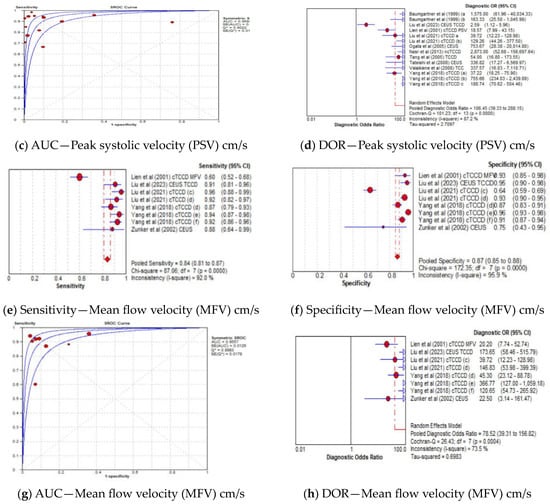
Figure 6.
Diagnostic Accuracy of TCCD technique for the two ultrasound parameters—Peak systolic velocity and Mean flow velocity. (a) Sensitivity—Peak systolic velocity (PSV) cm/s, (b) Specificity—Peak systolic velocity (PSV) cm/s, (c) AUC—Peak systolic velocity (PSV) cm/s, (d) DOR—Peak systolic velocity (PSV) cm/s, (e) Sensitivity—Mean flow velocity (MFV) cm/s, (f) Specificity—Mean flow velocity (MFV) cm/s, (g) AUC—Mean flow velocity (MFV) cm/s, (h) DOR—Mean flow velocity (MFV) cm/s. The position of the red circles corresponds to the diagnostic accuracy indicator value for each individual study, whilst the position of the red diamond shaped box represents the pooled diagnostic accuracy indicator value.
3.4.5. Diagnostic Accuracy of TCCD Based on Contrast-Enhanced and Non-Contrast TCCD
The pooled sensitivity, specificity, AUC, and DOR of utilizing non-contrast TCCD were 82% (95% CI: 80–84%), 88% (95% CI: 87–89%), 0.98, and 94 (95% CI: 56–160), respectively (Figure 7a–d), whereas the pooled sensitivity, specificity, AUC, and DOR of using contrast-enhanced TCCD were 87% (95% CI: 83–91%), 80% (95% CI: 76–83%), 0.95, and 87 (95% CI: 13–584), respectively (Figure 7e–h). The use of contrast enhancement did not improve the overall diagnostic accuracy of TCCD, although the pooled sensitivity was observed to improve (from 82% to 87%) with the use of contrast enhancement.
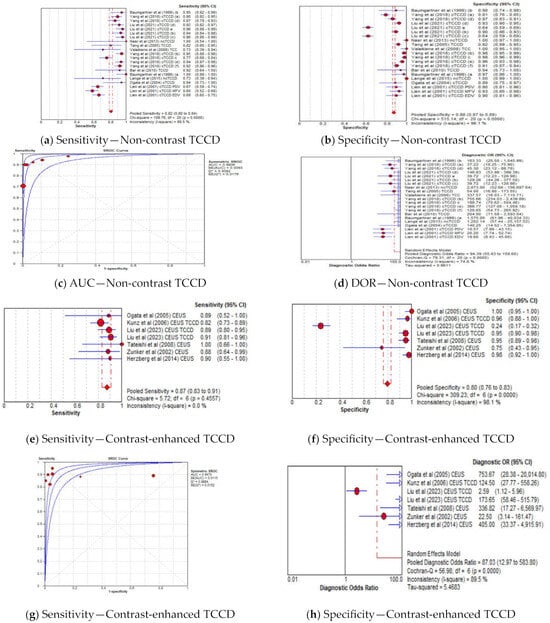
Figure 7.
Diagnostic Accuracy of TCCD for the two techniques of contrast-enhanced and non-contrast TCCD. (a) Sensitivity—Non-contrast TCCD, (b) Specificity—Non-contrast TCCD, (c) AUC—Non-contrast TCCD, (d) DOR—Non-contrast TCCD, (e) Sensitivity—Contrast-enhanced TCCD, (f) Specificity—Contrast-enhanced TCCD, (g) Sensitivity—Contrast-enhanced TCCD, (h) Specificity—Contrast-enhanced TCCD. The position of the red circles corresponds to the diagnostic accuracy indicator value for each individual study, whilst the position of the red diamond shaped box represents the pooled diagnostic accuracy indicator value.
The results showing the pooled diagnostic performance of TCCD in stratifying ICAs according to various categories are summarized in Table 5.

Table 5.
Summary of pooled diagnostic performance of TCCD in stratifying ICAs according to various categories.
4. Discussion
Although DSA still remains the gold standard for stratification of intracranial steno-occlusions, its limitations such as increased cost, invasiveness nature, and associated complications cannot be overemphasized [43,44], hence the need to investigate alternative imaging techniques. In the present study, we provided evidence pertaining to the diagnostic accuracy of the TCCD technique, which is non-invasive and inexpensive in stratifying ICASs in patients presenting with CVD.
The QUADAS assessment concluded there is high methodological quality within individual studies, as most of the studies had a low risk of bias across the four domains of patient selection, index test, reference standard, and flow and timing (Table 4). A majority of the studies (78%) adopted a prospective study design [19,20,22,24,26,27,28,29,30,31,32,38,39,40], whilst retrospective study design was used in only four studies (22%) [21,23,25,41]; this ensured a low risk of patient selection bias. Additionally, a single-center approach was adopted in all, except one study by Gerriets et al. [40], in which a multicenter approach was used, and this further reduced the possibility of bias and traps related to a multicenter study design [45]. Furthermore, a consecutive participants selection strategy was employed in all except for three studies [23,24,39], which further strengthened the methodological quality and consistency in the domain of patient selection among the included studies. We further observed a significant percentage in gender difference in the patients who underwent both the index and reference tests, (males = 68% versus females = 32%). The observed discrepancy could probably be attributed to previously reported differences in the prevalence rates of cerebrovascular disease across gender groups. Although temporal window failure was reported in previous studies to be higher in the female population compared to male counterparts, no formal analysis on temporal window status across gender was conducted in the current study due to limited information provided in the included studies.
The reference standard tests were clearly specified and described to allow for replication, as well as undertaken by teams, blinded to results of TCCD examination in all included studies. However, some studies utilized more than one reference standard for confirming the diagnosis [22,29,40]. Gerriets et al. [34] utilized three different reference standards (CTA, MRA, and DSA), whereas CTA and MRA were used in Schlachetzki et al. [22] and Herzberg et al. [29]. The use of multiple reference standards in a single study could introduce some applicability concerns with respect to the consistency in the reference index domain, as the accuracy of the three tests is reported to be different.
Notwithstanding, a low risk of bias observed within individual studies, a significant methodological variability across the studies with respect to mainly the (1) TCCD technique-with or without contrast enhancement, (2) ultrasound diagnostic parameter, (3) site assessed (4) degree of stenosis at which the accuracy was established, and (5) reference standard used (DSA, MRA, CTA) was noted. We observed a statistically significant heterogeneity within the study results when all studies were included (Figure 2a–d). The inconsistency index of ≥50% and p < 0.001 were deemed to indicate substantial between-study heterogeneity, as alluded to by Castaldo et al. [46]. This observation prompted the undertaking of various subgroup analyses. Although different subtypes of CVD such as AIS and TIA were reported in the included studies (Table 2), our study did not perform a subgroup analysis on different types of stroke, as it has been reported that there is no significant difference in the Doppler ultrasound diagnostic parameters among different types of stroke [38].
In the current meta-analysis, TCCD was observed to yield high diagnostic accuracy in the stratification of ICAS among patients with CVD, when compared to DSA as a reference standard, with a pooled sensitivity, specificity, AUC, and DOR of 90%, 86%, 0.97, and 121, respectively (Figure 3a–d). When studies utilizing DSA, MRA, CTA, or a combination of the three modalities as reference standards were considered, the pooled sensitivity decreased considerably from 90% to 83%, although the overall diagnostic accuracy between the two subgroups remained comparable (97% versus 96%, respectively). These results suggest that the accuracy of TCCD in the stratification of ICAS in patients with CVD is comparable to DSA; TCCD is non-invasive, relatively inexpensive, more acceptable to patients, and has a lower risk of post-examination complications when compared to DSA. We further found that both PSV and MFV are useful TCCD diagnostic parameters for stratifying ICASs, as they yield a comparable and high diagnostic accuracy (both AUC = 0.96) (Figure 6c,g). The diagnosis of ≥50% stenosis has clinical significance for timely treatment and intensive follow-up in our study; hence, pooled mean ultrasound parameter (PSV and MFV) cut-off values for ≥50% stenosis are reported to inform clinical decision making.
Although contrast-enhanced TCCD increased the visualization of intracranial vessels and had higher sensitivity compared to non-contrast TCCD (87% versus 82%, respectively) [22,24,39,40], our meta-analysis demonstrated that contrast enhancement did not result in improved overall diagnostic accuracy in stratifying ICASs (AUC = 0.95 versus 0.98, respectively).
There are limitations in this study. There were only a few studies that met the inclusion criteria, and this could restrict the generalizability of the study results. Notwithstanding the contributions of ICAS to ischemic stroke, recent evidence is pointing towards vulnerable atherosclerotic plaque rupture as a main mechanism of ischemic stroke [14,47]. There is need for future studies to interrogate the diagnostic accuracy of novel imaging techniques that may allow for the early and accurate characterization of plaque morphology, to which our current study was limited.
5. Conclusions
In conclusion, this systematic review and meta-analysis provide evidence that the TCCD imaging technique exhibits high diagnostic performance in the stratification of intracranial steno-occlusions among patients presenting with CVD, when compared to DSA as a reference standard. TCCD has potential to be used in stratifying ICASs in CVD patients, and could be considered in clinical cases where DSA is limited or contraindicated to patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13051507/s1, Table S1: Database search strings.
Author Contributions
Conceptualization, S.T.G. and M.T.-C.Y.; methodology, S.T.G., V.T.-K.N. and M.T.-C.Y.; data extraction, S.T.G., V.T.-K.N. and M.T.-C.Y.; validation, S.T.G., Z.C., X.H., X.C., M.Y.-C.P. and M.T.-C.Y.; data curation, S.T.G., V.T.-K.N. and J.H.-Y.Y.; formal analysis, S.T.G., J.H.-Y.Y., Z.C., X.H., X.C., M.Y.-C.P. and M.T.-C.Y.; writing—original draft, S.T.G.; writing—review and editing, S.T.G., Z.C., X.C., M.Y.-C.P. and M.T.-C.Y.; visualization, S.T.G., J.H.-Y.Y., V.T.-K.N., Z.C., X.H., X.C., M.Y.-C.P. and M.T.-C.Y.; supervision, X.C., M.Y.-C.P. and M.T.-C.Y.; project administration, S.T.G. and M.T.-C.Y.; funding acquisition, M.T.-C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by a research studentship grant (R006) and a research project grant (Project ID: P0035203) of The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong SAR, China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets of this study are presented in the article/Supplementary Materials. Any further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Díez-Tejedor, E.; del Brutto, O.H.; Álvarez-Sabín, J.; Muñoz-Collazos, M.; Abiusi, G.R.P. Clasificación de las enfermedades cerebrovasculares. Sociedad Iberoamericana de ECV. Rev. Neurol. 2001, 33, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Luo, H.; Zhou, J.; Yu, M.; Chen, X.; Tan, L.; Wei, W.; Li, J. Prevalence of stroke and stroke related risk factors: A population based cross sectional survey in southwestern China. BMC Neurol. 2020, 20, 5. [Google Scholar] [CrossRef]
- Esposito, L.; Sievers, M.; Sander, D.; Heider, P.; Wolf, O.; Greil, O.; Zimmer, C.; Poppert, H. Detection of unstable carotid artery stenosis using MRI. J. Neurol. 2007, 254, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Huston, J.; Rabinstein, A.A.; Kim, G.-M.; Lerman, A.; Lanzino, G. Contemporary carotid imaging: From degree of stenosis to plaque vulnerability. J. Neurosurg. 2016, 124, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.A.; Connolly, E.S. Indications for Carotid Endarterectomy in Patients with Asymptomatic and Symptomatic Carotid Stenosis. In Stroke, 7th ed.; Grotta, J.C., Albers, G.W., Broderick, J.P., Kasner, S.E., Lo, E.H., Sacco, R.L., Wong, L.K., Day, A.L., Eds.; Elsevier: Philadelphia, PA, USA, 2022; pp. 1084–1090.e2. [Google Scholar]
- Nicolaides, A.N.; Kakkos, S.K.; Kyriacou, E.; Griffin, M.; Sabetai, M.; Thomas, D.J.; Tegos, T.; Geroulakos, G.; Labropoulos, N.; Doré, C.J.; et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J. Vasc. Surg. 2010, 52, 1486–1496.e5. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, M.L.; Kissela, B.; Khoury, J.C.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Ferioli, S.; Adeoye, O.; Broderick, J.P.; et al. Carotid Artery Stenosis as a Cause of Stroke. Neuroepidemiology 2013, 40, 36–41. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Liu, L.; Soo, Y.O.; Pu, Y.; Pan, Y.; Wang, Y.; Zou, X.; Leung, T.W.; Cai, Y.; et al. Prevalence and Outcomes of Symptomatic Intracranial Large Artery Stenoses and Occlusions in China. Stroke 2014, 45, 663–669. [Google Scholar] [CrossRef]
- Nguyen-Huynh, M.N.; Wintermark, M.; English, J.; Lam, J.; Vittinghoff, E.; Smith, W.S.; Johnston, S.C. How Accurate Is CT Angiography in Evaluating Intracranial Atherosclerotic Disease? Stroke 2008, 39, 1184–1188. [Google Scholar] [CrossRef]
- Mattioni, A.; Brazzelli, M.; Cenciarelli, S.; Mazzoli, T.; Del Sette, M.; Gandolfo, C.; Marinoni, M.; Finocchi, C.; Saia, V.; Eusebi, P.; et al. Transcranial Doppler sonography for detecting stenosis or occlusion of intracranial arteries in people with acute ischaemic stroke. Cochrane Database Syst. Rev. 2020, 2, Cd010722. [Google Scholar] [CrossRef]
- Naylor, A.R.; Ricco, J.B.; De Borst, G.J.; Debus, S.; De Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Wong, J.C. A novel method to quantify carotid artery stenosis by Doppler ultrasound: Using the continuity principle. Int. J. Angiol. 2010, 19, e86–e90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saba, L.; Yuan, C.; Hatsukami, T.; Balu, N.; Qiao, Y.; DeMarco, J.; Saam, T.; Moody, A.; Li, D.; Matouk, C.; et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. Am. J. Neuroradiol. 2018, 39, E9–E31. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.E.; O’Brien, N.F. Transcranial Doppler Ultrasound, a Review for the Pediatric Intensivist. Children 2022, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E. Transcranial color-coded duplex ultrasonography in routine cerebrovascular diagnostics. Perspect. Med. 2012, 1, 325–330. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.R.; Bashmail, F.T.; Alzahrani, N.A.; Alharbi, S.I.; Anbar, R.; Alkharaiji, M. Contrast-enhanced ultrasound for the evaluation of symptomatic and asymptomatic carotid plaques: A systematic review and meta-analysis. Echocardiography 2022, 39, 1032–1043. [Google Scholar] [CrossRef]
- Baumgartner, R.W.; Mattle, H.P.; Schroth, G. Assessment of ≥50% and <50% Intracranial Stenoses by Transcranial Color-Coded Duplex Sonography. Stroke 1999, 30, 87–92. [Google Scholar]
- Bar, M.; Školoudík, D.; Roubec, M.; Hradílek, P.; Chmelová, J.; Czerný, D.; Procházka, V.; Langová, K.; Herzig, R. Transcranial duplex sonography and CT angiography in acute stroke patients. J. Neuroimaging 2010, 20, 240–245. [Google Scholar] [CrossRef]
- Nasr, N.; Ssi-Yan-Kai, G.; Guidolin, B.; Bonneville, F.; Larrue, V. Transcranial color-coded sonography to predict recurrent transient ischaemic attack/stroke. Eur. J. Neurol. 2013, 20, 1212–1217. [Google Scholar] [CrossRef]
- Schlachetzki, F.; Herzberg, M.; Hölscher, T.; Ertl, M.; Zimmermann, M.; Ittner, K.P.; Pels, H.; Bogdahn, U.; Boy, S. Transcranial ultrasound from diagnosis to early stroke treatment—Part 2: Prehospital neurosonography in patients with acute stroke—The regensburg stroke mobile project. Cerebrovasc. Dis. 2012, 33, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.C.; Bruch, T.P.; Pedrozo, J.C.; Maranha, L.; Sakae, T.M.; Pacheco, R.; Souza, P.C.; Zétola, V.F. The use of neurovascular ultrasound versus digital subtraction angiography in acute ischemic stroke. Arq. Neuro-Psiquiatr. 2015, 73, 218–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zunker, P.; Wilms, H.; Brossmann, J.; Georgiadis, D.; Weber, S.; Deuschl, G. Echo contrast-enhanced transcranial ultrasound: Frequency of use, diagnostic benefit, and validity of results compared with MRA. Stroke 2002, 33, 2600–2603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Hua, Y.; Li, X.; Gao, M.; Li, Q.; Liu, B.; Jiao, L. The Assessment of Diagnostic Accuracy for Basilar Artery Stenosis by Transcranial Color-Coded Sonography. Ultrasound Med. Biol. 2018, 44, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, Y.; Iguchi, Y.; Kimura, K.; Inoue, T.; Shibazaki, K.; Eguchi, K. Contrast-enhanced transcranial color-coded duplex sonography criteria for basilar artery stenosis. J. Neuroimaging 2008, 18, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Valaikiene, J.; Schuierer, G.; Ziemus, B.; Dietrich, J.; Bogdahn, U.; Schlachetzki, F. Transcranial color-coded duplex sonography for detection of distal internal carotid artery stenosis. Am. J. Neuroradiol. 2008, 29, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Hahn, G.; Mucha, D.; Müller, A.; Barrett, K.M.; Von Kummer, R.; Gahn, G. Echo-enhanced transcranial color-coded duplex sonography in the diagnosis of cerebrovascular events: A validation study. Am. J. Neuroradiol. 2006, 27, 2122–2127. [Google Scholar]
- Herzberg, M.; Boy, S.; Hölscher, T.; Ertl, M.; Zimmermann, M.; Ittner, K.P.; Pemmerl, J.; Pels, H.; Bogdahn, U.; Schlachetzki, F. Prehospital stroke diagnostics based on neurological examination and transcranial ultrasound. Crit. Ultrasound J. 2014, 6, 3. [Google Scholar] [CrossRef]
- Tang, S.C.; Jeng, J.S.; Yip, P.K.; Lu, C.J.; Hwang, B.S.; Lin, W.H.; Liu, H.M. Transcranial color-coded sonography for the detection of middle cerebral artery stenosis. J. Ultrasound Med. 2005, 24, 451–457. [Google Scholar] [CrossRef]
- Ogata, T.; Kimura, K.; Nakajima, M.; Ikeno, K.; Naritomi, H.; Minematsu, K. Transcranial color-coded real-time sonographic criteria for occlusion of the middle cerebral artery in acute ischemic stroke. Am. J. Neuroradiol. 2004, 25, 1680–1684. [Google Scholar]
- Lien, L.M.; Chen, W.H.; Chen, J.R.; Chiu, H.C.; Tsai, Y.F.; Choi, W.M.; Reynolds, P.S.; Tegeler, C.H. Comparison of transcranial color-coded sonography and magnetic resonance angiography in acute ischemic stroke. J. Neuroimaging 2001, 11, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Klötzsch, C.; Popescu, O.; Sliwka, U.; Mull, M.; Noth, J. Detection of stenoses in the anterior circulation using frequency-based transcranial color-coded sonography. Ultrasound Med. Biol. 2000, 26, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, T.; Seidel, G.; Fiss, I.; Modrau, B.; Kaps, M. Contrast-enhanced transcranial color-coded duplex sonography: Efficiency and validity. Neurology 1999, 52, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Brunser, A.M.; Lavados, P.M.; Hoppe, A.; Lopez, J.; Valenzuela, M.; Rivas, R. Accuracy of transcranial doppler compared with CT angiography in diagnosing arterial obstructions in acute ischemic strokes. Stroke 2009, 40, 2037–2041. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Sharma, V.K.; Hoover, S.L.; Lao, A.Y.; Ardelt, A.A.; Malkoff, M.D.; Alexandrov, A.V. Applications and advantages of power motion-mode Doppler in acute posterior circulation cerebral ischemia. Stroke 2008, 39, 1197–1204. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, B.-Z.; Mu, Y.; Jiang, D. Comparative study on transcranial color-duplex sonography and contrast-enhanced ultrasound with DSA in diagnosis of cerebral arterial stenosis. Chin. J. Med. Imaging Technol. 2006, 22, 880–882. [Google Scholar]
- Ogata, T.; Kimura, K.; Nakajima, M.; Naritomi, H.; Minematsu, K. Diagnosis of middle cerebral artery occlusive lesions with contrast-enhanced transcranial color-coded real-time sonography in acute stroke. Neuroradiology 2005, 47, 256–262. [Google Scholar] [CrossRef]
- Liu, S.; Huang, Z.L.; Sun, Y.R.; Liu, L.; Qi, H.; Wei, L.Y. Application value of transcranial contrast-enhanced ultrasonography in evaluating middle cerebral artery stenosis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 224–232. [Google Scholar]
- Gerriets, T.; Goertler, M.; Stolz, E.; Postert, T.; Sliwka, U.; Schlachetzki, F.; Seidel, G.; Weber, S.; Kaps, M. Feasibility and validity of transcranial duplex sonography in patients with acute stroke. J. Neurol. Neurosurg. Psychiatry 2002, 73, 17–20. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Hua, Y.; Yang, J.; Zhao, Y.; Tian, X.; Ma, Y.; Zhao, W. Transcranial Color-Coded Sonography Criteria for Moderate and Severe Middle Cerebral Artery Stenosis. Ultrasound Med. Biol. 2021, 47, 25–32. [Google Scholar] [CrossRef]
- Baratloo, A.; Hosseini, M.; Negida, A.; El Ashal, G. Part 1: Simple Definition and Calculation of Accuracy, Sensitivity and Specificity. Emergency 2015, 3, 48–49. [Google Scholar] [PubMed]
- Samuels, O.B.; Joseph, G.J.; Lynn, M.J.; Smith, H.A.; Chimowitz, M.I. A standardized method for measuring intracranial arterial stenosis. AJNR Am. J. Neuroradiol. 2000, 21, 643–646. [Google Scholar] [PubMed]
- Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trail Investigators. Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA): Design of a Prospective, Multicenter Trial of Diagnostic Tests. Neuroepidemiology 2004, 23, 23–32. [Google Scholar] [CrossRef]
- Jo, D. The interpretation bias and trap of multicenter clinical research. Korean J. Pain 2020, 33, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, R.; Cavaliere, C.; Soricelli, A.; Salvatore, M.; Pecchia, L.; Franzese, M. Radiomic and Genomic Machine Learning Method Performance for Prostate Cancer Diagnosis: Systematic Literature Review. J. Med. Internet Res. 2021, 23, e22394. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.; Jost, A. Carotid stenosis, stroke, and carotid artery revascularization. Prog. Cardiovasc. Dis. 2021, 65, 49–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).