Effect of Denosumab on Bone Health, Vascular Calcification, and Health-Related Quality of Life in Hemodialysis Patients with Osteoporosis: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Fracture Risk Assessment
2.4. Clinical and Laboratory Parameters
2.5. Determination of Serum Calcification Propensity (T50)
2.6. Vascular Calcification Score
2.7. Health-Related Quality of Life
2.8. Definition of Outcomes
2.9. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
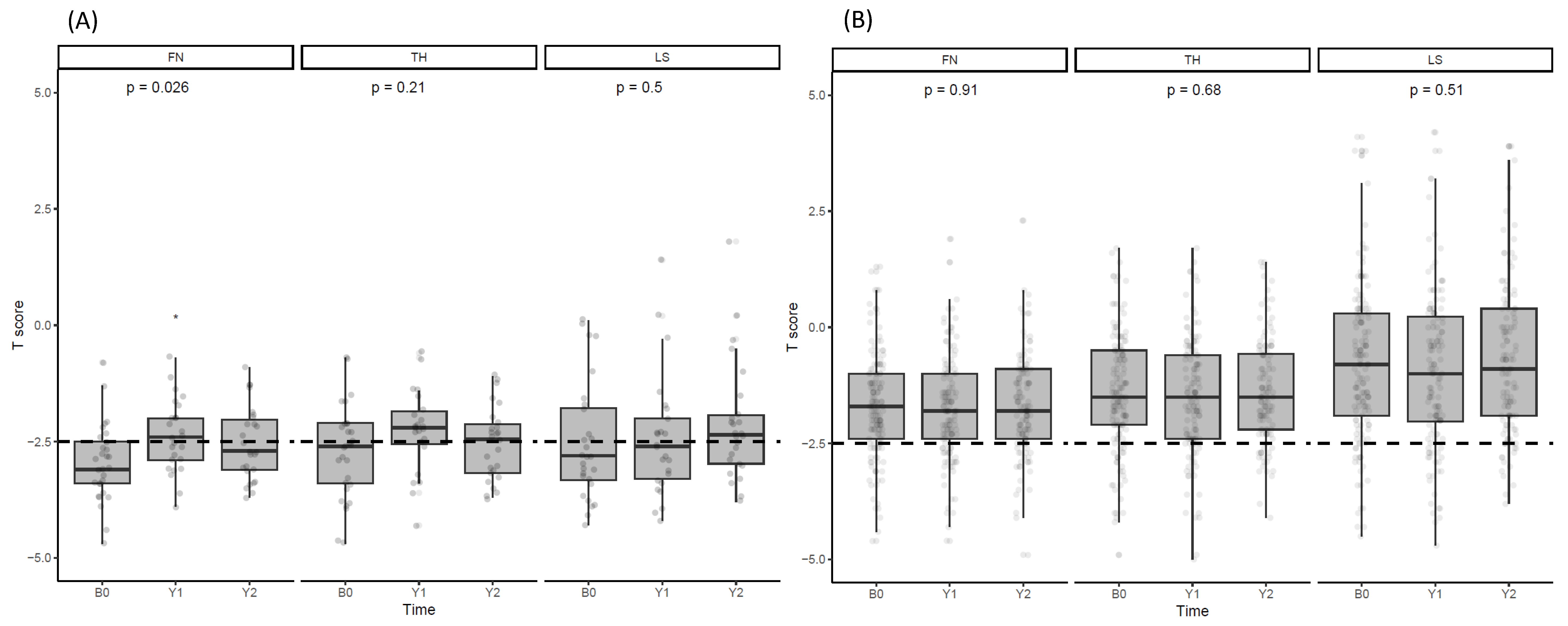
3.2. Changes in BMD after Denosumab Administration
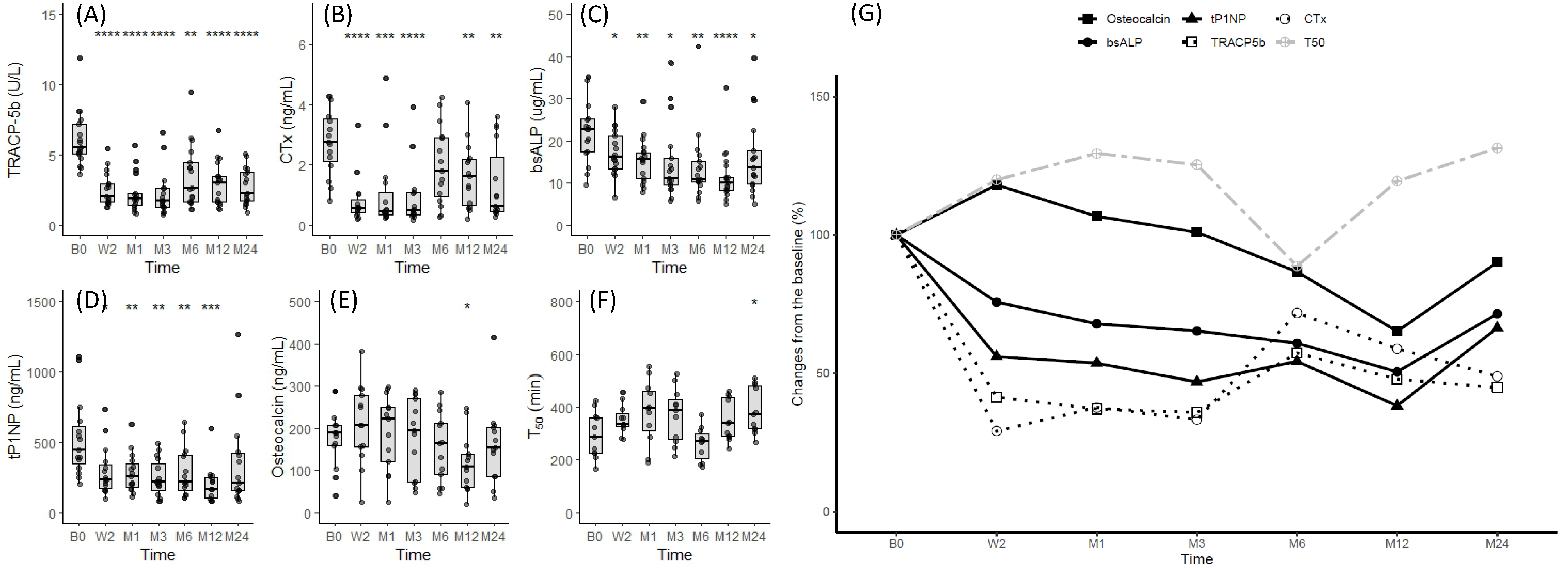
3.3. Changes in Bone Turnover Markers after Denosumab Administration
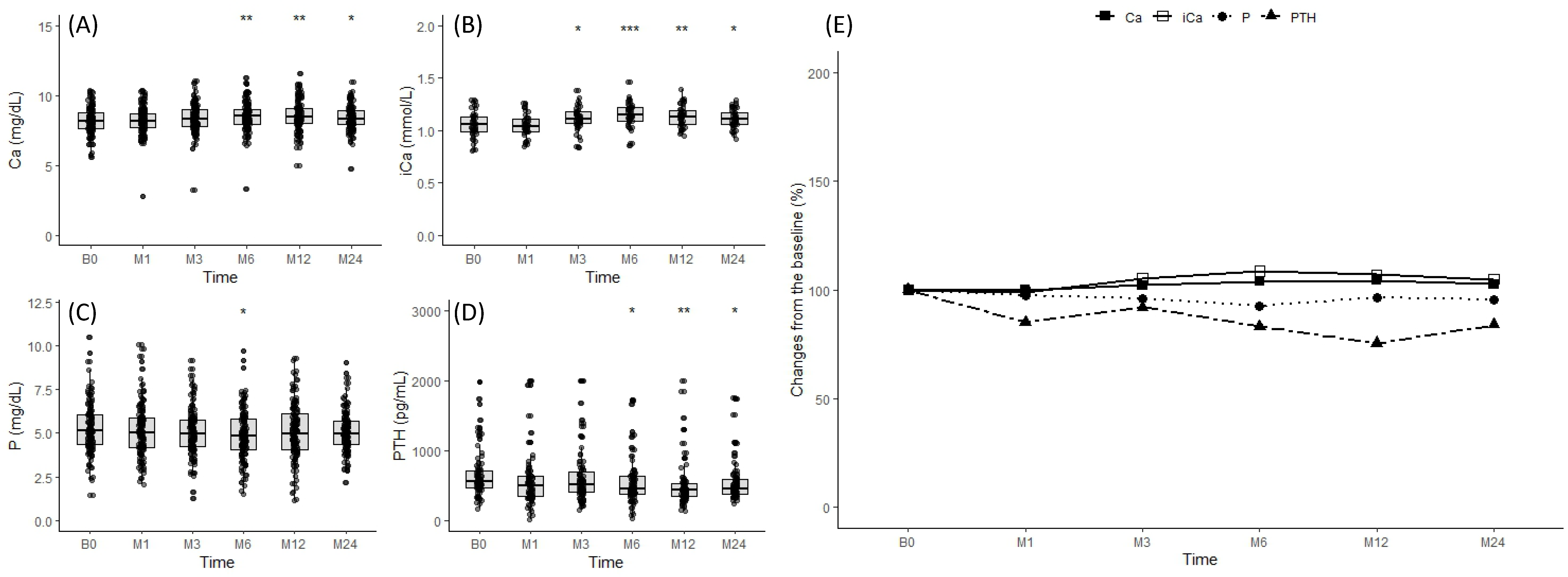
3.4. Changes in Mineral Parameters after Denosumab Administration
3.5. Relationship between T50, Mineral Parameters, and Bone Turnover Markers after Denosumab Administration
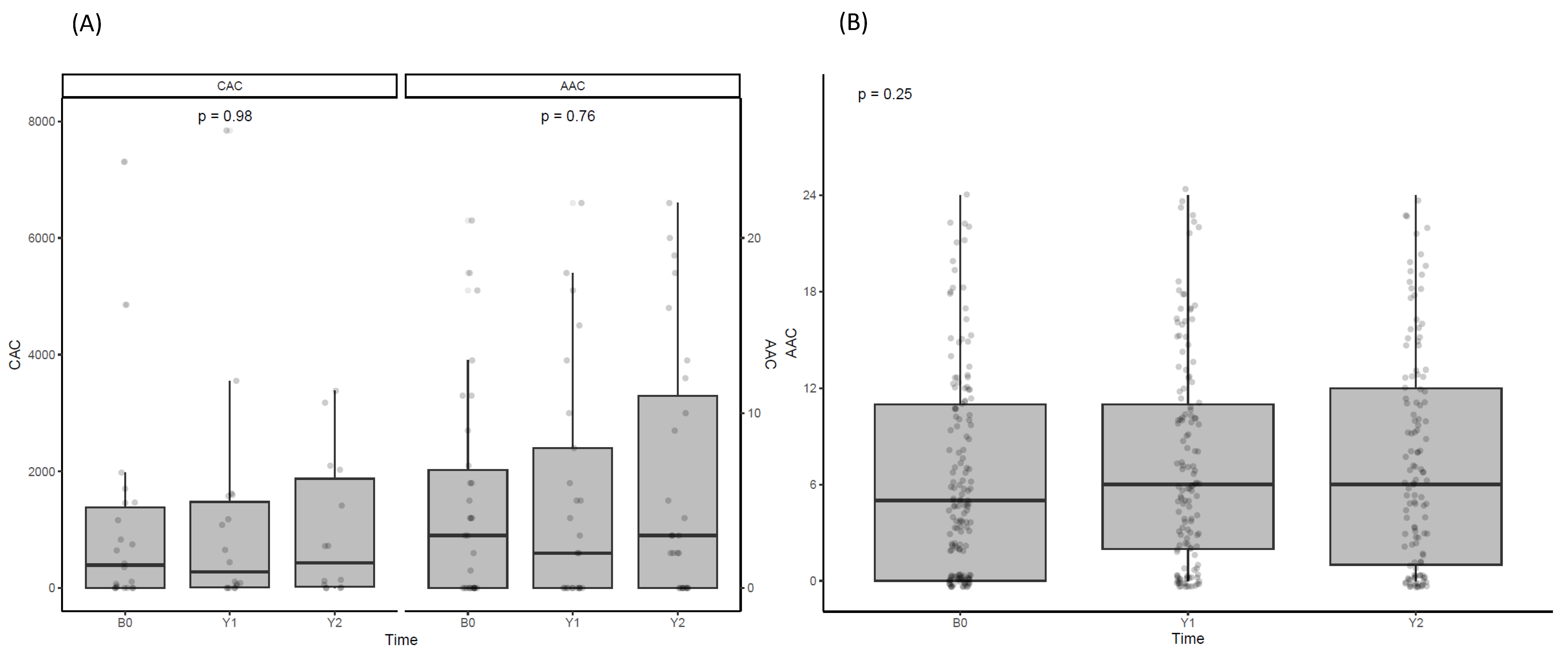
3.6. Effect of Denosumab on Vascular Calcification
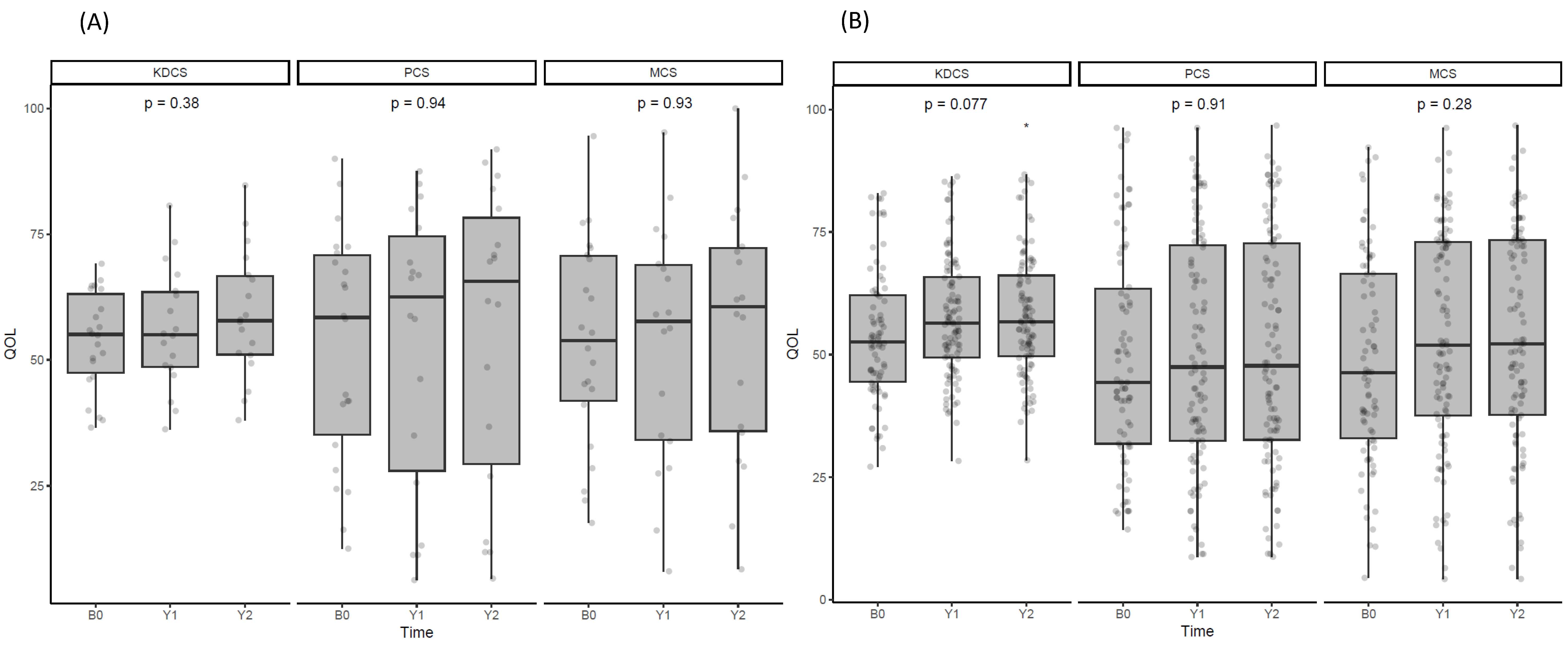
3.7. Effect of Denosumab on Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO 2017 Clinical Practice Guideline update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Khairallah, P.; Nickolas, T.L. Management of Osteoporosis in CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 962–969. [Google Scholar] [CrossRef]
- Festuccia, F.; Jafari, M.T.; Moioli, A.; Fofi, C.; Barberi, S.; Amendola, S.; Sciacchitano, S.; Punzo, G.; Mene, P. Safety and efficacy of denosumab in osteoporotic hemodialysed patients. J. Nephrol. 2017, 30, 271–279. [Google Scholar] [CrossRef]
- Kunizawa, K.; Hiramatsu, R.; Hoshino, J.; Mizuno, H.; Ozawa, Y.; Sekine, A.; Kawada, M.; Sumida, K.; Hasegawa, E.; Yamanouchi, M.; et al. Denosumab for dialysis patients with osteoporosis: A cohort study. Sci. Rep. 2020, 10, 2496. [Google Scholar] [CrossRef]
- Jamal, S.A.; Ljunggren, O.; Stehman-Breen, C.; Cummings, S.R.; McClung, M.R.; Goemaere, S.; Ebeling, P.R.; Franek, E.; Yang, Y.C.; Egbuna, O.I.; et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J. Bone Miner. Res. 2011, 26, 1829–1835. [Google Scholar] [CrossRef]
- Bover, J.; Bailone, L.; Lopez-Baez, V.; Benito, S.; Ciceri, P.; Galassi, A.; Cozzolino, M. Osteoporosis, bone mineral density and CKD-MBD: Treatment considerations. J. Nephrol. 2017, 30, 677–687. [Google Scholar] [CrossRef]
- Ahn, S.H.; Park, S.M.; Park, S.Y.; Yoo, J.I.; Jung, H.S.; Nho, J.H.; Kim, S.H.; Lee, Y.K.; Ha, Y.C.; Jang, S.; et al. Osteoporosis and Osteoporotic Fracture Fact Sheet in Korea. J. Bone Metab. 2020, 27, 281–290. [Google Scholar] [CrossRef]
- McCormick, B.B.; Davis, J.; Burns, K.D. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am. J. Kidney Dis. 2012, 60, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Bone, H.G.; Fang, L.; Lee, E.; Padhi, D. A single-dose study of denosumab in patients with various degrees of renal impairment. J. Bone Miner. Res. 2012, 27, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.B.; Little, A.J.; Williams, R.B.; Milner, J.R. Interpretation of serum calcium in patients with abnormal serum proteins. Br. Med. J. 1973, 4, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Kim, H.; Kim, A.J.; Ro, H.; Chang, J.H.; Lee, H.H.; Chung, W.; Jung, J.Y. Serum calcification propensity and its association with biochemical parameters and bone mineral density in hemodialysis patients. Kidney Res. Clin. Pract. 2023, 42, 262–271. [Google Scholar] [CrossRef]
- Kauppila, L.I.; Polak, J.F.; Cupples, L.A.; Hannan, M.T.; Kiel, D.P.; Wilson, P.W. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 1997, 132, 245–250. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.; Yong, J.S.; Han, S.S.; Yang, D.H.; Meguro, M.; Han, C.W.; Kohzuki, M. Reliability and validity of the Korean version of Kidney Disease Quality of Life instrument (KDQOL-SF). Tohoku J. Exp. Med. 2007, 211, 321–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Connelly, K.; Collister, D.; Tangri, N. Fracture risk and treatment in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2018, 27, 221–225. [Google Scholar] [CrossRef]
- Slouma, M.; Sahli, H.; Bahlous, A.; Laadhar, L.; Smaoui, W.; Rekik, S.; Gharsallah, I.; Sallami, M.; Moussa, F.B.; Elleuch, M.; et al. Mineral bone disorder and osteoporosis in hemodialysis patients. Adv. Rheumatol. 2020, 60, 15. [Google Scholar] [CrossRef]
- Jiang, X.; Gruner, M.; Trémollieres, F.; Pluskiewicz, W.; Sornay-Rendu, E.; Adamczyk, P.; Schnatz, P.F. Diagnostic accuracy of FRAX in predicting the 10-year risk of osteoporotic fractures using the USA treatment thresholds: A systematic review and meta-analysis. Bone 2017, 99, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Przedlacki, J.; Buczyńska-Chyl, J.; Koźmiński, P.; Niemczyk, E.; Wojtaszek, E.; Gieglis, E.; Żebrowski, P.; Podgórzak, A.; Wściślak, J.; Wieliczko, M.; et al. The utility of FRAX® in predicting bone fractures in patients with chronic kidney disease on hemodialysis: A two-year prospective multicenter cohort study. Osteoporos. Int. 2018, 29, 1105–1115. [Google Scholar] [CrossRef]
- Wilson, L.M.; Rebholz, C.M.; Jirru, E.; Liu, M.C.; Zhang, A.; Gayleard, J.; Chu, Y.; Robinson, K.A. Benefits and Harms of Osteoporosis Medications in Patients with Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2017, 166, 649–658. [Google Scholar] [CrossRef]
- Bezerra de Carvalho, K.S.; Vasco, R.F.V.; Custodio, M.R.; Jorgetti, V.; Moysés, R.M.A.; Elias, R.M. Chronic kidney disease is associated with low BMD at the hip but not at the spine. Osteoporos. Int. 2019, 30, 1015–1023. [Google Scholar] [CrossRef]
- Iimori, S.; Mori, Y.; Akita, W.; Kuyama, T.; Takada, S.; Asai, T.; Kuwahara, M.; Sasaki, S.; Tsukamoto, Y. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—A single-center cohort study. Nephrol. Dial. Transplant. 2012, 27, 345–351. [Google Scholar] [CrossRef]
- Iseri, K.; Mizobuchi, M.; Winzenrieth, R.; Humbert, L.; Saitou, T.; Kato, T.; Nakajima, Y.; Wakasa, M.; Shishido, K.; Honda, H. Long-Term Effect of Denosumab on Bone Disease in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2023, 18, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, R.; Ubara, Y.; Sawa, N.; Hoshino, J.; Hasegawa, E.; Kawada, M.; Imafuku, A.; Sumida, K.; Mise, K.; Hayami, N.; et al. Denosumab for low bone mass in hemodialysis patients: A noncontrolled trial. Am. J. Kidney Dis. 2015, 66, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Watanabe, M.; Yoshikawa, H.; Mitsui, H.; Endo, T.; Yamamoto, Y.; Iyoda, M.; Ryu, K.; Inaba, T.; Shibata, T. Effects of Denosumab and Alendronate on Bone Health and Vascular Function in Hemodialysis Patients: A Randomized, Controlled Trial. J. Bone Miner. Res. 2019, 34, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A.; Farese, S.; Gräber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef]
- Jang, S.M.; Anam, S.; Pringle, T.; Lahren, P.; Infante, S. Contrasting PTH Response of Denosumab Use in Dialysis Patients: A Report of 2 Cases. Pharmacy 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Makras, P.; Polyzos, S.A.; Papatheodorou, A.; Kokkoris, P.; Chatzifotiadis, D.; Anastasilakis, A.D. Parathyroid hormone changes following denosumab treatment in postmenopausal osteoporosis. Clin. Endocrinol. 2013, 79, 499–503. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, N.C.; Wu, F.Z.; Wu, M.T. Impact of denosumab on cardiovascular calcification in patients with secondary hyperparathyroidism undergoing dialysis: A pilot study. Osteoporos. Int. 2020, 31, 1507–1516. [Google Scholar] [CrossRef]
- Aleksova, J.; Kurniawan, S.; Vucak-Dzumhur, M.; Kerr, P.; Ebeling, P.R.; Milat, F.; Elder, G.J. Aortic vascular calcification is inversely associated with the trabecular bone score in patients receiving dialysis. Bone 2018, 113, 118–123. [Google Scholar] [CrossRef]
- Hung, K.C.; Chang, J.F.; Hsu, Y.H.; Hsieh, C.Y.; Wu, M.S.; Wu, M.Y.; Chiu, I.J.; Syu, R.S.; Wang, T.M.; Wu, C.C.; et al. Therapeutic Effect of Calcimimetics on Osteoclast-Osteoblast Crosslink in Chronic Kidney Disease and Mineral Bone Disease. Int. J. Mol. Sci. 2020, 21, 8712. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Fukuda, K.; Maeda, T.; Chinzei, N.; Kihara, S.; Miura, Y.; Sakai, Y.; Hashimoto, S.; Matsumoto, T.; Takayama, K.; et al. Denosumab Treatment Improved Health-Related Quality of Life in Osteoporosis: A Prospective Cohort Study. JBMR Plus 2019, 3, e10191. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 185) | Control (n = 155) | Denosumab (n = 30) | p-Value | |
|---|---|---|---|---|
| Age, yr | 61.0 ± 12.3 | 60.9 ± 12.1 | 62.1 ± 13.3 | 0.621 |
| Male, n (%) | 97 (52.4) | 90 (58.1) | 7 (23.3) | 0.001 |
| HD duration (month) | 108 (64–139) | 106 (64–136) | 119 (71–143) | 0.379 |
| BMI, kg/cm2 | 23.6 ± 3.8 | 23.8 ± 3.9 | 22.3 ± 3.3 | 0.046 |
| Smoking, n (%) | 29 (15.6) | 28 (18.0) | 1 (3.3) | 0.109 |
| DM, n (%) | 87 (47.0) | 75 (48.4) | 12 (40.0) | 0.520 |
| HTN, n (%) | 108 (58.4) | 91 (58.7) | 17 (56.7) | 0.996 |
| CVD, n (%) | 76 (41.1) | 64 (41.3) | 12 (40.0) | 1.000 |
| spKt/V | 1.6 [1.4–1.8] | 1.6 [1.4–1.8] | 1.7 [1.5–2.0] | 0.004 |
| RAS blockade, n (%) | 79 (42.7) | 69 (44.5) | 10 (33.3) | 0.351 |
| CCB, n (%) | 81 (43.8) | 70 (45.2) | 11 (36.7) | 0.511 |
| β-blocker, n (%) | 80 (43.2) | 71 (45.8) | 9 (30.0) | 0.162 |
| P binder | 132 (71.4) | 110 (71.0) | 22 (73.3) | 0.967 |
| Statin | 71 (38.4) | 61 (39.4) | 10 (33.3) | 0.678 |
| Vitamin D analogues | 123 (66.5) | 101 (65.2) | 22 (73.3) | 0.511 |
| Cinacalcet | 17 (9.1) | 14 (9.0) | 3 (10.0) | 0.873 |
| Hb, g/dL | 10.8 ± 1.3 | 10.9 ± 1.3 | 10.6 ± 1.1 | 0.221 |
| Alb, g/dL | 4.0 ± 0.3 | 4.0 ± 0.4 | 4.0 ± 0.3 | 0.696 |
| Cholesterol, mg/dL | 136.7 ± 34.8 | 136.2 ± 36.1 | 139.4 ± 27.6 | 0.645 |
| TG, mg/dL | 102.1 ± 72.6 | 104.2 ± 75.0 | 91.1 ± 58.1 | 0.367 |
| hsCRP, mg/dL | 0.2 [0.1–0.4] | 0.2 [0.1–0.4] | 0.1 [0.0–0.3] | 0.447 |
| Ca, mg/dL | 8.2 ± 0.9 | 8.2 ± 0.9 | 8.5 ± 0.9 | 0.123 |
| P, mg/dL | 5.3 ± 1.4 | 5.2 ± 1.4 | 5.5 ± 1.3 | 0.408 |
| VD25, ng/mL | 17.2 ± 9.8 | 17.5 ± 9.2 | 15.8 ± 12.3 | 0.490 |
| VD1,25, pg/mL | 5.9 ± 7.1 | 6.0 ± 7.2 | 5.6 ± 6.8 | 0.789 |
| PTH, pg/mL | 564.8 ± 380.0 | 560.4 ± 379.3 | 587.5 ± 388.9 | 0.721 |
| ALP, U/L | 108.3 ± 64.5 | 108.2 ± 66.9 | 109.3 ± 50.9 | 0.927 |
| T50, min | 296.3 ± 85.3 | 298.6 ± 86.5 | 284.8 ± 79.3 | 0.419 |
| BMD_LS, T score | –1.1 ± 1.7 | –0.8 ± 1.7 | –2.6 ± 1.3 | <0.001 |
| BMD_FN, T score | –1.9 ± 1.2 | –1.7 ± 1.1 | –2.9 ± 0.9 | <0.001 |
| BMD_TH, T score | –1.6 ± 1.3 | –1.4 ± 1.3 | –2.7 ± 1.0 | <0.001 |
| FRAX_MOF | 8.5 ± 6.3 | 7.6 ± 5.2 | 12.7 ± 9.0 | 0.006 |
| FRAX_HF | 3.8 ± 4.6 | 3.1± 3.4 | 6.9 ± 7.3 | 0.014 |
| Previous FX_Hx | 23 (12.4) | 17 (11.0) | 6 (20.0) | 0.285 |
| FX_Event | 7 (3.8) | 5 (3.3) | 2 (6.7) | 0.703 |
| AAC | 5.0 [0.0–11.0] | 5.0 [0.0–11.0] | 3.0 [0.0–9.0] | 0.248 |
| KDCS | 53.7 ± 12.6 | 53.9 ± 13.3 | 52.8 ± 9.5 | 0.725 |
| PCD | 49.8 ± 22.3 | 49.4 ± 51.2 | 51.2 ± 23.6 | 0.753 |
| MCS | 49.4 ± 21.0 | 48.7 ± 21.0 | 52.2 ± 21.2 | 0.507 |
| Age | spKtV | Alb | hsCRP a | Ca | P | PTH | ALP | BMD_LS | BMD_FN | BMD_TH | AAC | T50 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.000 | ||||||||||||
| spKtV | 0.196 * | 1.000 | |||||||||||
| Alb | –0.229 * | 0.001 | 1.000 | ||||||||||
| hsCRP a | 0.043 | –0.121 | –0.159 | 1.000 | |||||||||
| Ca | 0.002 | –0.010 | 0.291 * | –0.126 | 1.000 | ||||||||
| P | –0.340 * | –0.206 * | 0.243 * | 0.085 | 0.058 | 1.000 | |||||||
| PTH | –0.218 * | –0.196 * | 0.136 | –0.047 | 0.161 | 0.321 * | 1.000 | ||||||
| ALP | 0.044 | –0.051 | 0.078 | 0.028 | 0.064 | –0.009 | 0.341 * | 1.000 | |||||
| BMD_LS | –0.211 * | –0.215 * | 0.085 | 0.031 | –0.006 | 0.176 * | –0.094 | –0.258 * | 1.000 | ||||
| BMD_FN | –0.523 * | –0.364 * | 0.189 * | 0.025 | 0.077 | 0.158 | 0.041 | 0.040 | 0.590 * | 1.000 | |||
| BMD_TH | –0.434 * | –0.427 * | 0.212 * | 0.048 | 0.051 | 0.1531 | 0.012 | –0.091 | 0.632 * | 0.907 * | 1.000 | ||
| AAC a | 0.433 * | 0.002 | –0.098 | 0.080 | 0.097 | –0.038 | –0.050 | 0.122 | –0.012 | –0.311 * | –0.291 * | 1 | |
| T50 | –0.008 | –0.025 | 0.316 * | –0.207 * | 0.016 | –0.143 | 0.002 | –0.162 | 0.092 | 0.011 | 0.133 | –0.024 | 1 |
| Ca | P | PTH | ALP | BMD_LS | BMD_FN | BMD_TH | AAC | T50 | TRACP5b | CTx | BAP | tP1NP | OC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | 1.000 | |||||||||||||
| P | 0.032 | 1.000 | ||||||||||||
| PTH | 0.335 | 0.431 * | 1.000 | |||||||||||
| ALP | 0.221 | –0.247 | 0.339 | 1.000 | ||||||||||
| BMD_LS | 0.216 | 0.015 | 0.128 | –0.055 | 1.000 | |||||||||
| BMD_FN | 0.055 | 0.249 | 0.081 | –0.429 * | 0.016 | 1.000 | ||||||||
| BMD_TH | –0.075 | 0.257 | 0.065 | –0.429 * | 0.211 | 0.837 * | 1.000 | |||||||
| AAC a | –0.089 | –0.059 | –0.174 | 0.168 | 0.077 | –0.364 | –0.167 | 1.000 | ||||||
| T50 | –0.052 | –0.124 | –0.469 * | –0.062 | 0.083 | –0.098 | –0.008 | 0.496 * | 1.000 | |||||
| TRACP5b | 0.607 * | 0.183 | 0.582 * | 0.366 | 0.142 | 0.063 | 0.021 | –0.137 | –0.102 | 1.000 | ||||
| CTx | 0.443 * | –0.018 | 0.493 * | 0.458 * | –0.081 | 0.049 | –0.034 | –0.126 | –0.307 | 0.576 * | 1.000 | |||
| BAP | 0.258 | –0.232 | 0.384 * | 0.785 * | –0.069 | –0.205 | –0.274 | 0.055 | 0.011 | 0.553 * | 0.580 * | 1.000 | ||
| tP1NP | 0.520 * | 0.369 * | 0.745 * | 0.397 * | 0.158 | −0.064 | −0.047 | 0.060 | –0.253 | 0.720 * | 0.625 * | 0.543 * | 1.000 | |
| OC | 0.200 | –0.103 | –0.191 | 0.183 | 0.240 | −0.005 | 0.183 | 0.333 | 0.410 * | 0.013 | –0.119 | 0.103 | –0.050 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lee, E.J.; Woo, S.; Rho, S.; Jung, J.Y. Effect of Denosumab on Bone Health, Vascular Calcification, and Health-Related Quality of Life in Hemodialysis Patients with Osteoporosis: A Prospective Observational Study. J. Clin. Med. 2024, 13, 1462. https://doi.org/10.3390/jcm13051462
Kim H, Lee EJ, Woo S, Rho S, Jung JY. Effect of Denosumab on Bone Health, Vascular Calcification, and Health-Related Quality of Life in Hemodialysis Patients with Osteoporosis: A Prospective Observational Study. Journal of Clinical Medicine. 2024; 13(5):1462. https://doi.org/10.3390/jcm13051462
Chicago/Turabian StyleKim, Hyunsook, Eun Ju Lee, Siyun Woo, Sohee Rho, and Ji Yong Jung. 2024. "Effect of Denosumab on Bone Health, Vascular Calcification, and Health-Related Quality of Life in Hemodialysis Patients with Osteoporosis: A Prospective Observational Study" Journal of Clinical Medicine 13, no. 5: 1462. https://doi.org/10.3390/jcm13051462
APA StyleKim, H., Lee, E. J., Woo, S., Rho, S., & Jung, J. Y. (2024). Effect of Denosumab on Bone Health, Vascular Calcification, and Health-Related Quality of Life in Hemodialysis Patients with Osteoporosis: A Prospective Observational Study. Journal of Clinical Medicine, 13(5), 1462. https://doi.org/10.3390/jcm13051462






