Predictors for Dexmedetomidine Requirement for Sedation under Regional Anesthesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Standardized Regional Anesthesia Management
2.2. Intervention Protocol and Outcome Assessment
2.3. Sample Size Calculation and Statistical Analysis
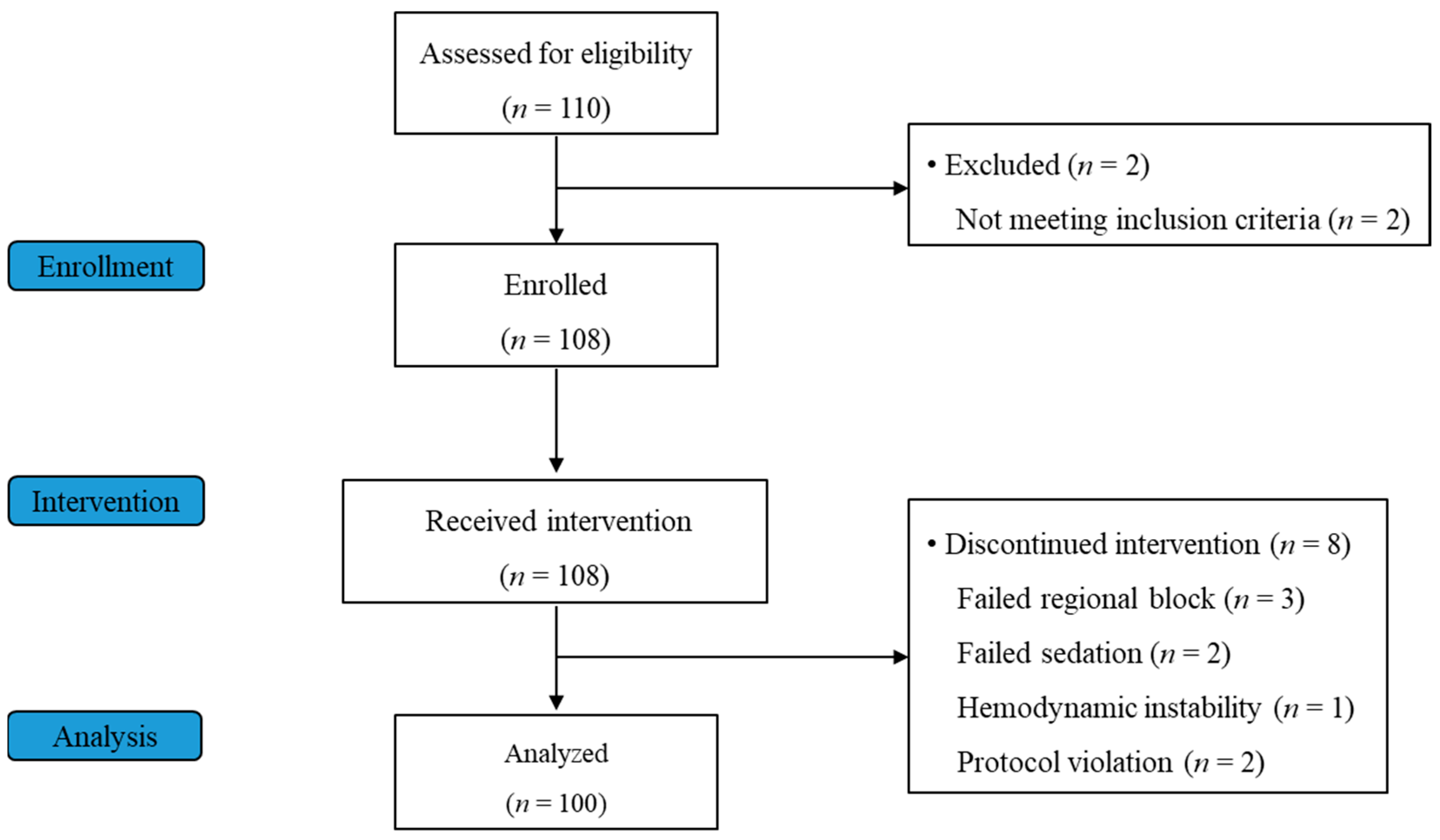
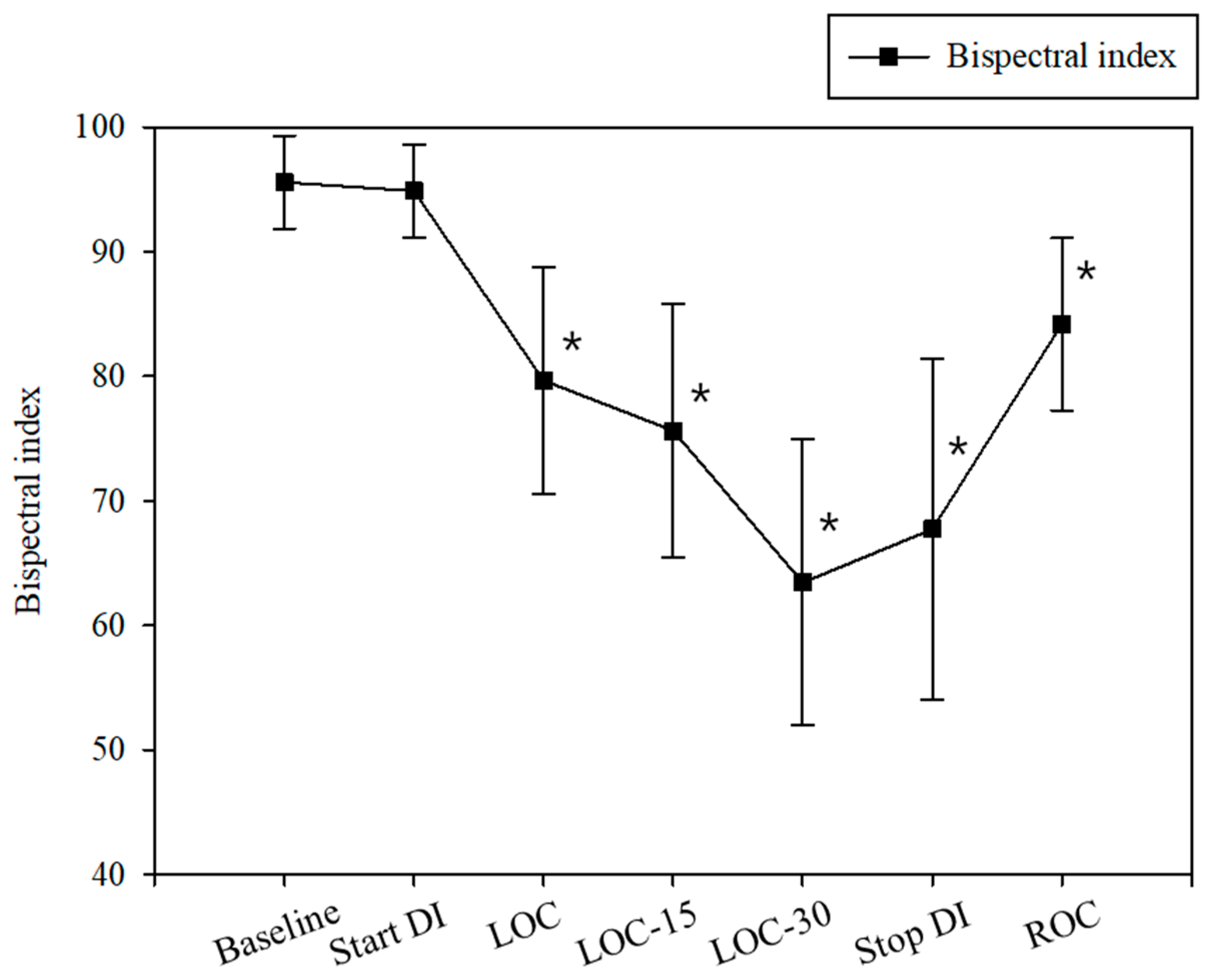
3. Results
3.1. Study Participants and Patient Characteristics
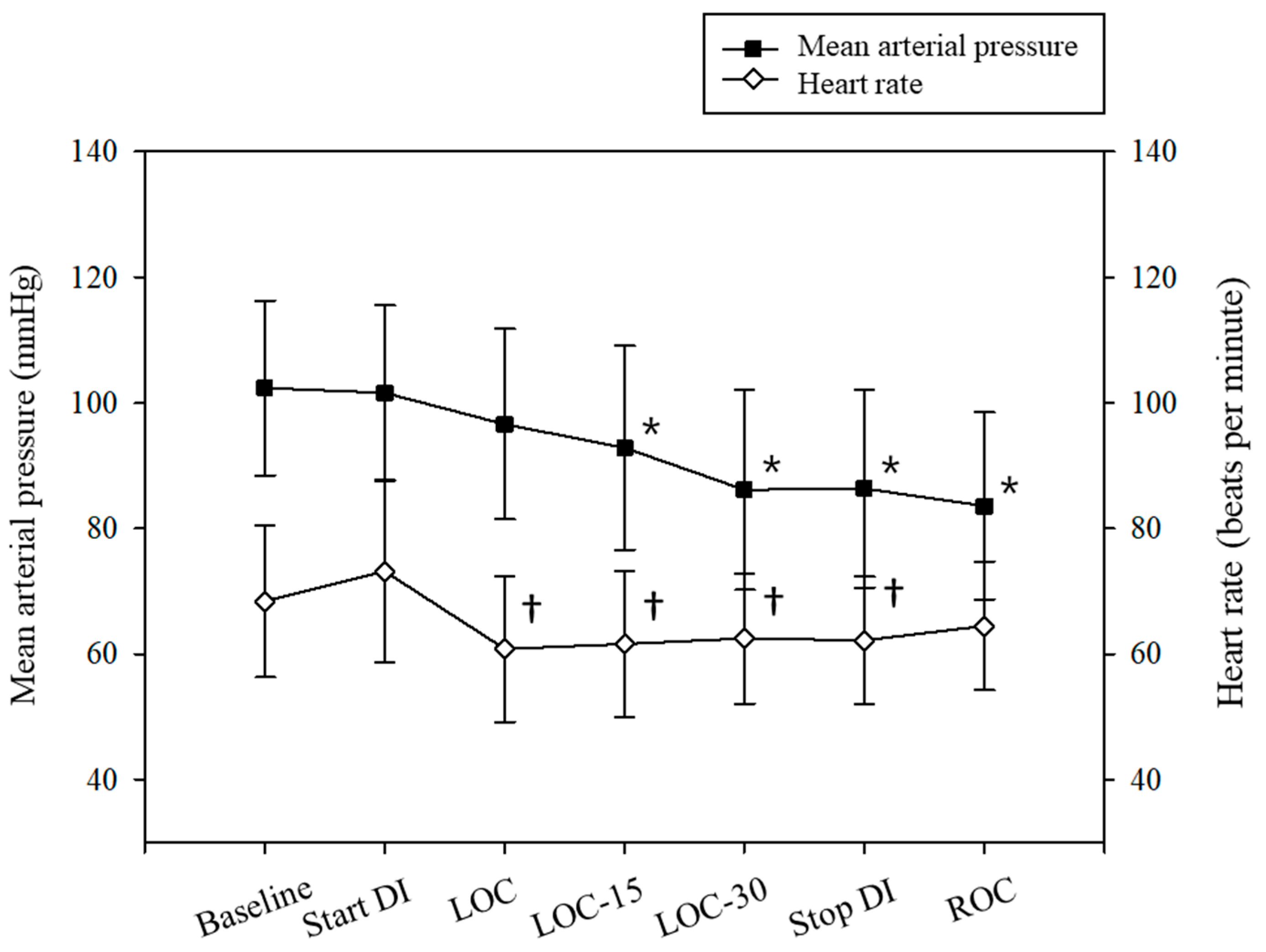
3.2. Study Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vorobeichik, L.; Brull, R.; Abdallah, F.W. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: A systematic review and meta-analysis of randomized controlled trials. Br. J. Anaesth. 2017, 118, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Grzywacz, V.P.; Ferreri, C.A.; Atrey, A.; Banfield, L.; Shaparin, N.; Vydyanathan, A. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: A systematic review and meta-analysis of 18 randomized controlled trials. Reg. Anesth. Pain Med. 2017, 42, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.W.; Dwyer, T.; Chan, V.W.; Niazi, A.U.; Ogilvie-Harris, D.J.; Oldfield, S.; Patel, R.; Oh, J.; Brull, R. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: A randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology 2016, 124, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Candiotti, K.A.; Bergese, S.D.; Bokesch, P.M.; Feldman, M.A.; Wisemandle, W.; Bekker, A.Y. Monitored anesthesia care with dexmedetomidine: A prospective, randomized, double-blind, multicenter trial. Anesth. Analg. 2010, 110, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ice, C.J.; Personett, H.A.; Frazee, E.N.; Dierkhising, R.A.; Kashyap, R.; Oeckler, R.A. Risk factors for dexmedetomidine-associated hemodynamic instability in noncardiac intensive care unit patients. Anesth. Analg. 2016, 122, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhou, D.; Ren, L.; Shulman, S.; Zhang, X.; Xiong, M. Pharmacokinetic and pharmacodynamics of intravenous dexmedetomidine in morbidly obese patients undergoing laparoscopic surgery. J. Anesth. 2017, 31, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Cheymol, G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin. Pharmacokinet. 2000, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Cortínez, L.I.; Anderson, B.J.; Holford, N.H.; Puga, V.; de la Fuente, N.; Auad, H.; Solari, S.; Allende, F.A.; Ibacache, M. Dexmedetomidine pharmacokinetics in the obese. Eur. J. Clin. Pharmacol. 2015, 71, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Rolle, A.; Paredes, S.; Cortínez, L.I.; Anderson, B.J.; Quezada, N.; Solari, S.; Allende, F.; Torres, J.; Cabrera, D.; Contreras, V.; et al. Dexmedetomidine metabolic clearance is not affected by fat mass in obese patients. Br. J. Anaesth. 2018, 120, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Jun, I.J.; Lee, S.; Lim, Y.; Yoo, B.; Kim, K.M. Effective dose of dexmedetomidine to induce adequate sedation in elderly patients under spinal anesthesia. Korean J. Anesthesiol. 2015, 68, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Iirola, T.; Ihmsen, H.; Laitio, R.; Kentala, E.; Aantaa, R.; Kurvinen, J.P.; Scheinin, M.; Schwilden, H.; Schüttler, J.; Olkkola, K.T. Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br. J. Anaesth. 2012, 108, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Hannivoort, L.N.; Eleveld, D.J.; Proost, J.H.; Reyntjens, K.M.; Absalom, A.R.; Vereecke, H.E.; Struys, M.M. Development of an optimized pharmacokinetic model of dexmedetomidine using target-controlled infusion in healthy volunteers. Anesthesiology 2015, 123, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Weerink, M.A.S.; Struys, M.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.O.; Kim, H.B.; Kil, H.K. Adequate sedation with single-dose dexmedetomidine in patients undergoing transurethral resection of the prostate with spinal anaesthesia: A dose-response study by age group. BMC Anesthesiol. 2015, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Doo, A.R.; Lee, H.; Baek, S.J.; Lee, J. Dexmedetomidine-induced hemodynamic instability in patients undergoing orthopedic upper limb surgery under brachial plexus block: A retrospective study. BMC Anesthesiol. 2021, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, B.H.; Lim, K.; Stalker, D.; Wisemandle, W.; Shin, S.G.; Jang, I.J.; Yu, K.S. Pharmacokinetics and pharmacodynamics of intravenous dexmedetomidine in healthy korean subjects. J. Clin. Pharm. Ther. 2012, 37, 698–703. [Google Scholar] [CrossRef] [PubMed]
| Men | Women | Total | |
|---|---|---|---|
| Number of patients | 49 | 51 | 100 |
| Age (years) | 41.3 ± 16.2 | 51.0 ± 17.7 | 46.3 ± 17.6 |
| Height (cm) | 172.1 ± 7.1 | 157.2 ± 6.1 | 164.4 ± 9.9 |
| Body weight (kg) | 78.5 ± 9.9 | 58.0 ± 7.8 | 68.9 ± 12.9 |
| BMI (kg/m2) | 26.7 (23.9–28.4) | 23.6 (21.9–26.1) | 25.4 (22.9–27.7) |
| ASA/PS 1/2 | 11/38 | 10/41 | 21/79 |
| Underlying disease [n (%)] | |||
| Hypertension | 8 (16.3%) | 12 (23.5%) | 20 (20.0%) |
| Diabetes mellitus | 4 (8.2%) | 2 (3.9%) | 6 (6.0%) |
| Type of anesthesia [n (%)] | |||
| Brachial plexus block | 25 (51.0%) | 29 (56.9%) | 54 (54.0%) |
| Spinal anesthesia | 23 (46.9%) | 20 (39.2%) | 43 (43.0%) |
| Femoral/sciatic block | 1 (2.0%) | 2 (3.9%) | 3 (3.0%) |
| Surgery time (min) | 47.0 (30.5–67.8) | 39.0 (30.0–65.0) | 44.0 (30.0–66.0) |
| Anesthesia time (min) | 77.5 (60.0–100.0) | 72.0 (60.0–100.0) | 75.0 (60.0–100.0) |
| Total infused fluid volume (mL) | 251.9 ± 135.0 | 217.6 ± 121.2 | 234.2 ± 128.6 |
| Men (n = 49) | Women (n = 51) | p-Value | |
|---|---|---|---|
| The administered dose of DMT for LOC (μg/kg) * | 0.83 (0.75–0.97) | 0.89 (0.75–1.02) | 0.303 |
| The elapsed time to LOC (min) † | 12.0 (10.1–13.6) | 12.8 (10.5–14.6) | 0.449 |
| BIS value at LOC | 82.0 (77.0–88.0) | 81.0 (72.0–86.0) | 0.249 |
| BIS value at the end of surgery ‡ | 69.7 ± 12.1 | 65.9 ± 15.0 | 0.162 |
| Cumulative dose of DMT (μg/kg) during surgery | 1.1 (0.9–1.3) | 1.2 (1.0–1.3) | 0.526 |
| The elapsed time to BIS 90 (min) § | 4.6 (2.9–9.6) | 5.0 (2.3–9.8) | 0.911 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | R2 | p-Value | β | SE | R2 | p-Value | |
| 0.259 | <0.001 † | |||||||
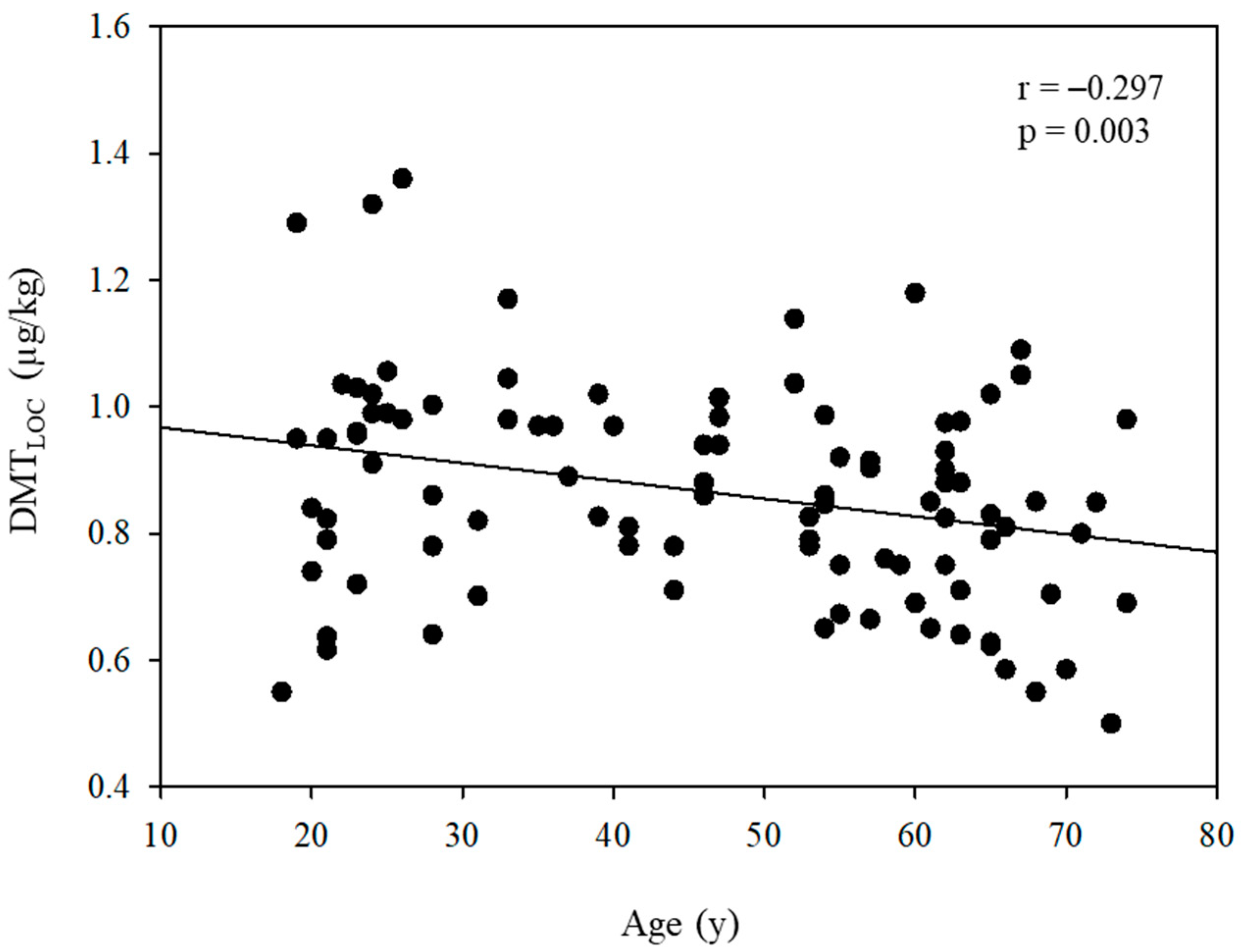
| Age, 10 yr | −0.032 | 0.010 | 0.088 | 0.003 * | −0.030 | 0.010 | 0.002 | |
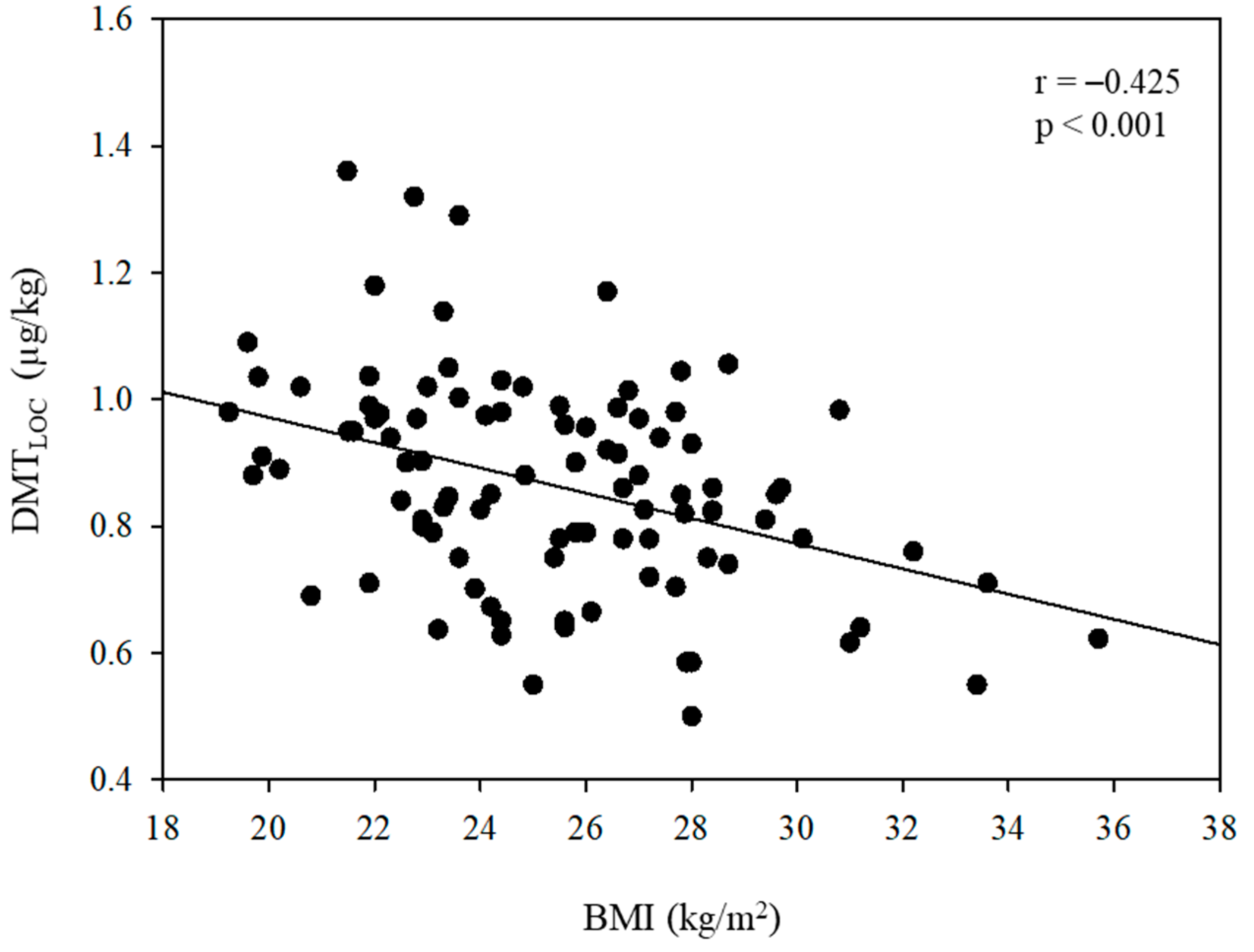
| BMI (kg/m2) | −0.020 | 0.005 | 0.181 | <0.001 * | −0.023 | 0.005 | <0.001 † | |
| Female | 0.041 | 0.038 | 0.012 | 0.280 | ||||
| Anesthesia type (spinal anesthesia) | −0.060 | 0.038 | 0.028 | 0.098 | ||||
| Variable | Univariate Analysis | |||
|---|---|---|---|---|
| β | SE | R2 | p-Value | |
| Age, 10 year | 0.412 | 0.540 | 0.006 | 0.447 |
| BMI (kg/m2) | 0.103 | 0.282 | 0.001 | 0.716 |
| Female | −0.515 | 1.894 | 0.001 | 0.786 |
| DMTCUM (µg/kg) | −5.244 | 3.146 | 0.028 | 0.099 |
| Normal Weight (n = 48) | Overweight (n = 43) | Obese (n = 9) | p-Value | |
|---|---|---|---|---|
| Age (years) | 49.5 (24.5–63.0) | 53.0 (31.0–62.0) | 45.5 (26.0–61.8) | 0.840 |
| Female sex [n (%)] | 31 (64.6%) | 17 (39.5%) | 3 (33.3%) | 0.032 * |
| The administered dose of DMT for LOC (μg/kg) | 0.94 (0.81–1.00) | 0.83 (0.75–0.94) | 0.68 (0.62–0.78) | <0.001 † |
| The elapsed time to LOC (min) | 13.0 (11.1–14.4) | 11.7 (10.2–13.6) | 9.0 (8.9–11.9) | 0.009 ‡ |
| BIS value at LOC | 82.0 (74.0–87.8) | 81.0 (73.0–85.0) | 78.5 (65.5–84.5) | 0.295 |
| BIS value at the end of surgery | 64.6 ± 13.2 | 71.5 ± 13.9 | 65.9 ± 12.8 | 0.051 |
| Cumulative dose of DMT (μg/kg) | 1.2 (1.0–1.3) | 1.1 (0.9–1.3) | 0.9 (0.7–1.1) | 0.006 § |
| The elapsed time to BIS 90 (min) | 5.0 (2.9–9.8) | 4.8 (2.6–10.5) | 3.2 (2.3–4.9) | 0.460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Jung, T.; Ko, S.; Doo, A. Predictors for Dexmedetomidine Requirement for Sedation under Regional Anesthesia. J. Clin. Med. 2024, 13, 1435. https://doi.org/10.3390/jcm13051435
Lee JH, Jung T, Ko S, Doo A. Predictors for Dexmedetomidine Requirement for Sedation under Regional Anesthesia. Journal of Clinical Medicine. 2024; 13(5):1435. https://doi.org/10.3390/jcm13051435
Chicago/Turabian StyleLee, Jun Ho, Taehyeon Jung, Seonghoon Ko, and Aram Doo. 2024. "Predictors for Dexmedetomidine Requirement for Sedation under Regional Anesthesia" Journal of Clinical Medicine 13, no. 5: 1435. https://doi.org/10.3390/jcm13051435
APA StyleLee, J. H., Jung, T., Ko, S., & Doo, A. (2024). Predictors for Dexmedetomidine Requirement for Sedation under Regional Anesthesia. Journal of Clinical Medicine, 13(5), 1435. https://doi.org/10.3390/jcm13051435





