Significant Reduction in Bone Density as Measured by Hounsfield Units in Patients with Ankylosing Spondylitis or Diffuse Idiopathic Skeletal Hyperostosis
Abstract
1. Introduction
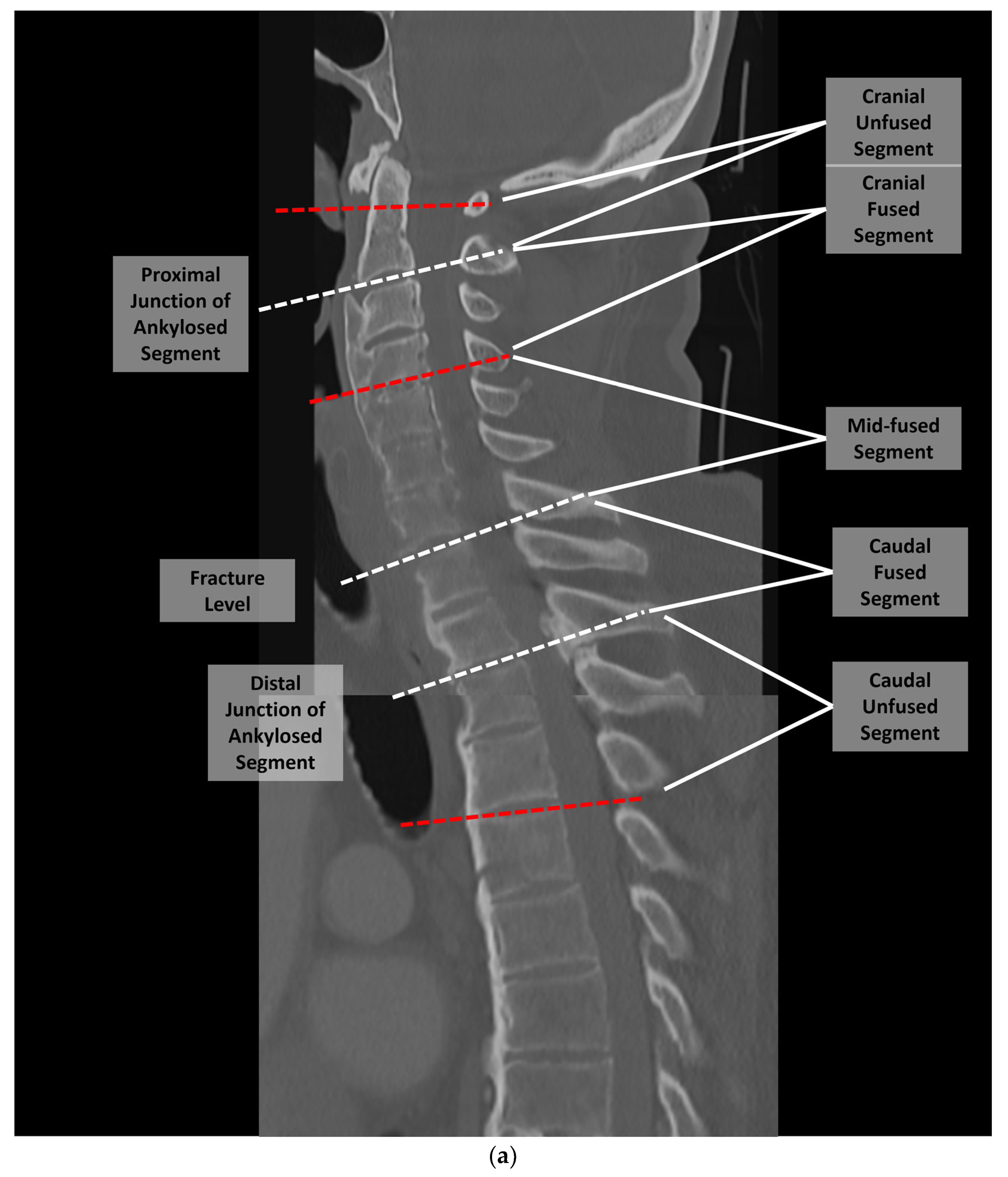
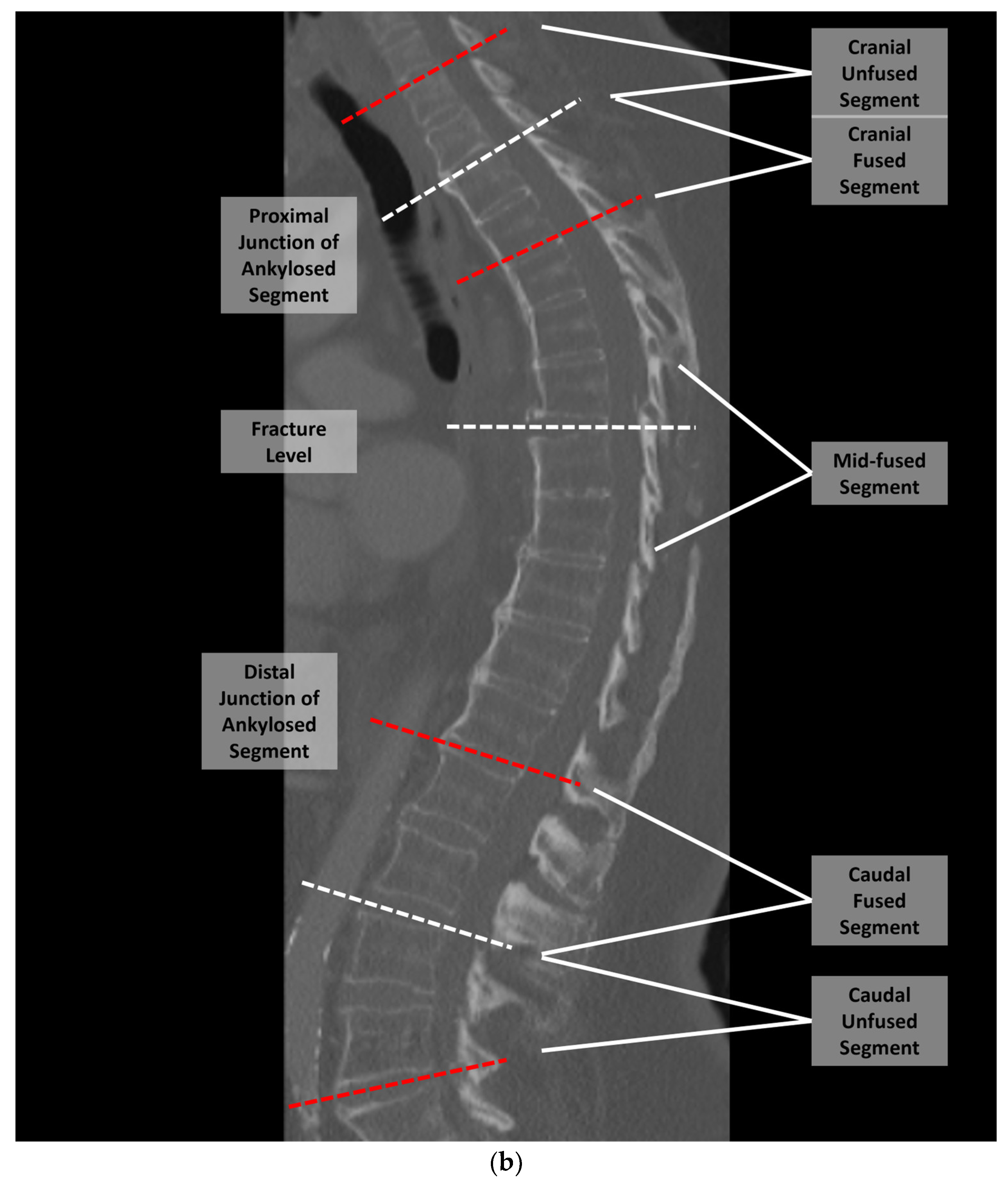
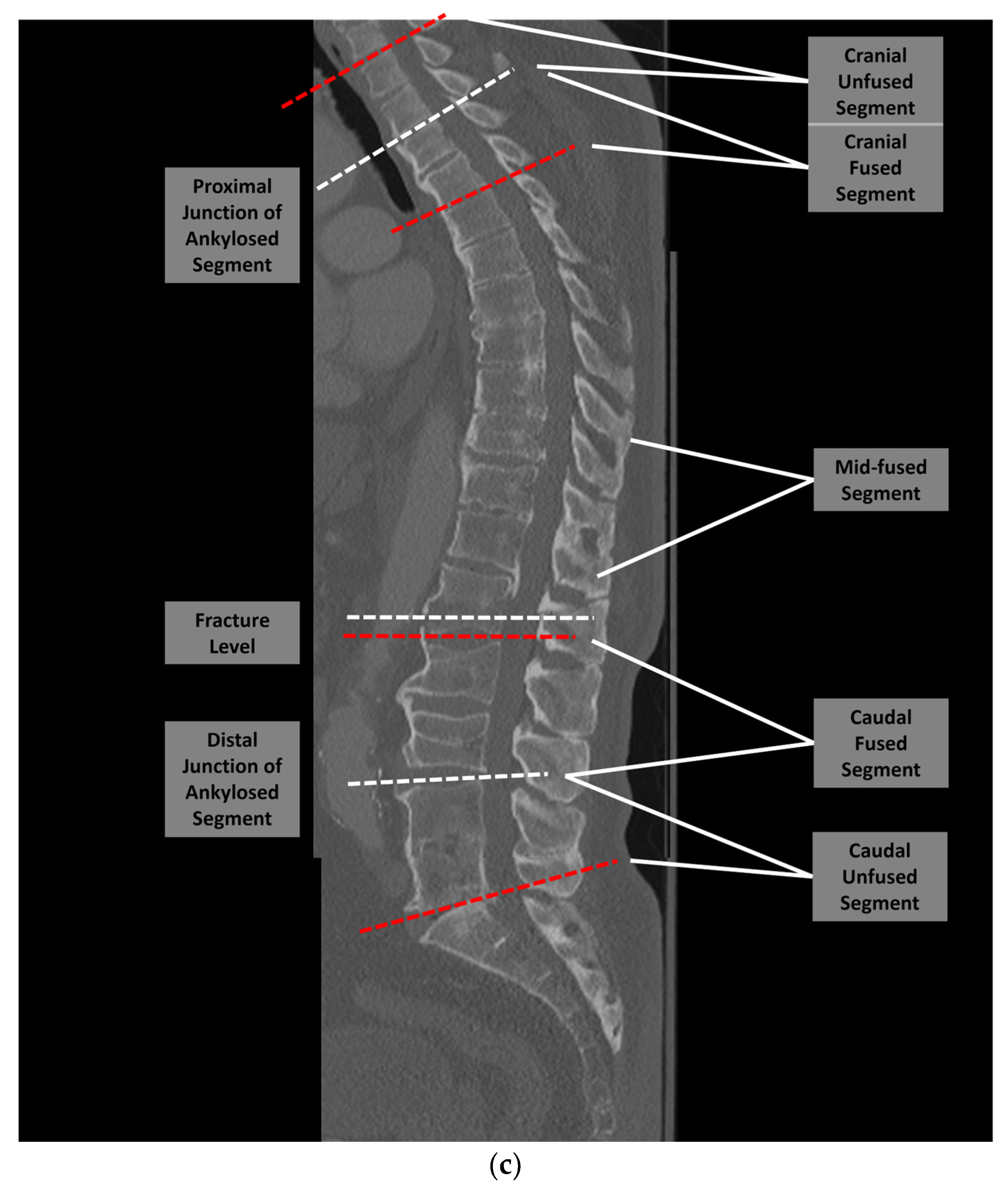
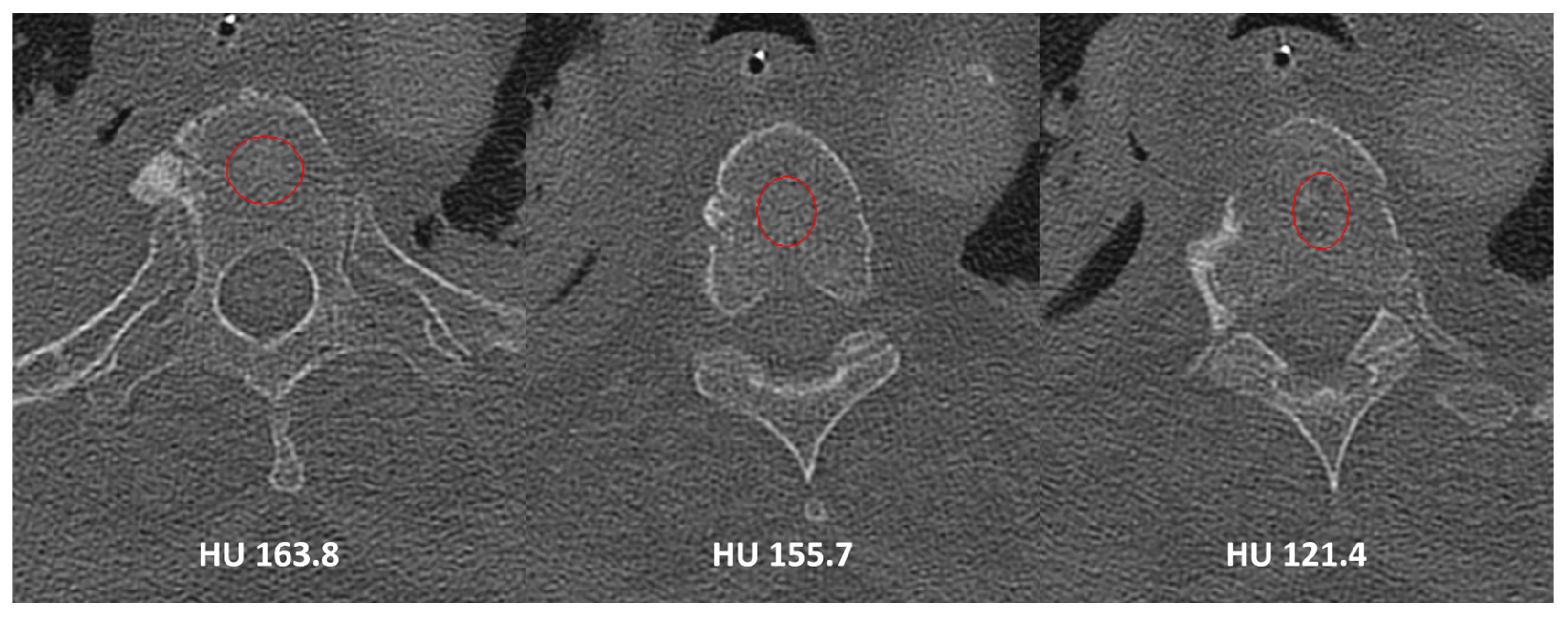
2. Materials and Methods
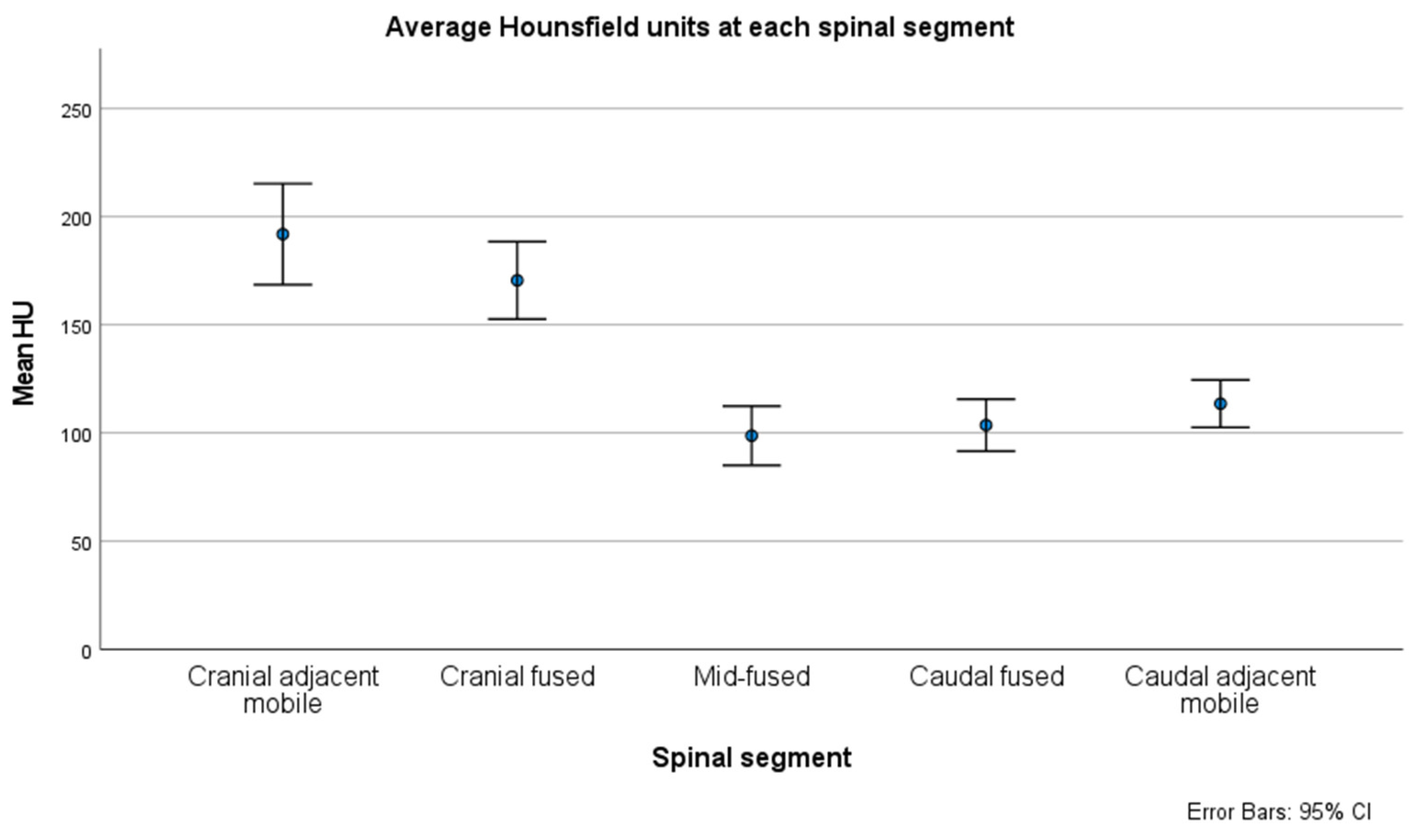
3. Results
4. Discussion
4.1. Basis of Fracture in Ankylosing Spine Disease
4.2. Management of Fractures in DISH and Ankylosing Spondylitis
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieper, J.; Braun, J.; Dougados, M.; Baeten, D. Axial spondyloarthritis. Nat. Rev. Dis. Prim. 2015, 1, 15013. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.E.; Jones, G.T.; MacDonald, A.G.; Downham, C.; Sturrock, R.D.; Macfarlane, G.J. Global prevalence of ankylosing spondylitis. Rheumatology 2014, 53, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Verlaan, J.J.; Buskila, D. Diffuse idiopathic skeletal hyperostosis: Clinical features and pathogenic mechanisms. Nat. Rev. Rheumatol. 2013, 9, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Le, H.V.; Wick, J.B.; Van, B.W.; Klineberg, E.O. Diffuse Idiopathic Skeletal Hyperostosis of the Spine: Pathophysiology, Diagnosis, and Management. J. Am. Acad. Orthop. Surg. 2021, 29, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Verlaan, J.J.; Eshed, I.; Jacome, B.-A.; Puttini, P.S.; Atzeni, F.; Buskila, D.; Reinshtein, E.; Novofastovski, I.; Fawaz, A.; et al. Diffuse idiopathic skeletal hyperostosis (DISH): Where we are now and where to go next. RMD Open 2017, 3, e000472. [Google Scholar] [CrossRef] [PubMed]
- Tam, L.S.; Gu, J.; Yu, D. Pathogenesis of ankylosing spondylitis. Nat. Rev. Rheumatol. 2010, 6, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.A.; Neto, H.M.; Gatto, L.A.M.; Lages, R.O.; Demartini, Z.; Koppe, G.L. Diffuse idiopathic skeletal hyperostosis: A review. Surg. Neurol. Int. 2014, 5, 122–125. [Google Scholar] [CrossRef]
- Takahashi, T.; Yoshii, T.; Mori, K.; Kobayashi, S.; Inoue, H.; Tada, K.; Tamura, N.; Hirai, T.; Sugimura, N.; Nagoshi, N.; et al. Comparison of radiological characteristics between diffuse idiopathic skeletal hyperostosis and ankylosing spondylitis: A multicenter study. Sci. Rep. 2023, 13, 1849. [Google Scholar] [CrossRef]
- Westerveld, L.A.; Verlaan, J.J.; Oner, F.C. Spinal fractures in patients with ankylosing spinal disorders: A systematic review of the literature on treatment, neurological status and complications. Eur. Spine J. 2009, 18, 145–156. [Google Scholar] [CrossRef]
- Caron, T.; Bransford, R.; Nguyen, Q.; Agel, J.; Chapman, J.; Bellabarba, C. Spine Fractures in Patients With Ankylosing Spinal Disorders. Spine 2010, 35, E458–E464. [Google Scholar] [CrossRef]
- Clunie, G.; Horwood, N. Loss and gain of bone in spondyloarthritis: What drives these opposing clinical features? Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X2096926. [Google Scholar] [CrossRef]
- Choi, M.K.; Kim, S.M.; Lim, J.K. Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: Correlation study between T-scores determined by DEXA scan and Hounsfield units from CT. Acta Neurochir. 2016, 158, 1421–1427. [Google Scholar] [CrossRef]
- Garg, M.; Kharb, S. Dual energy X-ray absorptiometry: Pitfalls in measurement and interpretation of bone mineral density. Indian J. Endocrinol. Metab. 2013, 17, 203. [Google Scholar] [CrossRef]
- Gregson, C.L.; Hardcastle, S.A.; Cooper, C.; Tobias, J.H. Friend or foe: High bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology 2013, 52, 968–985. [Google Scholar] [CrossRef]
- Pennington, Z.; Ehresman, J.; Lubelski, D.; Cottrill, E.; Schilling, A.; Ahmed, A.K.; Feghali, J.; Witham, T.F.; Sciubba, D.M. Assessing underlying bone quality in spine surgery patients: A narrative review of dual-energy X-ray absorptiometry (DXA) and alternatives. Spine J. 2021, 21, 321–331. [Google Scholar] [CrossRef]
- Anderson, P.A.; Polly, D.W.; Binkley, N.C.; Pickhardt, P.J. Clinical Use of Opportunistic Computed Tomography Screening for Osteoporosis. J. Bone Jt. Surg. 2018, 100, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Mikula, A.L.; Fogelson, J.L.; Lakomkin, N.; Flanigan, P.M.; Pinter, Z.W.; Doan, M.K.; Bydon, M.; Nassr, A.; Freedman, B.; Sebastian, A.S.; et al. Lower Hounsfield Units at the Upper Instrumented Vertebrae are Significantly Associated with Proximal Junctional Kyphosis and Failure Near the Thoracolumbar Junction. Oper. Neurosurg. 2021, 21, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, N.R.; Pickhardt, P.J.; Del Rio, A.M.; Rosas, H.G.; Anderson, P.A. Bone Mineral Density T-Scores Derived from CT Attenuation Numbers (Hounsfield Units): Clinical Utility and Correlation with Dual-energy X-ray Absorptiometry. Iowa Orthop. J. 2018, 38, 25–31. [Google Scholar] [PubMed]
- Lee, S.; Chung, C.K.; Oh, S.H.; Park, S.B. Correlation between Bone Mineral Density Measured by Dual-Energy X-Ray Absorptiometry and Hounsfield Units Measured by Diagnostic CT in Lumbar Spine. J. Korean Neurosurg. Soc. 2013, 54, 384. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield Units for Assessing Bone Mineral Density and Strength: A Tool for Osteoporosis Management. J. Bone Jt. Surg. 2011, 93, 1057–1063. [Google Scholar] [CrossRef]
- Zaidi, Q.; Danisa, O.A.; Cheng, W. Measurement Techniques and Utility of Hounsfield Unit Values for Assessment of Bone Quality Prior to Spinal Instrumentation. Spine 2019, 44, E239–E244. [Google Scholar] [CrossRef] [PubMed]
- Mikula, A.L.; Lakomkin, N.; Pennington, Z.; Pinter, Z.W.; Nassr, A.; Freedman, B.; Sebastian, A.S.; Abode-Iyamah, K.; Bydon, M.; Ames, C.P.; et al. Association between lower Hounsfield units and proximal junctional kyphosis and failure at the upper thoracic spine. J. Neurosurg. Spine 2022, 37, 694–702. [Google Scholar] [CrossRef]
- Pennington, Z.; Mikula, A.L.; Lakomkin, N.; Martini, M.; Clarke, M.J.; Sebastian, A.S.; Freedman, B.A.; Rose, P.S.; Karim, S.M.; Nassr, A.; et al. Comparison of Hounsfield units and vertebral bone quality score for the prediction of time to pathologic fracture in mobile spine metastases treated with radiotherapy. J. Neurosurg. Spine 2023, 40, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mikula, A.L.; Puffer, R.C.; Jeor, J.D.S.; Bernatz, J.T.; Fogelson, J.L.; Larson, A.N.; Nassr, A.; Sebastian, A.S.; Freedman, B.A.; Currier, B.L.; et al. Teriparatide treatment increases Hounsfield units in the lumbar spine out of proportion to DEXA changes. J. Neurosurg. Spine 2020, 32, 50–55. [Google Scholar] [CrossRef]
- Flanigan, P.M.; Mikula, A.L.; Peters, P.A.; Oushy, S.; Fogelson, J.L.; Bydon, M.; Freedman, B.A.; Sebastian, A.S.; Currier, B.L.; Nassr, A.; et al. Regional improvements in lumbosacropelvic Hounsfield units following teriparatide treatment. Neurosurg. Focus 2020, 49, E11. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kaul, R.; Chhabra, H.S.; Vaccaro, A.R.; Abel, R.; Tuli, S.; Shetty, A.P.; Das, K.D.; Mohapatra, B.; Nanda, A.; Sangondimath, G.M.; et al. Reliability assessment of AOSpine thoracolumbar spine injury classification system and Thoracolumbar Injury Classification and Severity Score (TLICS) for thoracolumbar spine injuries: Results of a multicentre study. Eur. Spine J. 2017, 26, 1470–1476. [Google Scholar] [CrossRef]
- Karamian, B.A.; Schroeder, G.D.; Lambrechts, M.J.; Canseco, J.A.; Oner, C.; Vialle, E.; Rajasekaran, S.; Dvorak, M.R.; Benneker, L.M.; Kandziora, F.; et al. An international validation of the AO spine subaxial injury classification system. Eur. Spine J. 2023, 32, 46–54. [Google Scholar] [CrossRef]
- Hirata, Y.; Inaba, Y.; Kobayashi, N.; Ike, H.; Fujimaki, H.; Saito, T. Comparison of Mechanical Stress and Change in Bone Mineral Density Between Two Types of Femoral Implant Using Finite Element Analysis. J. Arthroplast. 2013, 28, 1731–1735. [Google Scholar] [CrossRef]
- Ike, H.; Inaba, Y.; Kobayashi, N.; Hirata, Y.; Yukizawa, Y.; Aoki, C.; Choe, H.; Saito, T. Comparison between mechanical stress and bone mineral density in the femur after total hip arthroplasty by using subject-specific finite element analyses. Comput. Methods Biomech. Biomed. Engin. 2015, 18, 1056–1065. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [CrossRef]
- Srinivas, G.R.; Deb, A.; Kumar, M.N.; Kurnool, G. Long-Term Effects of Segmental Lumbar Spinal Fusion on Adjacent Healthy Discs: A Finite Element Study. Asian Spine J. 2016, 10, 205. [Google Scholar] [CrossRef]
- Razzouk, J.; Ramos, O.; Scolieri, J.; Bouterse, A.; Cabrera, A.; Shin, D.; Brandt, Z.; Carter, D.; Wycliffe, N.; Cheng, W.; et al. Correlations among Cervical, Thoracic, and lumbar Hounsfield Unit measurements for assessment of bone mineral density. J. Clin. Neurosci. 2024, 120, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Vosse, D.; Feldtkeller, E.; Erlendsson, J.; Geusens, P.; van der Linden, S. Clinical vertebral fractures in patients with ankylosing spondylitis. J. Rheumatol. 2004, 31, 1981–1985. [Google Scholar] [PubMed]
- Olerud, C.; Frost, A.; Bring, J. Spinal fractures in patients with ankylosing spondylitis. Eur. Spine J. 1996, 5, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Feldtkeller, E.; Vosse, D.; Geusens, P.; van der Linden, S. Prevalence and annual incidence of vertebral fractures in patients with ankylosing spondylitis. Rheumatol. Int. 2006, 26, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, G.; Engelken, F.; Marshall, L.M.; Peters, K.; Black, D.M.; Issever, A.S.; Barrett-Connor, E.; Orwoll, E.; Hamm, B. Diffuse idiopathic skeletal hyperostosis (DISH): Relation to vertebral fractures and bone density. Osteoporos. Int. 2011, 22, 1789–1797. [Google Scholar] [CrossRef]
- Murakami, Y.; Mashima, N.; Morino, T.; Fukuda, T.; Iwase, M.; Hino, M.; Misaki, H.; Miura, H. Association Between Vertebral Fracture and Diffuse Idiopathic Skeletal Hyperostosis. Spine 2019, 44, E1068–E1074. [Google Scholar] [CrossRef] [PubMed]
- Okada, E.; Tsuji, T.; Shimizu, K.; Kato, M.; Fukuda, K.; Kaneko, S.; Ogawa, J.; Watanabe, K.; Ishii, K.; Nakamura, M.; et al. CT-based morphological analysis of spinal fractures in patients with diffuse idiopathic skeletal hyperostosis. J. Orthop. Sci. 2017, 22, 3–9. [Google Scholar] [CrossRef]
- Nishida, N.; Mumtaz, M.; Tripathi, S.; Kumaran, Y.; Kelkar, A.; Sakai, T.; Goel, V.K. The Effect of Posterior Lumbar Interbody Fusion in Lumbar Spine Stenosis with Diffuse Idiopathic Skeletal Hyperostosis: A Finite Element Analysis. World Neurosurg. 2023, 176, e371–e379. [Google Scholar] [CrossRef]
- Okada, E.; Yoshii, T.; Yamada, T.; Watanabe, K.; Katsumi, K.; Hiyama, A.; Watanabe, M.; Nakagawa, Y.; Okada, M.; Endo, T.; et al. Spinal fractures in patients with Diffuse idiopathic skeletal hyperostosis: A nationwide multi-institution survey. J. Orthop. Sci. 2019, 24, 601–606. [Google Scholar] [CrossRef]
- Chen, S.R.; Munsch, M.A.; Chen, J.; Couch, B.K.; Wawrose, R.A.; Oyekan, A.A.; Adjei, J.; Donaldson, W.F.; Lee, J.Y.; Shaw, J.D. Spine Fractures of Patients with Ankylosing Spondylitis and Diffuse Idiopathic Skeletal Hyperostosis: Fracture Severity and Injury-Related Mortality at a Level I Trauma Center. Asian Spine J. 2023, 17, 549–558. [Google Scholar] [CrossRef]
- Lee, S.Y.; Song, R.; Yang, H.I.; Chung, S.W.; Lee, Y.-A.; Hong, S.-J.; Yun, S.J.; Lee, S.-H. The bone bridge significantly affects the decrease in bone mineral density measured with quantitative computed tomography in ankylosing spondylitis. PLoS ONE 2021, 16, e0249578. [Google Scholar] [CrossRef]
- Fauny, M.; Morizot, C.; Allado, E.; Verhoeven, F.; Albuisson, E.; Semaan, M.; Pinzano, A.; Chary-Valckenaere, I.; Loeuille, D. Consequences of spinal ankylosis on bone trabecular fragility assessed on CT scans in patients with ankylosing spondylitis. A retrospective study. Jt. Bone Spine 2020, 87, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J. Osteoporos. 2018, 2018, 1206235. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Melton, L.J.; O’Fallon, W.M.; Riggs, B.L. Risk factors for spinal osteoporosis in men. Am. J. Med. 1983, 75, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Liu, M.; Yin, X.; Liu, J.; Zhao, J.; Liu, P. Biomechanical influence of the surgical approaches, implant length and density in stabilizing ankylosing spondylitis cervical spine fracture. Sci. Rep. 2021, 11, 6023. [Google Scholar] [CrossRef] [PubMed]
- Himič, V.; Syrmos, N.; Ligarotti, G.K.I.; Kato, S.; Fehlings, M.G.; Ganau, M. The role of genetic and epigenetic factors in determining the risk of spinal fragility fractures: New insights in the management of spinal osteoporosis. Quant. Imaging Med. Surg. 2023, 13, 7632–7645. [Google Scholar] [CrossRef] [PubMed]
- Wáng, Y.X.J. Fragility fracture prevalence among elderly Chinese is no more than half of that of elderly Caucasians. Quant. Imaging Med. Surg. 2022, 12, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yuan, H.; Cheng, J.; Weng, X.; Xu, H.; Gao, J.; Huang, M.; Wáng, Y.X.J.; Wu, Y.; Xu, W.; et al. Chinese expert consensus on the diagnosis of osteoporosis by imaging and bone mineral density. Quant. Imaging Med. Surg. 2020, 10, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
| Gender (count, %) | |
| Male | 55 (74.3) |
| Female | 19 (25.7) |
| Age at presentation (years, SD) | 76 (11.4) |
| BMI (kg/m2, SD) | 33.4 (8.2) |
| Smoking history (count, %) | |
| Current/former | 23 (31.5) |
| Never | 50 (68.5) |
| Unavailable | 1 (1.4) |
| DM (count, %) | 34 (45.9) |
| Type of ankylosis (count, %) | |
| AS | 33 (44.6) |
| DISH | 33 (44.6) |
| AS/DISH | 8 (10.8) |
| Fracture Location | |
| Cervical | 8 (10.8%) |
| Thoracic | 63 (85.1%) |
| Lumbar | 3 (4.1%) |
| AO Fracture Morphology [29,30] | |
| A0 | 0 (0%) |
| A1 | 8 (10.8%) |
| A2 | 0 (0%) |
| A3 | 6 (8.1%) |
| A4 | 0 (0%) |
| B1 | 2 (2.7%) |
| B2 | 13 (17.6%) |
| B3 | 40 54.1%) |
| C | 5 (6.8%) |
| CCI (score, SD) | 5.8 (2.5) |
| Variable | β | p-Value | 95% CI |
|---|---|---|---|
| Age at presentation (years) | −3.3 | <0.001 | −4.3 to −2.4 |
| Current or former smoker | −30.0 | 0.009 | −52.3 to −7.7 |
| CCI | −4.8 | 0.071 | −10.1 to 0.4 |
| BMI | 1.0 | 0.142 | −0.4 to 2.5 |
| Male gender | 9.2 | 0.460 | −15.4 to 33.8 |
| Variable | AS | DISH | p-Value |
|---|---|---|---|
| Male gender (count, %) | 23 (70) | 24 (73) | 0.79 |
| Age (years, SD) | 76.3 (11.8) | 74.8 (10.7) | 0.6 |
| BMI (SD) | 33 (7) | 34 (10) | 0.53 |
| History of current or former smoking (count, %) | 5 (16) | 12 (36) | 0.06 |
| DM (count, %) | 11 (33) | 19 (58) | <0.05 |
| CCI (score, SD) | 5.9 (2.7) | 5.6 (2.5) | 0.67 |
| Mid-fused segment HUs (SD) | 74 (53) | 123 (48) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swart, A.; Hamouda, A.; Pennington, Z.; Lakomkin, N.; Mikula, A.L.; Martini, M.L.; Shafi, M.; Subramaniam, T.; Sebastian, A.S.; Freedman, B.A.; et al. Significant Reduction in Bone Density as Measured by Hounsfield Units in Patients with Ankylosing Spondylitis or Diffuse Idiopathic Skeletal Hyperostosis. J. Clin. Med. 2024, 13, 1430. https://doi.org/10.3390/jcm13051430
Swart A, Hamouda A, Pennington Z, Lakomkin N, Mikula AL, Martini ML, Shafi M, Subramaniam T, Sebastian AS, Freedman BA, et al. Significant Reduction in Bone Density as Measured by Hounsfield Units in Patients with Ankylosing Spondylitis or Diffuse Idiopathic Skeletal Hyperostosis. Journal of Clinical Medicine. 2024; 13(5):1430. https://doi.org/10.3390/jcm13051430
Chicago/Turabian StyleSwart, Alexander, Abdelrahman Hamouda, Zach Pennington, Nikita Lakomkin, Anthony L. Mikula, Michael L. Martini, Mahnoor Shafi, Thirusivapragasam Subramaniam, Arjun S. Sebastian, Brett A. Freedman, and et al. 2024. "Significant Reduction in Bone Density as Measured by Hounsfield Units in Patients with Ankylosing Spondylitis or Diffuse Idiopathic Skeletal Hyperostosis" Journal of Clinical Medicine 13, no. 5: 1430. https://doi.org/10.3390/jcm13051430
APA StyleSwart, A., Hamouda, A., Pennington, Z., Lakomkin, N., Mikula, A. L., Martini, M. L., Shafi, M., Subramaniam, T., Sebastian, A. S., Freedman, B. A., Nassr, A. N., Fogelson, J. L., & Elder, B. D. (2024). Significant Reduction in Bone Density as Measured by Hounsfield Units in Patients with Ankylosing Spondylitis or Diffuse Idiopathic Skeletal Hyperostosis. Journal of Clinical Medicine, 13(5), 1430. https://doi.org/10.3390/jcm13051430







