Displacing the Burden: A Review of Protein-Bound Uremic Toxin Clearance Strategies in Chronic Kidney Disease
Abstract
1. Introduction
2. Definition and Classification of Uremic Toxins
- (a)
- Chemical Identification and Analysis: The compound must be chemically identifiable, with quantitative analysis feasible in biological fluids.
- (b)
- Elevated Levels in Uremia: The total and plasma levels should be higher in uremic subjects than in non-uremic individuals.
- (c)
- Clinical Relevance: Elevated concentrations should correlate with specific uremic dysfunctions and/or symptoms that decrease or disappear when the concentration is reduced.
- (d)
- Biological Activity: There must be evidence of biological activity, consistent with clinical changes observed in uremic syndrome, demonstrated in in vivo, ex vivo, or in vitro studies.
- (e)
- Concentration Consistency: Concentrations in these studies should reflect those found in bodily fluids or tissues of uremic patients.
- Small Hydrophilic Toxins (<500 Da): These include compounds like urea (60 Da) and uric acid. Conventional hemodialysis effectively removes them using diffusion as the primary transport mechanism [12].
- Medium-Sized Toxins (≥500 Da): Examples are β2 microglobulin (11.8 kDa) and parathyroid hormone (9.5 kDa). While convective transport can remove some of these toxins, their size hinders efficient elimination [13].
- Protein-Bound Toxins (PBUTs): This category encompasses molecules with low molecular weight, such as indoxyl sulfate and p-cresyl sulfate, which exhibit more than 80% plasma protein binding. Despite their inherently low molecular weight, clearance is negatively affected due to the lower concentration of unbound toxin at the dialysate side surface of the membrane.
3. Protein-Bound Toxins: Main Types and Molecular Weight
- (a)
- Post-Translational Modifications: Glycation, oxidation, and other changes in albumin can alter its structure and, therefore, its binding capacity. In patients with chronic kidney disease, these modifications are more common and can affect albumin’s transport function [20].
- (b)
- (c)
- Changes in pH and Electrolytes: Variations in the blood pH and electrolyte levels, common in patients with renal insufficiency, can modify albumin’s structure and its affinity for uremic toxins. Albumin’s spatial structures are sensitive to changes in the acid–base balance, common in patients with renal insufficiency, and their tertiary structures change considerably with pH variations [25,26].
4. Effects of Protein-Bound Uremic Toxins
- -
- Endothelial Dysfunction: Endothelial dysfunction caused by PBUTs is closely related to the development of cardiovascular diseases in patients with chronic kidney disease. PBUTs can cause structural damage, inflammation, and a decrease in endothelium-dependent vasodilation [29,30]. Furthermore, endothelial dysfunction is associated with the progression of chronic kidney disease and albuminuria [31]. Patients undergoing dialysis for chronic kidney disease exhibit a markedly diminished endothelial response to stimuli when compared to a control group of healthy individuals. This reduced response is evident across various assessment parameters, including both shear stress and biochemical agents, indicative of compromised endothelial function [32]. PBUTs can decrease nitric oxide production in endothelial cells by inhibiting endothelial nitric oxide synthase (eNOS) activity and expression [33]. PBUTs, like indoxyl sulfate, act as prooxidant and proinflammatory agents, which are associated with changes in the hemostatic system, increased oxidative stress, and monocyte activation. Additionally, this leads to a prothrombotic state through the activation of prothrombotic factors such as tissue factor and factor Xa [34], and the formation of endothelial microparticles.
- -
- Prooxidant and Proinflammatory Actions: Indoxyl sulfate acts as both a prooxidant and proinflammatory agent, linked with changes in the hemostatic system, increased oxidative stress, and monocyte activation. This leads to a prothrombotic state through the activation of prothrombotic factors such as tissue factor and factor Xa [34], and the formation of endothelial microparticles.
- -
- Cardiorenal Syndrome: The accumulation of PBUTs, particularly IS, in cardiomyocytes is linked to increased production of inflammatory cytokines such as IL1, IL6, and TNF-α [38]. These toxins have been associated with pro-arrhythmogenic effects and atrial fibrillation [35]. Studies have also noted structural and functional changes in cardiomyocytes, including reduced spontaneous contraction and irregularity, following exposure to toxins like p-cresol sulfate (PCS) [39].
- -
- Immune System Dysfunction: Patients with chronic kidney disease present immune system dysfunction due to various causes, such as the dialysis process, vitamin D deficiency, and a sustained systemic inflammatory state due to elevated PBUT, which can alter the innate immune response [40]. Among the main PBUTs related to immune system activation are IS, PCS, and p-cresyl glucuronide, among the most well-known [41,42].
Kidney
5. PBUT Clearance Strategies
5.1. Conventional Dialysis Efficacy on Protein-Bound Uremic Toxins (PBUTs)
5.2. Conventional Dialysis: Importance of Dialysis Time
5.3. Importance of Residual Renal Function
5.4. Online Hemodiafiltration: Role of Convection
5.5. Expanded HD
5.6. Adsorptive Therapies
Challenges and Future Directions of Adsorptive Therapies
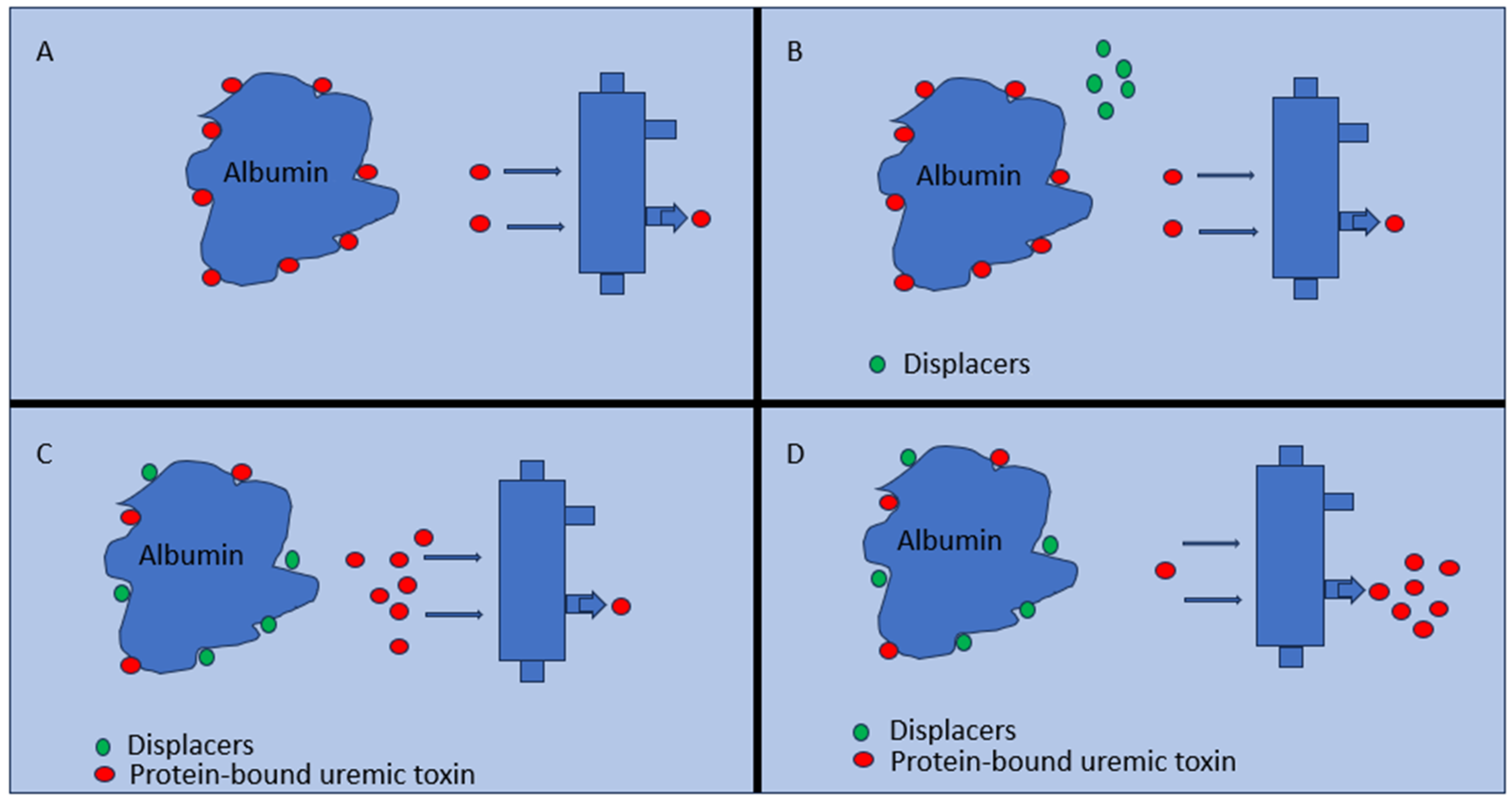
6. Protein-Bound Uremic Toxin Displacers
6.1. The Role of Displacers
6.2. The Affinity for Albumin of Uremic Toxins (UTs)
6.3. Major Described Displacers
- -
- Ibuprofen: A nonsteroidal anti-inflammatory drug (NSAID) with high protein-binding capacity, ibuprofen effectively displaces PBUTs such as p-cresyl sulfate (pCS) and indoxyl sulfate (IS) from albumin. However, its long-term use poses risks like gastrointestinal and renal complications [83]. Cellulose membranes embedded with ibuprofen have been developed, which exhibit a 1.2-fold increase in the removal of protein-bound uremic toxins (PBUTs). This performance is slightly lower than that achieved with ibuprofen perfusion, yet it comes without the associated potential risks [84].
- -
- Furosemide: This diuretic shows a high affinity for albumin and can increase the free fraction of certain UTs like hippuric acid. Combined with ibuprofen, it enhances the displacement of toxins like IS [80].
- -
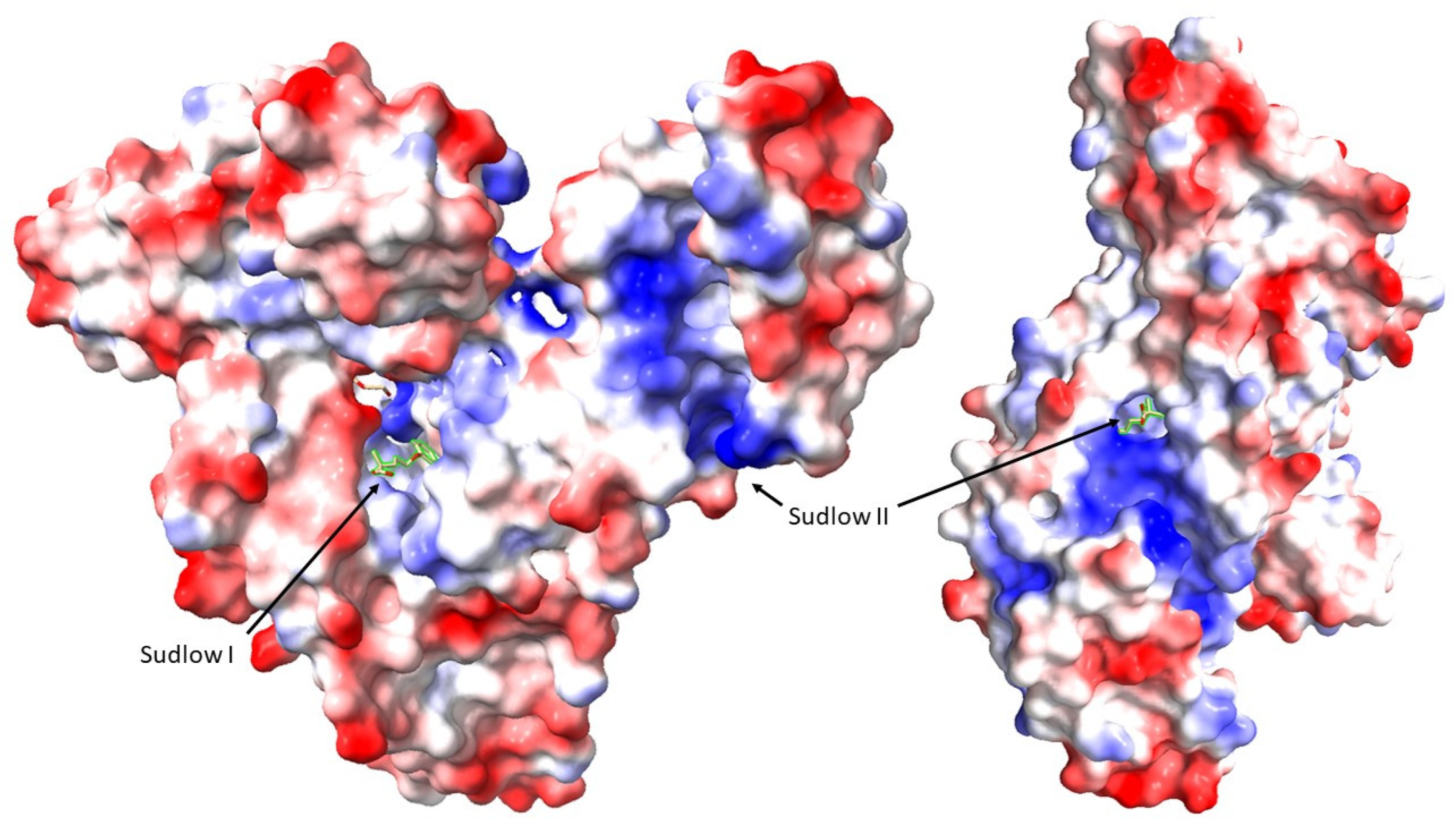
- Tryptophan: Being the precursor of IS through intestinal microbiota metabolism, tryptophan shares structural similarities with some uremic toxins. It can bind to the Sudlow site II on albumin. A concentration of 1 mM of tryptophan increases the free fraction of IS and p-CS by a factor of 2, demonstrating its ability to compete with these toxins for albumin-binding sites [85].
- -
- Non-Esterified Fatty Acids (NEFA): NEFAs have shown a high capacity to increase the free fraction of UTs such as IS and pCS [86]. However, high concentrations of these molecules are required to achieve this effect, which may predispose patients to adverse effects, and in the case of NEFA, there is a high risk of hemolysis at the concentrations necessary for the displacing effect on UTs.
| Displacer | Effect on PBUT Removal | Considerations | References |
|---|---|---|---|
| Ibuprofen (1 mM) | Free fraction of IS and pCS increased by a factor 3 No impact on HA removal | Handling high doses can be a risk for HD patients | [80] |
| Furosemide (1 mM) | Free fraction of IS and pCS increased by a factor of 1.3 HA by a factor of 1.5 | Side effects such as ototoxicity | [80] |
| Ibuprofen + Furosemide | Increased the removal of IS by a factor of 3 and IAA by a factor of 2 | Enhanced PBUT displacement but increased the risk of side effects | [80] |
| Tryptophan (1 mM) | Free fraction of IS and pCS increased by a factor of 2.0 No impact on HA removal | Could increase uremic syndrome | [80,85] |
| Non-esterified fatty acids (NEFAs) | High capacity to increase free fraction of IS and pCS | High doses required Risk of hemolysis | [86] |
| Salvianolic acids | In vitro, increased the dialysis efficiency of IS and pCS by 99.13% and 142.00%, and in vivo (rats), by 135.61% and 272.13% | Need to test these results in patients | [87] |
- -
- Salvianolic Acids: Salvianolic acids, including lithospermic acid (LA), salvianolic acid A (SaA), tanshinol (DSS), caffeic acid (CA), salvianolic acid B (SaB), protocatechuic aldehyde (PA), and rosmarinic acid (RA), are molecules with high affinity for albumin receptors, significantly increasing the free concentration of UTs. This effect depends on their plasma concentration [87].
6.4. Efficacy and Safety
6.5. Future Directions for Displacer Use
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A Single Number for Advocacy and Communication-Worldwide More than 850 Million Individuals Have Kidney Diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Cockwell, P.; Fisher, L.-A. The Global Burden of Chronic Kidney Disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting Life Expectancy, Years of Life Lost, and All-Cause and Cause-Specific Mortality for 250 Causes of Death: Reference and Alternative Scenarios for 2016-40 for 195 Countries and Territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. European Uremic Toxin Work Group Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Nutrients Turned into Toxins: Microbiota Modulation of Nutrient Properties in Chronic Kidney Disease. Nutrients 2017, 9, 489. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef]
- Popkov, V.A.; Silachev, D.N.; Zalevsky, A.O.; Zorov, D.B.; Plotnikov, E.Y. Mitochondria as a Source and a Target for Uremic Toxins. Int. J. Mol. Sci. 2019, 20, 3094. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on Uremic Toxins: Classification, Concentration, and Interindividual Variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An Update on Uremic Toxins. Int. Urol. Nephrol. 2013, 45, 139–150. [Google Scholar] [CrossRef]
- Clark, W.R.; Dehghani, N.L.; Narsimhan, V.; Ronco, C. Uremic Toxins and Their Relation to Dialysis Efficacy. Blood Purif. 2019, 48, 299–314. [Google Scholar] [CrossRef]
- List of Uremic Solutes—Uremic Solutes Database. Available online: https://database.uremic-toxins.org/soluteList.php (accessed on 4 December 2023).
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tian, H.; Wang, Y.; Shen, Y.; Zhu, Q.; Ding, F. Effect of Ionic Strength, pH and Chemical Displacers on the Percentage Protein Binding of Protein-Bound Uremic Toxins. Blood Purif. 2019, 47, 351–360. [Google Scholar] [CrossRef]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic Kidney Disease and the Gut Microbiome. Am. J. Physiol. Renal Physiol. 2019, 316, F1211–F1217. [Google Scholar] [CrossRef]
- Kragh-Hansen, U.; Chuang, V.T.G.; Otagiri, M. Practical Aspects of the Ligand-Binding and Enzymatic Properties of Human Serum Albumin. Biol. Pharm. Bull. 2002, 25, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Murugan, N.A.; Saraswathi, N.T. Advanced Glycation End Products Modulate Structure and Drug Binding Properties of Albumin. Mol. Pharm. 2015, 12, 3312–3322. [Google Scholar] [CrossRef]
- Viaene, L.; Annaert, P.; de Loor, H.; Poesen, R.; Evenepoel, P.; Meijers, B. Albumin Is the Main Plasma Binding Protein for Indoxyl Sulfate and P-Cresyl Sulfate. Biopharm. Drug Dispos. 2013, 34, 165–175. [Google Scholar] [CrossRef]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The Characterization of Two Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar]
- Meijers, B.K.I.; De Loor, H.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. P-Cresyl Sulfate and Indoxyl Sulfate in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1932–1938. [Google Scholar] [CrossRef]
- Sakai, T.; Takadate, A.; Otagiri, M. Characterization of Binding Site of Uremic Toxins on Human Serum Albumin. Biol. Pharm. Bull. 1995, 18, 1755–1761. [Google Scholar] [CrossRef]
- Zaidi, N.; Ahmad, E.; Rehan, M.; Rabbani, G.; Ajmal, M.R.; Zaidi, Y.; Subbarao, N.; Khan, R.H. Biophysical Insight into Furosemide Binding to Human Serum Albumin: A Study to Unveil Its Impaired Albumin Binding in Uremia. J. Phys. Chem. B 2013, 117, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Sasahara, K.; Domon, M.; Yamaguchi, K.; Ito, T.; Goto, S.; Goto, Y.; Narita, I. pH-Dependent Protein Binding Properties of Uremic Toxins In Vitro. Toxins 2021, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Bulavin, L.A.; Khorolskyi, O.V. Concentration Dependences of Macromolecular Sizes in Aqueous Solutions of Albumins. Ukr. J. Phys. 2020, 65, 619. [Google Scholar] [CrossRef]
- Liberi, S.; Linciano, S.; Moro, G.; De Toni, L.; Cendron, L.; Angelini, A. Structural Analysis of Human Serum Albumin in Complex with the Fibrate Drug Gemfibrozil. Int. J. Mol. Sci. 2022, 23, 1769. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-H.; Yu, M.-C.; Wei, M.-J.; Kuo, K.-L. The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease. Toxins 2021, 13, 573. [Google Scholar] [CrossRef]
- Zhu, Y.-B.; Zhang, Y.-P.; Zhang, J.; Zhang, Y.-B. Evaluation of Vitamin C Supplementation on Kidney Function and Vascular Reactivity Following Renal Ischemic Injury in Mice. Kidney Blood Press. Res. 2016, 41, 460–470. [Google Scholar] [CrossRef]
- Vila Cuenca, M.; van Bezu, J.; Beelen, R.H.J.; Vervloet, M.G.; Hordijk, P.L. Stabilization of Cell-Cell Junctions by Active Vitamin D Ameliorates Uraemia-Induced Loss of Human Endothelial Barrier Function. Nephrol. Dial. Transplant. 2019, 34, 252–264. [Google Scholar] [CrossRef]
- Seliger, S.L.; Salimi, S.; Pierre, V.; Giffuni, J.; Katzel, L.; Parsa, A. Microvascular Endothelial Dysfunction Is Associated with Albuminuria and CKD in Older Adults. BMC Nephrol. 2016, 17, 82. [Google Scholar] [CrossRef]
- Alexandrou, M.-E.; Gkaliagkousi, Ε.; Loutradis, C.; Dimitriadis, C.; Mitsopoulos, E.; Lazaridis, A.; Nikolaidou, B.; Dolgiras, P.; Douma, S.; Papagianni, A.; et al. Haemodialysis and Peritoneal Dialysis Patients Have Severely Impaired Post-Occlusive Skin Forearm Vasodilatory Response Assessed with Laser Speckle Contrast Imaging. Clin. Kidney J. 2021, 14, 1419–1427. [Google Scholar] [CrossRef]
- Tumur, Z.; Niwa, T. Indoxyl Sulfate Inhibits Nitric Oxide Production and Cell Viability by Inducing Oxidative Stress in Vascular Endothelial Cells. Am. J. Nephrol. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic Uremic Solutes Increase Tissue Factor Production in Endothelial Cells by the Aryl Hydrocarbon Receptor Pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef]
- Lekawanvijit, S. Cardiotoxicity of Uremic Toxins: A Driver of Cardiorenal Syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.C.; Dinatale, B.C.; Murray, I.A.; Flaveny, C.A.; Liu, Q.; Laurenzana, E.M.; Lin, J.M.; Strom, S.C.; Omiecinski, C.J.; Amin, S.; et al. The Uremic Toxin 3-Indoxyl Sulfate Is a Potent Endogenous Agonist for the Human Aryl Hydrocarbon Receptor. Biochemistry 2010, 49, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Poitevin, S.; Sallée, M.; Addi, T.; Gondouin, B.; McKay, N.; Denison, M.S.; Jourde-Chiche, N.; Duval-Sabatier, A.; Cerini, C.; et al. Aryl Hydrocarbon Receptor Is Activated in Patients and Mice with Chronic Kidney Disease. Kidney Int. 2018, 93, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Krum, H. Cardiorenal Syndrome: Role of Protein-Bound Uremic Toxins. J. Ren. Nutr. 2015, 25, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-S.; Ding, H.-C.; Lin, Y.-T.; Syu, J.-P.; Chen, Y.; Wang, S.-M. Uremic Toxin P-Cresol Induces Disassembly of Gap Junctions of Cardiomyocytes. Toxicology 2012, 302, 11–17. [Google Scholar] [CrossRef]
- Azevedo, M.L.V.; Bonan, N.B.; Dias, G.; Brehm, F.; Steiner, T.M.; Souza, W.M.; Stinghen, A.E.M.; Barreto, F.C.; Elifio-Esposito, S.; Pecoits-Filho, R.; et al. P-Cresyl Sulfate Affects the Oxidative Burst, Phagocytosis Process, and Antigen Presentation of Monocyte-Derived Macrophages. Toxicol. Lett. 2016, 263, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Vanholder, R. Special Issue: Immune Dysfunction in Uremia. Toxins 2021, 13, 70. [Google Scholar] [CrossRef]
- Fujii, H.; Goto, S.; Fukagawa, M. Role of Uremic Toxins for Kidney, Cardiovascular, and Bone Dysfunction. Toxins 2018, 10, 202. [Google Scholar] [CrossRef]
- Niwa, T. Uremic Toxicity of Indoxyl Sulfate. Nagoya J. Med. Sci. 2010, 72, 1–11. [Google Scholar]
- Sun, C.-Y.; Hsu, H.-H.; Wu, M.-S. P-Cresol Sulfate and Indoxyl Sulfate Induce Similar Cellular Inflammatory Gene Expressions in Cultured Proximal Renal Tubular Cells. Nephrol. Dial. Transplant. 2013, 28, 70–78. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. P-Cresyl Sulfate Causes Renal Tubular Cell Damage by Inducing Oxidative Stress by Activation of NADPH Oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef]
- Krieter, D.H.; Hackl, A.; Rodriguez, A.; Chenine, L.; Moragues, H.L.; Lemke, H.-D.; Wanner, C.; Canaud, B. Protein-Bound Uraemic Toxin Removal in Haemodialysis and Post-Dilution Haemodiafiltration. Nephrol. Dial. Transplant. 2010, 25, 212–218. [Google Scholar] [CrossRef]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-Bound Uremic Toxins in Hemodialysis Patients Measured by Liquid Chromatography/Tandem Mass Spectrometry and Their Effects on Endothelial ROS Production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Donadio, C.; Tramonti, G. Progress in Hemodialysis: From Emergent Biotechnology to Clinical Practice; IntechOpen Ltd.: London, UK, 2011; ISBN 978-953-307-377-4. Available online: https://www.intechopen.com/books/353 (accessed on 4 December 2023).
- Fagugli, R.M.; De Smet, R.; Buoncristiani, U.; Lameire, N.; Vanholder, R. Behavior of Non-Protein-Bound and Protein-Bound Uremic Solutes during Daily Hemodialysis. Am. J. Kidney Dis. 2002, 40, 339–347. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, M.K.; Middel, I.R.; Vernooij, R.W.M.; Bots, M.L.; Verhaar, M.C.; Masereeuw, R.; Grooteman, M.P.; Nubé, M.J.; van den Dorpel, M.A.; Blankestijn, P.J.; et al. Protein-Bound Uremic Toxins in Hemodialysis Patients Relate to Residual Kidney Function, Are Not Influenced by Convective Transport, and Do Not Relate to Outcome. Toxins 2020, 12, 234. [Google Scholar] [CrossRef]
- Cupisti, A.; Bolasco, P.; D’Alessandro, C.; Giannese, D.; Sabatino, A.; Fiaccadori, E. Protection of Residual Renal Function and Nutritional Treatment: First Step Strategy for Reduction of Uremic Toxins in End-Stage Kidney Disease Patients. Toxins 2021, 13, 289. [Google Scholar] [CrossRef]
- Tiong, M.K.; Krishnasamy, R.; Smith, E.R.; Hutchison, C.A.; Ryan, E.G.; Pascoe, E.M.; Hawley, C.M.; Hewitson, T.D.; Jardine, M.J.; Roberts, M.A.; et al. Effect of a Medium Cut-off Dialyzer on Protein-Bound Uremic Toxins and Mineral Metabolism Markers in Patients on Hemodialysis. Hemodial. Int. 2021, 25, 322–332. [Google Scholar] [CrossRef]
- Maheshwari, V.; Thijssen, S.; Tao, X.; Fuertinger, D.H.; Kappel, F.; Kotanko, P. In Silico Comparison of Protein-Bound Uremic Toxin Removal by Hemodialysis, Hemodiafiltration, Membrane Adsorption, and Binding Competition. Sci. Rep. 2019, 9, 909. [Google Scholar] [CrossRef]
- Tetali, S.D.; Jankowski, V.; Luetzow, K.; Kratz, K.; Lendlein, A.; Jankowski, J. Adsorption Capacity of Poly(Ether Imide) Microparticles to Uremic Toxins. Clin. Hemorheol. Microcirc. 2016, 61, 657–665. [Google Scholar] [CrossRef]
- Falconi, C.A.; Junho, C.V.C.; Fogaça-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef]
- Ronco, C.; Clark, W.R. Haemodialysis Membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Neri, M.; Lorenzin, A.; Garzotto, F.; Clark, W.R. Multidimensional Classification of Dialysis Membranes. Contrib. Nephrol. 2017, 191, 115–126. [Google Scholar] [CrossRef]
- Deltombe, O.; Van Biesen, W.; Glorieux, G.; Massy, Z.; Dhondt, A.; Eloot, S. Exploring Protein Binding of Uremic Toxins in Patients with Different Stages of Chronic Kidney Disease and during Hemodialysis. Toxins 2015, 7, 3933–3946. [Google Scholar] [CrossRef]
- Schulman, G.; Agarwal, R.; Acharya, M.; Berl, T.; Blumenthal, S.; Kopyt, N. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study of AST-120 (Kremezin) in Patients with Moderate to Severe CKD. Am. J. Kidney Dis. 2006, 47, 565–577. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kazama, J.J.; Omori, K.; Matsuo, K.; Takahashi, Y.; Kawamura, K.; Matsuto, T.; Watanabe, H.; Maruyama, T.; Narita, I. Continuous Reduction of Protein-Bound Uraemic Toxins with Improved Oxidative Stress by Using the Oral Charcoal Adsorbent AST-120 in Haemodialysis Patients. Sci. Rep. 2015, 5, 14381. [Google Scholar] [CrossRef]
- Meijers, B.K.; Weber, V.; Bammens, B.; Dehaen, W.; Verbeke, K.; Falkenhagen, D.; Evenepoel, P. Removal of the Uremic Retention Solute P-Cresol Using Fractionated Plasma Separation and Adsorption. Artif. Organs 2008, 32, 214–219. [Google Scholar] [CrossRef]
- Brettschneider, F.; Tölle, M.; von der Giet, M.; Passlick-Deetjen, J.; Steppan, S.; Peter, M.; Jankowski, V.; Krause, A.; Kühne, S.; Zidek, W.; et al. Removal of Protein-Bound, Hydrophobic Uremic Toxins by a Combined Fractionated Plasma Separation and Adsorption Technique. Artif. Organs 2013, 37, 409–416. [Google Scholar] [CrossRef]
- Pavlenko, D.; Giasafaki, D.; Charalambopoulou, G.; van Geffen, E.; Gerritsen, K.G.F.; Steriotis, T.; Stamatialis, D. Carbon Adsorbents With Dual Porosity for Efficient Removal of Uremic Toxins and Cytokines from Human Plasma. Sci. Rep. 2017, 7, 14914. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Cosola, C.; di Bari, I.; Magnani, S.; Galleggiante, V.; Scandiffio, L.; Dalfino, G.; Netti, G.S.; Atti, M.; Corciulo, R.; et al. Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-Cresol Sulfate in Hemodialysis Patients: Results From an In Vitro Study and An In Vivo Pilot Trial (Xuanro4-Nature 3.2). Toxins 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of Increasing Dietary Fiber on Plasma Levels of Colon-Derived Solutes in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Hai, X.; Landeras, V.; Dobre, M.A.; DeOreo, P.; Meyer, T.W.; Hostetter, T.H. Mechanism of Prominent Trimethylamine Oxide (TMAO) Accumulation in Hemodialysis Patients. PLoS ONE 2015, 10, e0143731. [Google Scholar] [CrossRef] [PubMed]
- Krieter, D.H.; Kerwagen, S.; Rüth, M.; Lemke, H.-D.; Wanner, C. Differences in Dialysis Efficacy Have Limited Effects on Protein-Bound Uremic Toxins Plasma Levels over Time. Toxins 2019, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, S.H.; Jung, S.W.; Jung, G.T.; Lim, H.J.; Kim, K.P.; Jo, Y.-I.; Jin, K.; Moon, J.Y. The Medium Cut-Off Membrane Does Not Lower Protein-Bound Uremic Toxins. Toxins 2022, 14, 779. [Google Scholar] [CrossRef]
- Magnani, S.; Atti, M. Uremic Toxins and Blood Purification: A Review of Current Evidence and Future Perspectives. Toxins 2021, 13, 246. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sato, M.; Sato, Y.; Wakamatsu, T.; Takahashi, Y.; Iguchi, A.; Omori, K.; Suzuki, Y.; Ei, I.; Kaneko, Y.; et al. Adsorption of Protein-Bound Uremic Toxins Through Direct Hemoperfusion With Hexadecyl-Immobilized Cellulose Beads in Patients Undergoing Hemodialysis. Artif. Organs 2018, 42, 88–93. [Google Scholar] [CrossRef]
- Lee, S.; Sirich, T.L.; Meyer, T.W. Improving Clearance for Renal Replacement Therapy. Kidney360 2021, 2, 1188–1195. [Google Scholar] [CrossRef]
- Bammens, B.; Evenepoel, P.; Verbeke, K.; Vanrenterghem, Y. Removal of the Protein-Bound Solute p-Cresol by Convective Transport: A Randomized Crossover Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2004, 44, 278–285. [Google Scholar] [CrossRef]
- Cornelis, T.; Eloot, S.; Vanholder, R.; Glorieux, G.; van der Sande, F.M.; Scheijen, J.L.; Leunissen, K.M.; Kooman, J.P.; Schalkwijk, C.G. Protein-Bound Uraemic Toxins, Dicarbonyl Stress and Advanced Glycation End Products in Conventional and Extended Haemodialysis and Haemodiafiltration. Nephrol. Dial. Transplant. 2015, 30, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Luo, F.J.-G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Selectively Increasing the Clearance of Protein-Bound Uremic Solutes. Nephrol. Dial. Transplant. 2012, 27, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Funk, B.A.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Prominent Accumulation in Hemodialysis Patients of Solutes Normally Cleared by Tubular Secretion. J. Am. Soc. Nephrol. 2014, 25, 615–622. [Google Scholar] [CrossRef]
- Sandeman, S.R.; Zheng, Y.; Ingavle, G.C.; Howell, C.A.; Mikhalovsky, S.V.; Basnayake, K.; Boyd, O.; Davenport, A.; Beaton, N.; Davies, N. A Haemocompatible and Scalable Nanoporous Adsorbent Monolith Synthesised Using a Novel Lignin Binder Route to Augment the Adsorption of Poorly Removed Uraemic Toxins in Haemodialysis. Biomed. Mater. 2017, 12, 035001. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Peattie, J.W.T.; Miller, J.D.; Dinh, D.C.; Recht, N.S.; Walther, J.L.; Hostetter, T.H. Increasing the Clearance of Protein-Bound Solutes by Addition of a Sorbent to the Dialysate. J. Am. Soc. Nephrol. 2007, 18, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. Clin. J. Am. Soc. Nephrol. 2019, 14, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sirich, T.L.; Meyer, T.W. Improving Solute Clearances by Hemodialysis. Blood Purif. 2022, 51, 20–31. [Google Scholar] [CrossRef]
- Tao, X.; Thijssen, S.; Kotanko, P.; Ho, C.-H.; Henrie, M.; Stroup, E.; Handelman, G. Improved Dialytic Removal of Protein-Bound Uraemic Toxins with Use of Albumin Binding Competitors: An in Vitro Human Whole Blood Study. Sci. Rep. 2016, 6, 23389. [Google Scholar] [CrossRef]
- Carter, D.C.; Ho, J.X. Structure of Serum Albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [CrossRef]
- Maheshwari, V.; Tao, X.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review. Toxins 2021, 13, 622. [Google Scholar] [CrossRef]
- Rodrigues, F.S.C.; Faria, M. Adsorption- and Displacement-Based Approaches for the Removal of Protein-Bound Uremic Toxins. Toxins 2023, 15, 110. [Google Scholar] [CrossRef]
- Rodrigues, F.S.C.; Brilhante, D.; Macêdo, A.; Pires, R.F.; Faria, M. Ibuprofen-Immobilized Thin Films: A Novel Approach to Improve the Clearance of Protein-Bound Uremic Toxins. ACS Appl. Mater. Interfaces 2024, 16, 6589–6604. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, A.; Mingrone, G.; Gandolfi, A.; Greco, A.V.; Ringoir, S.; Vanholder, R. Binding of Indole-3-Acetic Acid to Human Serum Albumin and Competition with L-Tryptophan. Clin. Chim. Acta 1997, 265, 183–192. [Google Scholar] [CrossRef]
- de Loor, H.; Meijers, B.K.I.; Meyer, T.W.; Bammens, B.; Verbeke, K.; Dehaen, W.; Evenepoel, P. Sodium Octanoate to Reverse Indoxyl Sulfate and P-Cresyl Sulfate Albumin Binding in Uremic and Normal Serum during Sample Preparation Followed by Fluorescence Liquid Chromatography. J. Chromatogr. A 2009, 1216, 4684–4688. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xu, X.; Cao, W.; Shen, Z.; Wang, N.; Leng, J.; Zou, N.; Shang, E.; Zhu, Z.; et al. Improved Dialysis Removal of Protein-Bound Uremic Toxins by Salvianolic Acids. Phytomedicine 2019, 57, 166–173. [Google Scholar] [CrossRef]
- Saar-Kovrov, V.; Zidek, W.; Orth-Alampour, S.; Fliser, D.; Jankowski, V.; Biessen, E.A.L.; Jankowski, J. Reduction of Protein-Bound Uraemic Toxins in Plasma of Chronic Renal Failure Patients: A Systematic Review. J. Intern. Med. 2021, 290, 499–526. [Google Scholar] [CrossRef] [PubMed]
- Florens, N.; Yi, D.; Juillard, L.; Soulage, C.O. Using Binding Competitors of Albumin to Promote the Removal of Protein-Bound Uremic Toxins in Hemodialysis: Hope or Pipe Dream? Biochimie 2018, 144, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Uremic Toxin | Albumin Binding % |
|---|---|
| Indoxyl Sulfate (IS) | 90–95% |
| p-Cresyl Sulfate (pCS) | 90–95% |
| Hippuric Acid (HA) | >40% |
| Indole-3-Acetic (IAA) | >30% |
| 3-Carboxy-4-Methyl-5-Propyl-2-Furanpropionate (CMPF) | >40% |
| Clearance Strategy | Tested PBUT | Clearance Efficiency | Clinical Effects and Conclusions | Ref. |
|---|---|---|---|---|
| Dialysis Techniques | ||||
| Convectional HD | pCS and IS | Less than 50% | Alternative strategies promise to be more efficient | [48] |
| pCS, IS and CMPF | 29%, 32% and 0%, respectively | [49] | ||
| pCS, IS and inorganic phosphate | No significant clearance | Need to focus on different approaches | [50] | |
| Prolonged Convectional HD | pCS, IS, IAA, CMPF and HA | IAA, IS and pCS at the borderline of significance | [47] | |
| pCS | Significantly less than other soluble molecules | Convection can provide superior protein-bound solute removal compared with high-flux HD | [52] | |
| Residual Renal Function | IS, pCS, IAA, HA, p-cresyl glucuronide, kynurenine, kynurenic acid | Only IS decreased by 8.0% | RRF is an important determinant of PBUT plasma concentrations in HD patients | [53] |
| pCS and IS | 1.7% and 2%, respectively | The implementation of theOWHD plus LPD strategy may be useful for lowering PBUTs | [54] | |
| IS, pCS, HA and phenylacetylglutamine | Significantly less than the rates of urea and creatinine | An increase in treatment frequency would be required to significantly reduce the plasma levels of PBUTs | [55] | |
| Online HDF | pCS and IS | Free IS and free and total pCS remained unaltered | Current HDF techniques have only limited impact on IS and pCS plasma levels in the short and also long term | [56] |
| Expanded HD | IS and pCS | No statistically significant clearance | The clearance did not differ between the HF-HD, post-OL-HDF, and MCO-HD | [57] |
| pCS and IS | No statistically significant clearance | [58] | ||
| Adsorptive Therapies | ||||
| Oral absorbents (AST-120) | IS | Dose dependent decreased levels | To determine whether this effect can attenuate the progression of CKD | [59] |
| pCS, IS and phenyl sulfate | Reduction of IS (total 45.7%; free 70.4%) pCS (total 31.1%: free, 63.5%) and phenyl sulfate (free 50.6%) | AST-120 has additive effects on the continuous reduction of some PBUTs in anuric patients in HD | [60] | |
| Activated charcoal | pCS and IS | Increase in the clearance of protein-bound solutes without altering the clearance of unbound solutes | Increasing the dialysate flow without the addition of sorbent, had a similar effect | [61] |
| Hexadecyl-immobilized cellulose bead (HICB) | IS, pCS, IAA and phenyl sulfate | 34% decrease in free form, no change in total | Need to develop more effective materials to adsorb PBUTs selectively | [62] |
| Ordered nanoporous adsorbent material (CMK-3 type) | IS and HA | Significant reduction in the free form but not the total form | The IS removal is slightly lower than the corresponding one for HA | [63] |
| Divinylbenzene-polyvinylpyrrolidone (DVB-PVP) | IS and pCS | In vitro 54% IS and 56% PCS, In vivo efficient only for IS plasma levels | Symbiotic treatment with DVB-PVP HD decreased IS and pCS; this study provides the first line of evidence on the synergistic action of gut microbiota modulation and an absorption-based approach | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ospina, D.; Mas-Fontao, S.; Gracia-Iguacel, C.; Avello, A.; González de Rivera, M.; Mujika-Marticorena, M.; Gonzalez-Parra, E. Displacing the Burden: A Review of Protein-Bound Uremic Toxin Clearance Strategies in Chronic Kidney Disease. J. Clin. Med. 2024, 13, 1428. https://doi.org/10.3390/jcm13051428
Sánchez-Ospina D, Mas-Fontao S, Gracia-Iguacel C, Avello A, González de Rivera M, Mujika-Marticorena M, Gonzalez-Parra E. Displacing the Burden: A Review of Protein-Bound Uremic Toxin Clearance Strategies in Chronic Kidney Disease. Journal of Clinical Medicine. 2024; 13(5):1428. https://doi.org/10.3390/jcm13051428
Chicago/Turabian StyleSánchez-Ospina, Didier, Sebastián Mas-Fontao, Carolina Gracia-Iguacel, Alejandro Avello, Marina González de Rivera, Maddalen Mujika-Marticorena, and Emilio Gonzalez-Parra. 2024. "Displacing the Burden: A Review of Protein-Bound Uremic Toxin Clearance Strategies in Chronic Kidney Disease" Journal of Clinical Medicine 13, no. 5: 1428. https://doi.org/10.3390/jcm13051428
APA StyleSánchez-Ospina, D., Mas-Fontao, S., Gracia-Iguacel, C., Avello, A., González de Rivera, M., Mujika-Marticorena, M., & Gonzalez-Parra, E. (2024). Displacing the Burden: A Review of Protein-Bound Uremic Toxin Clearance Strategies in Chronic Kidney Disease. Journal of Clinical Medicine, 13(5), 1428. https://doi.org/10.3390/jcm13051428







