Cardiac Mechanics and Valvular and Vascular Abnormalities in Hypereosinophilic Syndrome
Abstract
1. Hypereosinophilic Syndrome and the Heart
- −
- hereditary (familial clustering, pathogenesis unknown);
- −
- primary (clonal, neoplastic, according to WHO criteria, underlying stem cell, myeloid or eosinophilic neoplasm, and eosinophils are considered neoplastic cells);
- −
- secondary (reactive, non-clonal, including infections, asthma, and allergies, parasitic infestations, cytokine infusions, respiratory diseases, vasculitis, drug reactions non-hematological malignant diseases, connective tissue diseases, and non-Hodgkin’s and Hodgkin’s lymphomas);
- −
- hypereosionophilia of an undetermined significance.
- (1)
- Necrotic (acute) stage: characterized by the infiltration and deposition of eosinophil cells, resulting in cytokine-mediated damage to the endocardium,
- (2)
- Thrombotic stage: during which a layered thrombus is formed as a result of the activation of the tissue factor by the eosinophil cells accumulating in the myocardium;
- (3)
- Fibrotic stage: the stage of myocardial fibrosis and its consequential wall stiffness.
2. Cardiovascular Imaging and MAGYAR-Path Study
3. The Left Heart and the Aorta
3.1. Left Ventricle
3.1.1. Under Healthy Circumstances
3.1.2. In the Hypereosinophilic Syndrome
LV Structure, Volumes, and Hypertrophy
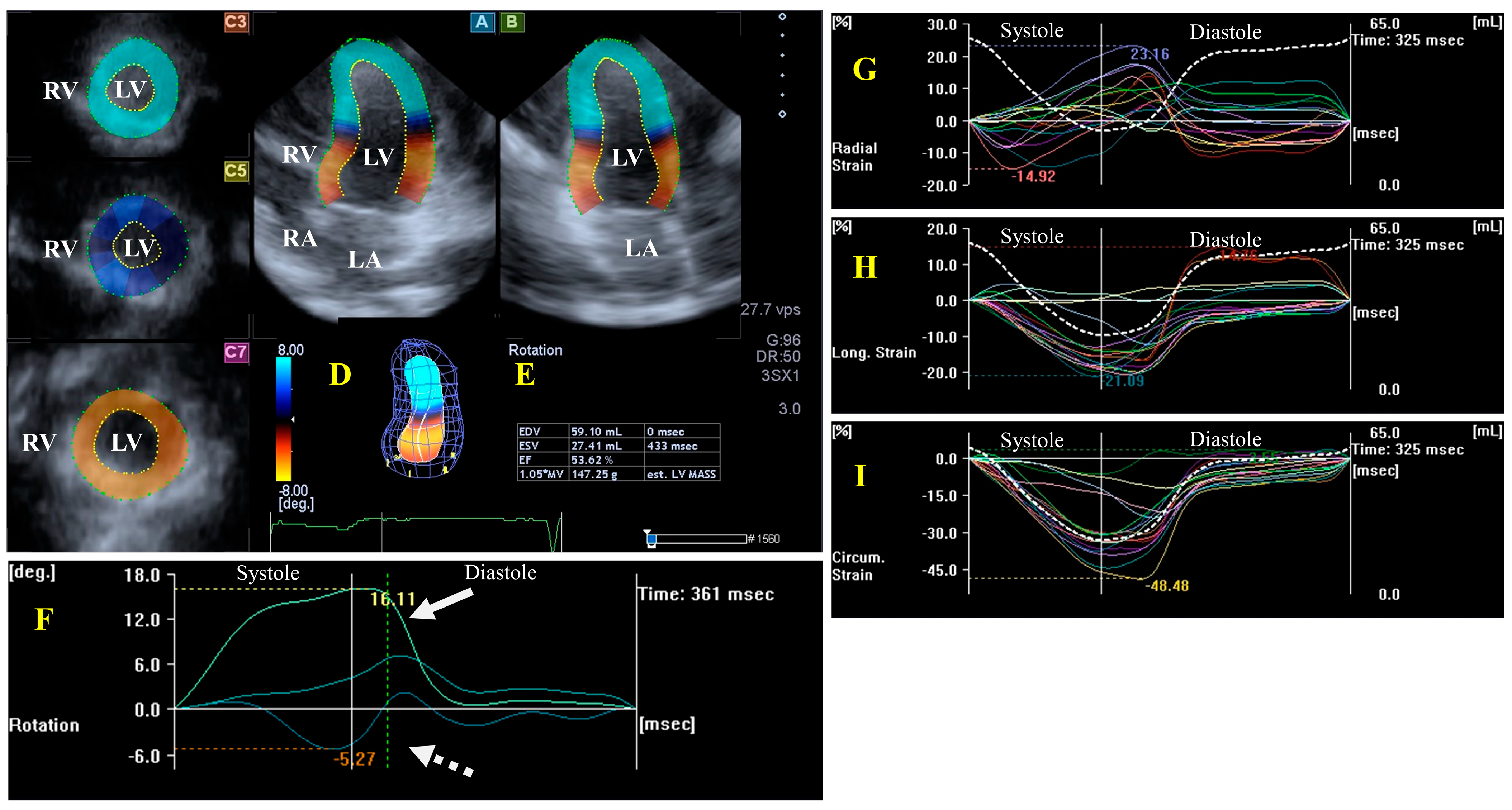
LV Functional Properties, Strains, and Rotational Mechanics
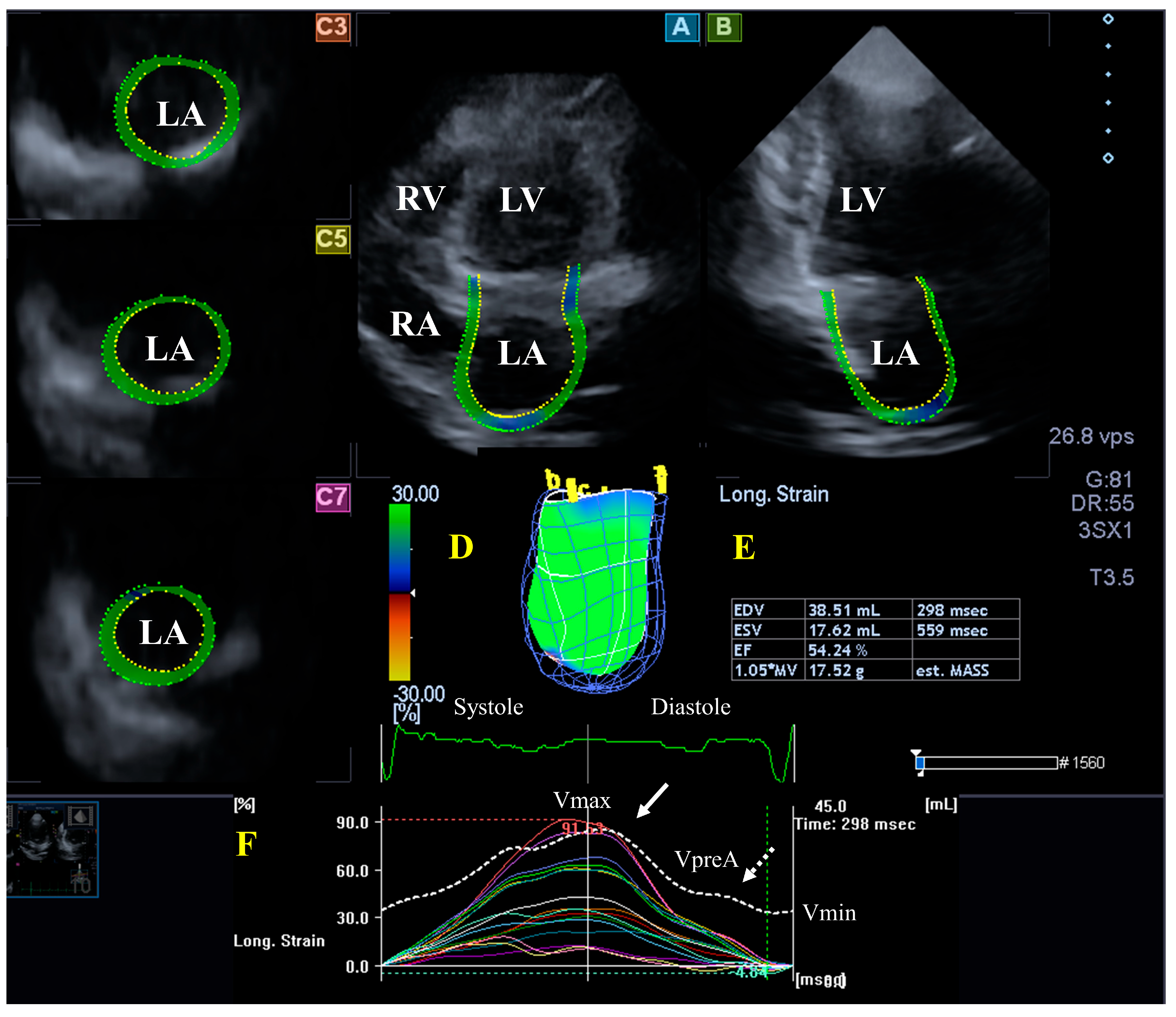
3.2. Left Atrium
3.2.1. Under Healthy Circumstances
3.2.2. In the Hypereosinophilic Syndrome
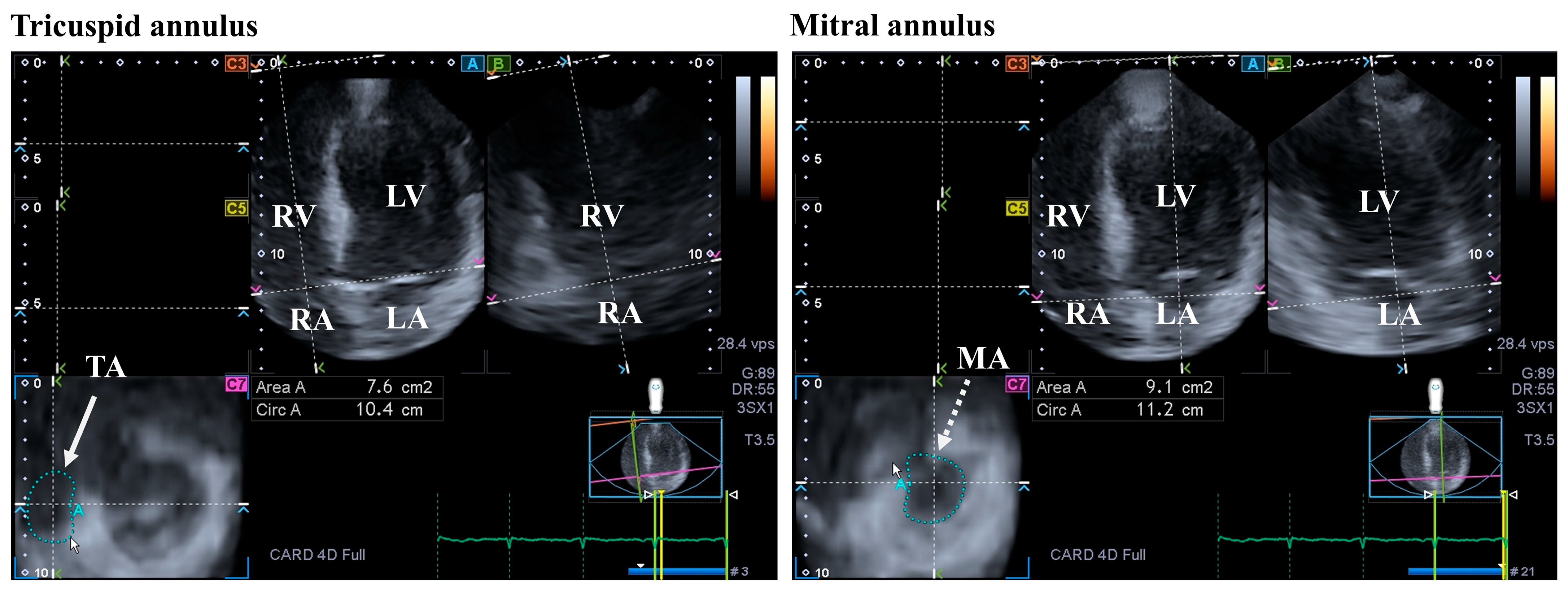
3.3. Mitral Valve
3.3.1. Under Healthy Circumstances
3.3.2. In the Hypereosinophilic Syndrome
3.4. Aortic Valve
3.4.1. Under Healthy Circumstances
3.4.2. In the Hypereosinophilic Syndrome
3.5. Aorta
3.5.1. Under Healthy Circumstances
3.5.2. In the Hypereosinophilic Syndrome
4. The Right Heart and the Pulmonary Artery
4.1. Right Ventricle
4.1.1. Under Healthy Circumstances
4.1.2. In the Hypereosinophilic Syndrome
4.2. Right Atrium
4.2.1. In Healthy Subjects
4.2.2. In the Hypereosinophilic Syndrome
4.3. Tricuspid Valve
4.3.1. In Healthy Circumstances
4.3.2. In the Hypereosinophilic Syndrome
4.4. Pulmonary Valve
4.4.1. Under Healthy Circumstances
4.4.2. In the Hypereosinophilic Syndrome
4.5. Pulmonary Artery
4.5.1. Under Healthy Circumstances
4.5.2. In the Hypereosinophilic Syndrome
5. Pathophysiologic Background
6. Clinical Implications
7. Conclusions
Funding
Conflicts of Interest
References
- Reiter, A.; Gotlib, J. Myeloid neoplasms with eosinophilia. Blood 2017, 129, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Klion, A.D.; Horny, H.P.; Roufosse, F.; Gotlib, J.; Weller, P.F.; Hellmann, A.; Metzgeroth, G.; Leiferman, K.M.; Arock, M.; et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012, 130, 607–612.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A.; Orazi, A.; Gotlib, J.; Reiter, A.; Tzankov, A.; Hasserjian, R.P.; Arber, D.A.; Tefferi, A. The international consensus classification of eosinophilic disorders and systemic mastocytosis. Am. J. Hematol. 2023, 98, 1286–1306. [Google Scholar] [CrossRef] [PubMed]
- Brito-Babapulle, F. The eosinophilias, including the idiopathic hypereosinophilic syndrome. Br. J. Haematol. 2003, 121, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Hardy, W.R.; Anderson, R.E. The hypereosinophilic syndromes. Ann. Intern. Med. 1968, 68, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, J.E. Heart disease and the eosinophil. N. Engl. J. Med. 1990, 323, 1560–1561. [Google Scholar] [CrossRef] [PubMed]
- Kleinfeldt, T.; Nienaber, C.A.; Kische, S.; Akin, I.; Turan, R.G.; Körber, T.; Schneider, H.; Ince, H. Cardiac manifestation of the hypereosinophilic syndrome: New insights. Clin. Res. Cardiol. 2010, 99, 419–427. [Google Scholar] [CrossRef]
- Parrillo, J.E.; Borer, J.S.; Henry, W.L.; Wolff, S.M.; Fauci, A.S. The cardiovascular manifestations of the hypereosinophilic syndrome. Prospective study of 26 patients, with review of the literature. Am. J. Med. 1979, 67, 572–582. [Google Scholar] [CrossRef]
- Al-Kaisey, A.M.; Meher-Homji, Z.; Hayward, P.; Jones, E. Mitral and tricuspid valve repair in hypereosinophilic syndrome. BMJ Case Rep. 2019, 12, e228951. [Google Scholar] [CrossRef]
- Löffler, W. Scientific raisins from 125 years SMW (Swiaa Medical Weekly). 2nd international medical week dedicated in Switzerland. Luzern, 31 August–5 September 1936. Fibroplastic parietal endocarditis with eosinophilia. An unusual disease. 1936. Schweiz Med. Wochenschr. 1995, 125, 1837–1840. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef] [PubMed]
- Kormányos, Á.; Domsik, P.; Kalapos, A.; Marton, I.; Földeák, D.; Modok, S.; Gyenes, N.; Borbényi, Z.; Nemes, A. Left ventricular deformation in cardiac light-chain amyloidosis and hypereosinophilic syndrome. Results from the MAGYAR-Path Study. Orv. Hetil. 2020, 161, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Ambrus, N.; Modok, S.; Borbényi, Z.; Marton, I. Left ventricular rotational mechanics in hypereosinophilic syndrome—Analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Echocardiography 2019, 36, 2064–2069. [Google Scholar] [CrossRef]
- Nemes, A.; Kalapos, A.; Domsik, P.; Marton, I.; Borbényi, Z.; Forster, T. Three-dimensional speckle-tracking echocardiography in Loeffler endocarditis: Case report from the MAGYAR-Path Study. Herz 2014, 39, 722–724. [Google Scholar] [CrossRef]
- Nemes, A.; Marton, I.; Domsik, P.; Kalapos, A.; Pósfai, É.; Modok, S.; Borbényi, Z.; Forster, T. Characterization of left atrial dysfunction in hypereosinophilic syndrome—Insights from the Motion analysis of the heart and great vessels by three-dimensional speckle tracking echocardiography in pathological cases (MAGYAR-Path) Study. Rev. Port. Cardiol. 2016, 35, 277–283. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Rácz, G.; Ambrus, N.; Marton, I.; Borbényi, Z. Mitral and Tricuspid Annular Abnormalities in Hypereosinophilic Syndrome—Insights from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Path Study. Rev. Cardiovasc. Med. 2023, 24, 115. [Google Scholar] [CrossRef]
- Nemes, A.; Marton, I.; Domsik, P.; Kalapos, A.; Pósfai, É.; Modok, S.; Kormányos, Á.; Ambrus, N.; Borbényi, Z.; Forster, T. The right atrium in idiopathic hypereosinophilic syndrome. Insights from the 3D speckle tracking echocardiographic MAGYAR-Path Study. Herz 2019, 44, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.; Addetia, K. An introduction to left ventricular strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S. Left ventricular rotation and twist: Why should we learn? J. Cardiovasc. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Tajik, A.J.; Chandrasekaran, K.; Khandheria, B.K. Twist mechanics of the left ventricle: Principles and application. JACC Cardiovasc. Imaging 2008, 1, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á. Prevalence of left ventricular ‘rigid body rotation’, the near absence of left ventricular twist (insights from the MAGYAR studies). Rev. Cardiovasc. Med. 2022, 23, 5. [Google Scholar] [CrossRef]
- Oh, J.K.; Seward, J.B.; Tajik, A.J. The Echo Manual, 2nd ed.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Braunwald, E. The cardiomyopathies. In Braunwald’s Heart Disease, 7th ed.; Zipes, D., Ed.; Saunders: Philadelphia, PA, USA, 2005. [Google Scholar]
- Tian, Z.; Fang, Q.; Zhao, D.C.; Cui, Q.C.; Liu, Y.T.; Zeng, Y.; Li, M.T.; Jiang, X.C. The clinico-pathological manifestation of cardiac involvement in eosinophilic diseases. Zhonghua Nei Ke Za Zhi 2010, 49, 684–687. [Google Scholar] [PubMed]
- Syed, I.S.; Martinez, M.W.; Feng, D.L.; Glockner, J.F. Cardiac magnetic resonance imaging of eosinophilic endomyocardial disease. Int. J. Cardiol. 2008, 126, e50–e52. [Google Scholar] [CrossRef]
- Miszalski-Jamka, T.; Szczeklik, W.; Karwat, K.; Sokołowska, B.; Gąsior, J.; Rucińska, M.; Mazur, W.; Skotnicki, A.; Kereiakes, D.J.; Urbańczyk, M.; et al. MRI-based evidence for myocardial involvement in women with hypereosinophilic syndrome. Magn. Reson. Med. Sci. 2015, 14, 107–114. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tanaka, H.; Kurimoto, C.; Imanishi, T.; Hyashi, N.; Saegusa, J.; Morinobu, A.; Hirata, K.I.; Kawano, S. Very early stage left ventricular endocardial dysfunction of patients with hypereosinophilic syndrome. Int. J. Cardiovasc. Imaging 2016, 32, 1357–1361. [Google Scholar] [CrossRef]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Badano, L.P.; Nour, A.; Muraru, D. Left atrium as a dynamic three-dimensional entity: Implications for echocardiographic assessment. Rev. Esp. Cardiol. 2013, 66, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dal-Bianco, J.P.; Levine, R.A. Anatomy of the mitral valve apparatus: Role of 2D and 3D echocardiography. Cardiol. Clin. 2013, 31, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J.; Bazaz, R. The anatomic substrate of mitral annular contraction. Int. J. Cardiol. 2020, 306, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.; Muraru, D.; Miglioranza, M.H.; Piasentini, E.; Peluso, D.; Cucchini, U.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Normal mitral annulus dynamics and its relationships with left ventricular and left atrial function. Int. J. Cardiovasc. Imaging 2015, 31, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eurointervention 2022, 17, e1126–e1196. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.Y. Arterial-cardiac interaction: The concept and implications. J. Cardiovasc. Ultrasound 2011, 19, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83. [Google Scholar] [CrossRef]
- Nemes, A.; Marton, I.; Domsik, P.; Kalapos, A.; Pósfai, É.; Modok, S.; Borbényi, Z.; Forster, T. Aortic stiffness is increased in patients with hypereosinophilic syndrome being in early necrotic phase. Quant. Imaging Med. Surg. 2017, 7, 636–640. [Google Scholar] [CrossRef]
- Foale, R.; Nihoyannopoulos, P.; McKenna, W.; Kleinebenne, A.; Nadazdin, A.; Rowland, E.; Smith, G. Echocardiographic measurement of the normal adult right ventricle. Br. Heart J. 1986, 56, 33–44. [Google Scholar] [CrossRef]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92 (Suppl. S1), i2–i13. [Google Scholar] [CrossRef]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, Part I. Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Tadic, M. The right atrium, a forgotten cardiac chamber: An updated review of multimodality imaging. J. Clin. Ultrasound 2015, 43, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Dahou, A.; Levin, D.; Reisman, M.; Hahn, R.T. Anatomy and physiology of the tricuspid valve. JACC Cardiovasc. Imaging 2019, 12, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Kivelitz, D.E.; Dohmen, P.M.; Lembcke, A.; Kroencke, T.J.; Klingebiel, R.; Hamm, B.; Konertz, W.; Taupitz, M. Visualization of the pulmonary valve using cine MR imaging. Acta Radiol. 2003, 44, 172–176. [Google Scholar] [CrossRef]
- Kawashima, A.; Kimura, A.; Katsuda, S.; Sumita, R.; Yachie, A.; Nonomura, A.; Nakanishi, I. Pulmonary vasculitis with hypereosinophilia and episodic pulmonary hypertension: Report of three siblings. Pathol. Int. 1995, 45, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gidon, L.; Yousefi, S.; Karaulov, A.; Simon, H.U. Mechanisms of toxicity mediated by neutrophil and eosinophil granule proteins. Allergol. Int. 2021, 70, 30–38. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A. Cardiac Mechanics and Valvular and Vascular Abnormalities in Hypereosinophilic Syndrome. J. Clin. Med. 2024, 13, 1403. https://doi.org/10.3390/jcm13051403
Nemes A. Cardiac Mechanics and Valvular and Vascular Abnormalities in Hypereosinophilic Syndrome. Journal of Clinical Medicine. 2024; 13(5):1403. https://doi.org/10.3390/jcm13051403
Chicago/Turabian StyleNemes, Attila. 2024. "Cardiac Mechanics and Valvular and Vascular Abnormalities in Hypereosinophilic Syndrome" Journal of Clinical Medicine 13, no. 5: 1403. https://doi.org/10.3390/jcm13051403
APA StyleNemes, A. (2024). Cardiac Mechanics and Valvular and Vascular Abnormalities in Hypereosinophilic Syndrome. Journal of Clinical Medicine, 13(5), 1403. https://doi.org/10.3390/jcm13051403





