An Observational Study in the Real Clinical Practice of the Treatment of Noninfectious Uveitis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Patients with Noninfectious Uveitis (NIU)
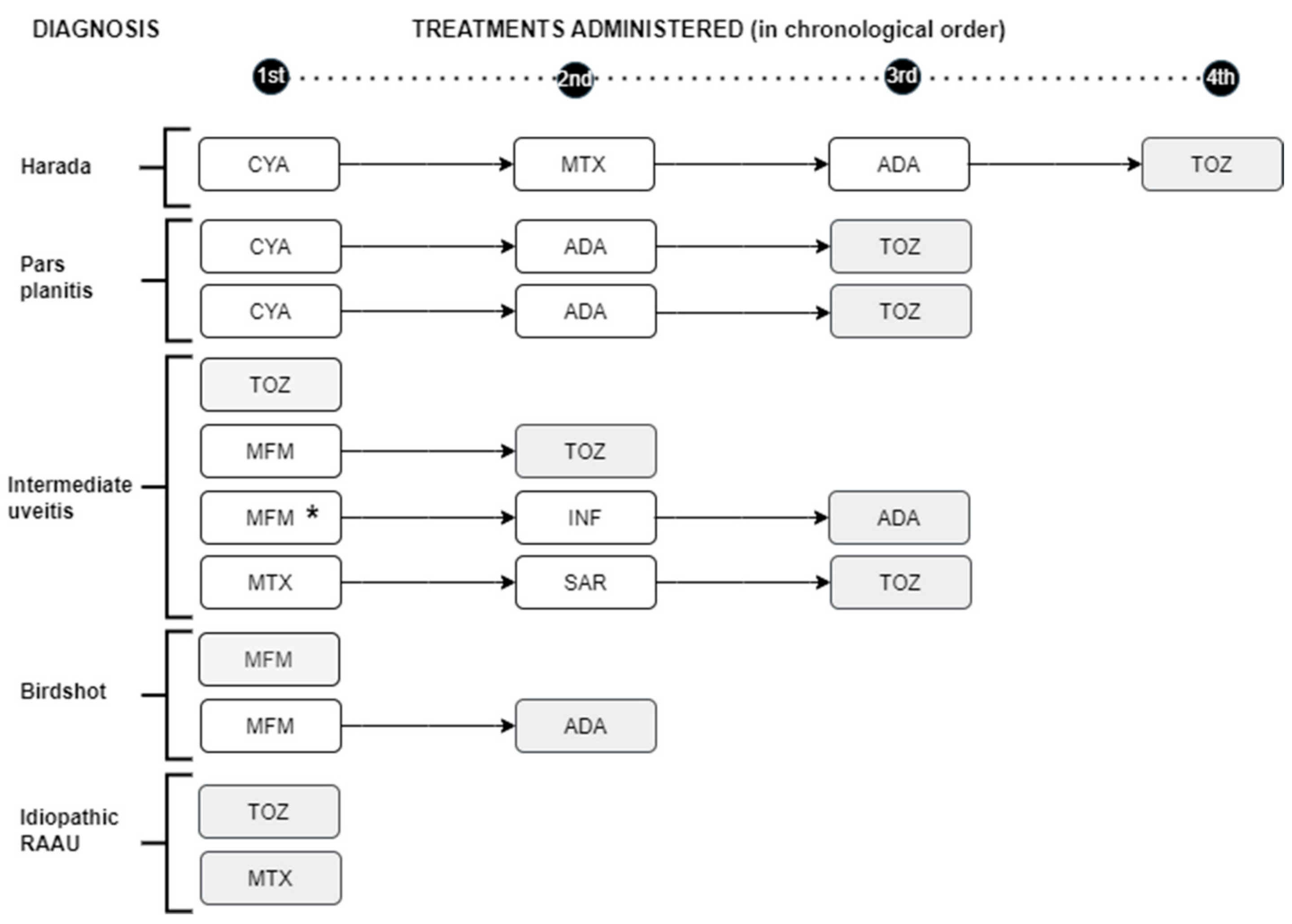
3.2. Need for IS Treatment and/or BT
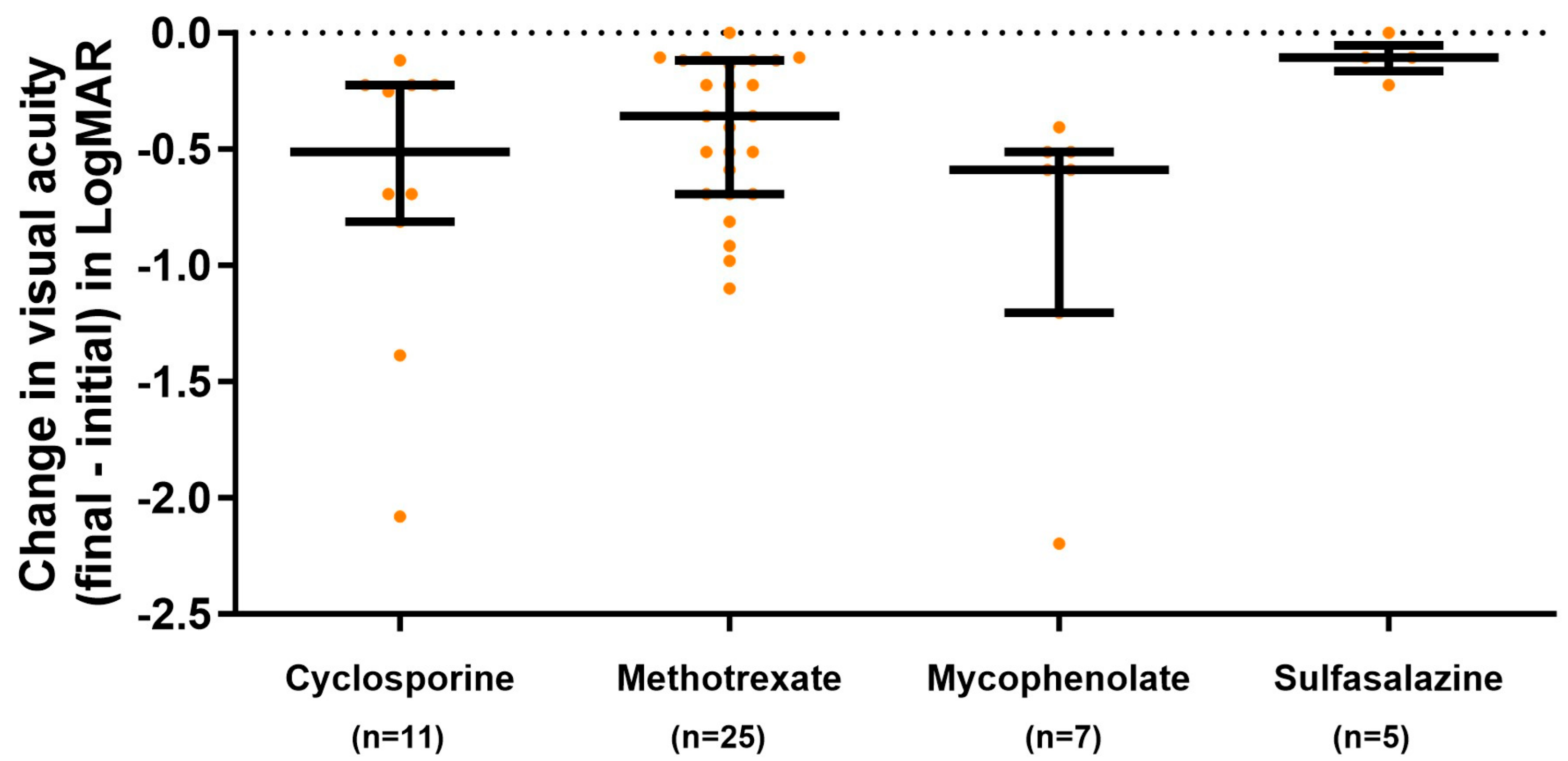
3.3. Response to Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunningham, E.T.E.; Zierhut, M. Vision Loss in Uveitis. Ocul. Immunol. Inflamm. 2021, 29, 1037–1039. [Google Scholar] [CrossRef]
- Tomkins-Netzer, O.; Talat, L.; Bar, A.; Lula, A.; Taylor, S.R.J.; Joshi, L.; Lightman, S. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology 2014, 121, 2387–2392. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Bodaghi, B.; Couto, C.; Zierhut, M.; Acharya, N.; Pavesio, C.; Tay-Kearney, M.-L.; Neri, P.; Douglas, K.; Pathai, S.; et al. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: A review. Semin. Arthritis Rheum. 2019, 49, 438–445. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.D.; Taylor, S.R.J.; Bodaghi, B.; Miserocchi, E.; Murray, P.I.; Pleyer, U.; Zierhut, M.; Barisani-Asenbauer, T.; LeHoang, P.; Lightman, S. Understanding uveitis: The impact of research on visual outcomes. Prog. Retin. Eye Res. 2011, 30, 452–470. [Google Scholar] [CrossRef]
- van Laar, J.A.M.; Rothova, A.; Missotten, T.; Kuijpers, R.W.A.M.; van Hagen, P.M.; van Velthoven, M.E.J. Diagnosis and treatment of uveitis; not restricted to the ophthalmologist. J. Clin. Transl. Res. 2015, 1, 94–99. [Google Scholar]
- Lowder, C.; Belfort, R.; Lightman, S.; Foster, C.S.; Robinson, M.R.; Schiffman, R.M.; Li, X.-Y.; Cui, H.; Whitcup, S.M. Ozurdex HURON Study Group Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch. Ophthalmol. 2011, 129, 545–553. [Google Scholar] [CrossRef]
- Callanan, D.G.; Jaffe, G.J.; Martin, D.F.; Pearson, P.A.; Comstock, T.L. Treatment of posterior uveitis with a fluocinolone acetonide implant: Three-year clinical trial results. Arch. Ophthalmol. 2008, 126, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Rosenbaum, J.T.; Foster, C.S.; Holland, G.N.; Jaffe, G.J.; Louie, J.S.; Nussenblatt, R.B.; Stiehm, E.R.; Tessler, H.; Van Gelder, R.N.; et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am. J. Ophthalmol. 2000, 130, 492–513. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Hatef, E.; Kayen, B.; Macahilig, C.P.; Ibrahim, M.; Wang, J.; Shaikh, O.; Bodaghi, B. A cross-sectional study of the current treatment patterns in noninfectious uveitis among specialists in the United States. Ophthalmology 2011, 118, 184–190. [Google Scholar] [CrossRef]
- Dick, A.D.; Rosenbaum, J.T.; Al-Dhibi, H.A.; Belfort, R.; Brézin, A.P.; Chee, S.P.; Davis, J.L.; Ramanan, A.V.; Sonoda, K.-H.; Carreño, E.; et al. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis: Fundamentals Of Care for UveitiS (FOCUS) Initiative. Ophthalmology 2018, 125, 757–773. [Google Scholar] [CrossRef]
- Suhler, E.B.; Adán, A.; Brézin, A.P.; Fortin, E.; Goto, H.; Jaffe, G.J.; Kaburaki, T.; Kramer, M.; Lim, L.L.; Muccioli, C.; et al. Safety and Efficacy of Adalimumab in Patients with Noninfectious Uveitis in an Ongoing Open-Label Study: VISUAL III. Ophthalmology 2018, 125, 1075–1087. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kezuka, T.; Sugita, S.; Keino, H.; Namba, K.; Kaburaki, T.; Maruyama, K.; Nakai, K.; Hijioka, K.; Shibuya, E.; et al. Evaluation of the long-term efficacy and safety of infliximab treatment for uveitis in Behçet’s disease: A multicenter study. Ophthalmology 2014, 121, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Levy-Clarke, G.; Jabs, D.A.; Read, R.W.; Rosenbaum, J.T.; Vitale, A.; Van Gelder, R.N. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 2014, 121, 785–796.e3. [Google Scholar] [CrossRef]
- Sepah, Y.J.; Sadiq, M.A.; Chu, D.S.; Dacey, M.; Gallemore, R.; Dayani, P.; Hanout, M.; Hassan, M.; Afridi, R.; Agarwal, A.; et al. Primary (Month-6) Outcomes of the STOP-Uveitis Study: Evaluating the Safety, Tolerability, and Efficacy of Tocilizumab in Patients With Noninfectious Uveitis. Am. J. Ophthalmol. 2017, 183, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, M.; Andrillon, A.; Maalouf, G.; Sève, P.; Bielefeld, P.; Gueudry, J.; Sené, T.; Moulinet, T.; Rouvière, B.; Sène, D.; et al. Anti-Tumor Necrosis Factor α versus Tocilizumab in the Treatment of Refractory Uveitic Macular Edema: A Multicenter Study from the French Uveitis Network. Ophthalmology 2022, 129, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, J.; Murray, P.I.; Rao, N.A.; Nussenblatt, R.B. International Uveitis Study Group International Uveitis Study Group (IUSG): Clinical classification of uveitis. Ocul. Immunol. Inflamm. 2008, 16, 1–2. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef]
- Pistilli, M.; Joffe, M.M.; Gangaputra, S.S.; Pujari, S.S.; Jabs, D.A.; Levy-Clarke, G.A.; Nussenblatt, R.B.; Rosenbaum, J.T.; Sen, H.N.; Suhler, E.B.; et al. Visual Acuity Outcome over Time in Non-Infectious Uveitis. Ocul. Immunol. Inflamm. 2021, 29, 1064–1071. [Google Scholar] [CrossRef]
- Hunter, R.S.; Skondra, D.; Papaliodis, G.; Sobrin, L. Role of OCT in the diagnosis and management of macular edema from uveitis. Semin. Ophthalmol. 2012, 27, 236–241. [Google Scholar] [CrossRef]
- Espinosa, G.; Muñoz-Fernández, S.; García Ruiz de Morales, J.M.; Herreras, J.M.; Cordero-Coma, M. Treatment recommendations for non-infectious anterior uveitis. Med. Clin. 2017, 149, 552.e1–552.e12. [Google Scholar] [CrossRef]
- Espinosa, G.; Herreras, J.M.; Muñoz-Fernández, S.; García Ruiz de Morales, J.M.; Cordero-Coma, M. Recommendations statement on the immunosuppressive treatment of non-infectious, non-neoplastic, non-anterior uveitis. Med. Clín. Engl. 2020, 155, 220.e1–220.e12. [Google Scholar] [CrossRef]
- Gómez-Gómez, A.; Loza, E.; Rosario, M.P.; Espinosa, G.; de Morales, J.M.G.R.; Herreras, J.M.; Muñoz-Fernández, S.; Cordero-Coma, M. Efficacy and safety of immunomodulatory drugs in patients with anterior uveitis: A systematic literature review. Medicine 2017, 96, e8045. [Google Scholar] [CrossRef]
- Gómez-Gómez, A.; Loza, E.; Rosario, M.P.; Espinosa, G.; de Morales, J.M.G.R.; Herrera, J.M.; Muñoz-Fernández, S.; Rodríguez-Rodríguez, L.; Cordero-Coma, M. Spanish Society of Ocular Inflammation (SEIOC) Efficacy and safety of immunomodulatory drugs in patients with non-infectious intermediate and posterior uveitis, panuveitis and macular edema: A systematic literature review. Semin. Arthritis Rheum. 2020, 50, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- García-Aparicio, A.; Alonso Martín, L.; López Lancho, R.; Quirós Zamorano, R.; Del Olmo Perez, L.; Sánchez Fernández, S.; Otón, T.; Jiménez Escribano, R.; González Del Valle, F.; Muñoz-Fernández, S. Epidemiology of Uveitis in a Spanish Region: Prevalence and Etiology. Ophthalmic Epidemiol. 2021, 28, 227–236. [Google Scholar] [CrossRef]
- Millán-Longo, C.; Peiteado, D.; Schlincker, A.; Hidalgo, V.; Pieren, A.; Balsa, A.; de Miguel, E. Use of Immunomodulatory Drugs at a Uveitis Clinic. Reumatol. Clin. 2019, 15, 271–276. [Google Scholar] [CrossRef]
- Chang, J.H.-M.; Wakefield, D. Uveitis: A global perspective. Ocul. Immunol. Inflamm. 2002, 10, 263–279. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.; Zheng, Y.; Li, Q.; Yuan, X.; Hua, X. Analysis of the clinical diagnosis and treatment of uveitis. Ann. Palliat. Med. 2021, 10, 12782–12788. [Google Scholar] [CrossRef]
- Muñoz-Fernández, S.; García-Aparicio, A.M.; Hidalgo, M.V.; Platero, M.; Schlincker, A.; Bascones, M.L.; Pombo, M.; Morente, P.; Sanpedro, J.; Martín-Mola, E. Methotrexate: An option for preventing the recurrence of acute anterior uveitis. Eye Lond. Engl. 2009, 23, 1130–1133. [Google Scholar] [CrossRef]
- Bajwa, A.; Osmanzada, D.; Osmanzada, S.; Khan, I.; Patrie, J.; Xin, W.; Reddy, A.K. Epidemiology of uveitis in the mid-Atlantic United States. Clin. Ophthalmol. 2015, 9, 889–901. [Google Scholar] [CrossRef]
- Foster, C.S.; Kothari, S.; Anesi, S.D.; Vitale, A.T.; Chu, D.; Metzinger, J.L.; Cerón, O. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv. Ophthalmol. 2016, 61, 1–17. [Google Scholar] [CrossRef]
- Guillot, X.; Prati, C.; Sondag, M.; Wendling, D. Etanercept for treating axial spondyloarthritis. Expert Opin. Biol. Ther. 2017, 17, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, N.-S. Etanercept therapy-associated acute uveitis: A case report and literature review. Clin. Exp. Rheumatol. 2009, 27, 838–839. [Google Scholar]
- Benitez-Del-Castillo, J.M.; Garcia-Sanchez, J.; Iradier, T.; Bañares, A. Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye Lond. Engl. 2000, 14 Pt 3A, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.; Rathinam, S.R.; Babu, M.; Kanakath, A.; Thundikandy, R.; Lee, S.M.; Browne, E.N.; Porco, T.C.; Acharya, N.R. Outcomes of Vogt-Koyanagi-Harada Disease: A Subanalysis From a Randomized Clinical Trial of Antimetabolite Therapies. Am. J. Ophthalmol. 2016, 168, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fardeau, C.; Champion, E.; Massamba, N.; LeHoang, P. Uveitic macular edema. Eye Lond. Engl. 2016, 30, 1277–1292. [Google Scholar] [CrossRef]
- Vegas-Revenga, N.; Calvo-Río, V.; Mesquida, M.; Adán, A.; Hernández, M.V.; Beltrán, E.; Valls Pascual, E.; Díaz-Valle, D.; Díaz-Cordovés, G.; Hernandez-Garfella, M.; et al. Anti-IL6-Receptor Tocilizumab in Refractory and Noninfectious Uveitic Cystoid Macular Edema: Multicenter Study of 25 Patients. Am. J. Ophthalmol. 2019, 200, 85–94. [Google Scholar] [CrossRef]
- Deuter, C.M.E.; Zierhut, M.; Igney-Oertel, A.; Xenitidis, T.; Feidt, A.; Sobolewska, B.; Stuebiger, N.; Doycheva, D. Tocilizumab in Uveitic Macular Edema Refractory to Previous Immunomodulatory Treatment. Ocul. Immunol. Inflamm. 2017, 25, 215–220. [Google Scholar] [CrossRef]
- Heissigerová, J.; Callanan, D.; de Smet, M.D.; Srivastava, S.K.; Karkanová, M.; Garcia-Garcia, O.; Kadayifcilar, S.; Ozyazgan, Y.; Vitti, R.; Erickson, K.; et al. Efficacy and Safety of Sarilumab for the Treatment of Posterior Segment Noninfectious Uveitis (SARIL-NIU): The Phase 2 SATURN Study. Ophthalmology 2019, 126, 428–437. [Google Scholar] [CrossRef]
- Karkhur, S.; Hasanreisoglu, M.; Vigil, E.; Halim, M.S.; Hassan, M.; Plaza, C.; Nguyen, N.V.; Afridi, R.; Tran, A.T.; Do, D.V.; et al. Interleukin-6 inhibition in the management of non-infectious uveitis and beyond. J. Ophthalmic Inflamm. Infect. 2019, 9, 17. [Google Scholar] [CrossRef]

| Total | No TTO IS/BT | With TTO IS/BT | p-Value | |
|---|---|---|---|---|
| n = 356 | n = 290 (81.5%) | n = 66 (18.5%) | ||
| Female | 180 (50.6) | 146 (50.3) | 34 (51.5) | 0.864 |
| Male | 176 (49.4) | 144 (49.7) | 32 (48.5) | |
| Age (median [Q1Q3]) | ||||
| At diagnosis | 42.8 [31.5, 54.0] | 42.0 [32.0, 55.0] | 38.0 [30.0, 47.0] | 0.027 |
| At the start of treatment | 42.5 [34.0, 50.0] | Not applicable | ||
| Time diagnosis–initiation TTO | 2 [1, 4] | Not applicable | ||
| n. outbreaks | ||||
| 1 outbreak | 157 (44.1) | 134 (46.2) | 23 (34.9) | 0.197 |
| 2 outbreak | 35 (9.8) | 30 (10.3) | 5 (7.6) | |
| ≥3 outbreak | 164 (45.9) | 126 (43.4) | 38 (57.6) | |
| Laterality | ||||
| Unilateral | 271 (76.1) | 236 (81.4) | 31 (47.0) | <0.001 |
| Bilateral | 85 (23.9) | 54 (18.6) | 35 (53.0) | |
| Anatomic Location | ||||
| Anterior | 265 (74.4) | 233 (80.3) | 32 (48.5) | <0.001 |
| Intermedia | 34 (9.6) | 22 (7.6) | 12 (18.2) | |
| Posterior | 28 (7.9) | 18 (6.2) | 10 (15.2) | |
| Panuveitis | 29 (8.2) | 17 (5.9) | 12 (18.2) | |
| Etiology | ||||
| Idiopathic | 154 (43.3) | 133 (45.9) | 21 (31.8) | Not applicable |
| AS HLA B27+ | 76 (21.4) | 58 (20.0) | 18 (27.3) | |
| Pars planitis | 19 (5.3) | 14 (4.8) | 5 (7.6) | |
| Associated with HLA B27 | 17 (4.8) | 16 (5.5) | 1 (1.5) | |
| IBD | 13 (3.7) | 10 (3.5) | 3 (4.6) | |
| AS HLA B27- | 12 (3.4) | 11 (3.8) | 1 (1.5) | |
| Behcet | 11 (3.1) | 5 (1.7) | 6 (9.1) | |
| Other white dot syndrome | 8 (2.3) | 7 (2.4) | 1 (1.52) | |
| Posner | 7 (2.0) | 7 (2.4) | 0 | |
| Sarcoidosis | 7 (2.0) | 7 (2.4) | 0 | |
| Psoriatic arthritis | 6 (1.7) | 4 (1.4) | 2 (3.0) | |
| Harada syndrome | 5 (1.4) | 3 (1.0) | 2 (3.0) | |
| Birdshot uveitis | 4 (1.1) | 0 | 4 (6.1) | |
| APPC | 4 (1.1) | 4 (1.4) | 0 | |
| For drugs | 4 (1.1) | 4 (1.4) | 0 | |
| Intermedia-associated MS | 3 (0.8) | 1 (0.3) | 2 (3.0) | |
| Associated Sjögren 1º | 2 (0.6) | 2 (0.7) | 0 | |
| Cogan’s syndrome | 1 (0.3) | 1 (0.3) | 0 | |
| Systemic lupus erythematosus | 1 (0.3) | 1 (0.3) | 0 | |
| TINU | 1 (0.3) | 1 (0.3) | 0 | |
| Undetermined | 1 (0.3) | 1 (0.3) | 0 | |
| Systemic CS treatment | 43 (12.1) | 19 (6.5) | 24 (36.4) | |
| Orals | 26 (7) | 14 (4.8) | 12 (18.2) | <0.001 |
| Intravenous | 17 (4.8) | 5 (1.7) | 12 (18.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban-Ortega, M.; Steiner, M.; Andreu-Vázquez, C.; Thuissard-Vasallo, I.; Díaz-Rato, A.; Muñoz-Fernández, S. An Observational Study in the Real Clinical Practice of the Treatment of Noninfectious Uveitis. J. Clin. Med. 2024, 13, 1402. https://doi.org/10.3390/jcm13051402
Esteban-Ortega M, Steiner M, Andreu-Vázquez C, Thuissard-Vasallo I, Díaz-Rato A, Muñoz-Fernández S. An Observational Study in the Real Clinical Practice of the Treatment of Noninfectious Uveitis. Journal of Clinical Medicine. 2024; 13(5):1402. https://doi.org/10.3390/jcm13051402
Chicago/Turabian StyleEsteban-Ortega, Mar, Martina Steiner, Cristina Andreu-Vázquez, Israel Thuissard-Vasallo, Alvaro Díaz-Rato, and Santiago Muñoz-Fernández. 2024. "An Observational Study in the Real Clinical Practice of the Treatment of Noninfectious Uveitis" Journal of Clinical Medicine 13, no. 5: 1402. https://doi.org/10.3390/jcm13051402
APA StyleEsteban-Ortega, M., Steiner, M., Andreu-Vázquez, C., Thuissard-Vasallo, I., Díaz-Rato, A., & Muñoz-Fernández, S. (2024). An Observational Study in the Real Clinical Practice of the Treatment of Noninfectious Uveitis. Journal of Clinical Medicine, 13(5), 1402. https://doi.org/10.3390/jcm13051402






