Transcatheter Edge-to-Edge Mitral Valve Repair versus Minimally Invasive Mitral Valve Surgery: An Observational Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Patient Cohort
2.2. Procedure
2.3. Study Endpoints
2.4. Ethical Consideration
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural Results
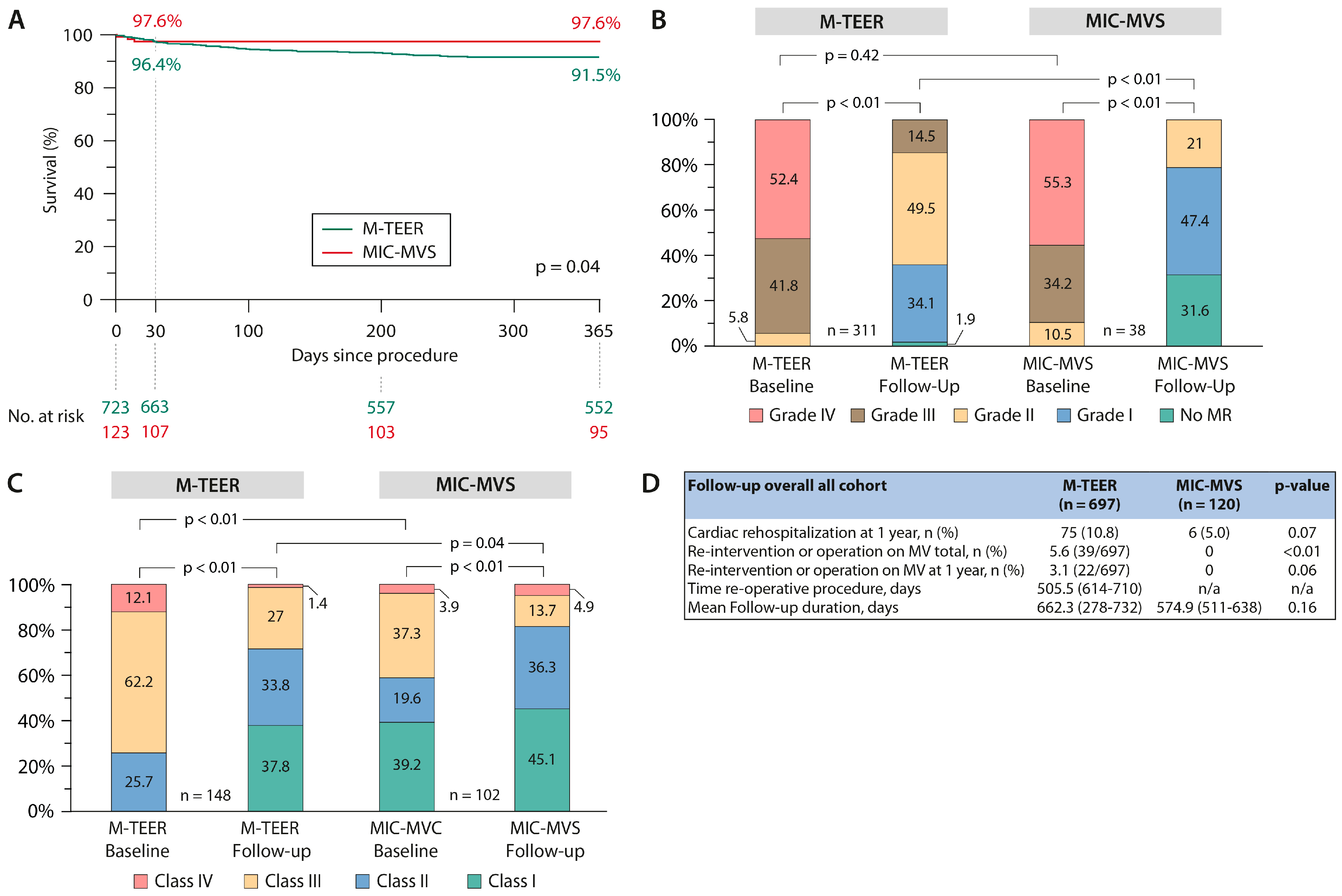
3.3. Primary Endpoints
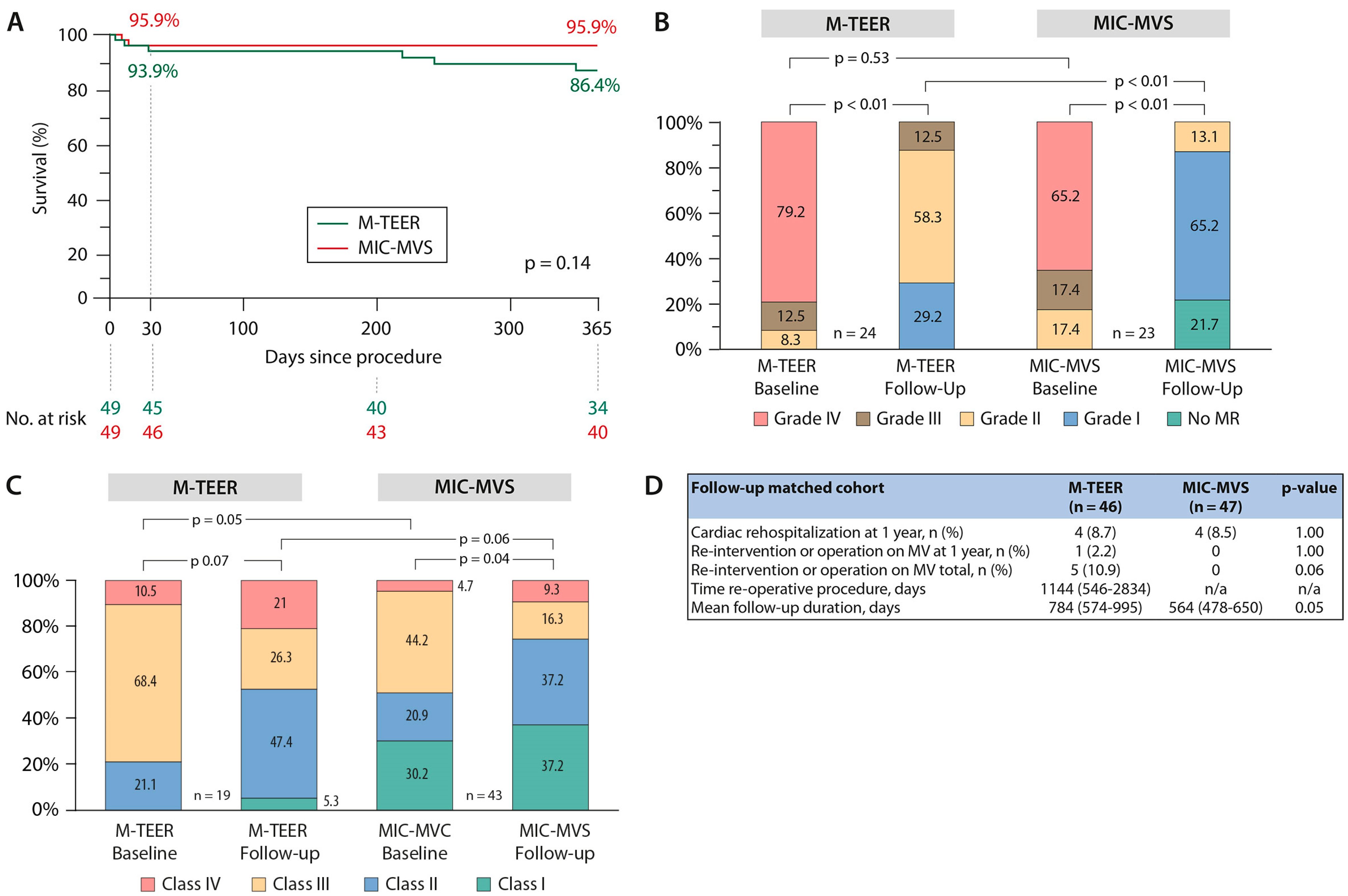
3.4. Sensitivity Analysis—Case Control Matching
3.5. Patients with Degenerative Mitral Valve Disease
4. Discussion
Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldstone, A.B.; Atluri, P.; Szeto, W.Y.; Trubelja, A.; Howard, J.L.; MacArthur, J.W., Jr.; Newcomb, C.; Donnelly, J.P.; Kobrin, D.M.; Sheridan, M.A.; et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: A propensity-matched comparison. J. Thorac. Cardiovasc. Surg. 2013, 145, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.; Martin, J.; Lal, A.; Diegeler, A.; Folliguet, T.A.; Nifong, L.W.; Perier, P.; Raanani, E.; Smith, J.M.; Seeburger, J.; et al. Minimally invasive versus conventional open mitral valve surgery: A meta-analysis and systematic review. Innovations 2011, 6, 84–103. [Google Scholar] [PubMed]
- Falk, V.; Cheng, D.C.; Martin, J.; Diegeler, A.; Folliguet, T.A.; Nifong, L.W.; Perier, P.; Raanani, E.; Smith, J.M.; Seeburger, J. Minimally invasive versus open mitral valve surgery: A consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations 2011, 6, 66–76. [Google Scholar] [PubMed]
- Sündermann, S.H.; Sromicki, J.; Biefer, H.R.C.; Seifert, B.; Holubec, T.; Falk, V.; Jacobs, S. Mitral valve surgery: Right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1989–1995.e4. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Blaßfeld, D.; Böning, A. German Heart Surgery Report 2022: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2023, 71, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [PubMed]
- Beyersdorf, F.; Vahanian, A.; Milojevic, M.; Praz, F.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef] [PubMed]
- Del Forno, B.; De Bonis, M.; Agricola, E.; Melillo, F.; Schiavi, D.; Castiglioni, A.; Montorfano, M.; Alfieri, O. Mitral valve regurgitation: A disease with a wide spectrum of therapeutic options. Nat. Rev. Cardiol. 2020, 17, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; Atik, F.A.; Cosgrove, D.M.; Blackstone, E.H.; Rajeswaran, J.; Krishnaswamy, G.; Jin, U.; Gillinov, A.M.; Griffin, B.; Navia, J.L.; et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J. Thorac. Cardiovasc. Surg. 2010, 139, 926–932.e2. [Google Scholar] [CrossRef] [PubMed]
- Misfeld, M.; Davierwala, P.; Banusch, J.; Ender, J.; Mohr, F.-W.; Pfannmüller, B. Minimally invasive, beating heart tricuspid valve surgery in a redo case. Ann. Cardiothorac. Surg. 2017, 6, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Braun, D.; Unterhuber, M.; Spirito, A.; Orban, M.; Brugger, N.; Brinkmann, I.; Spring, K.; Moschovitis, A.; Nabauer, M.; et al. Edge-to-Edge Mitral Valve Repair With Extended Clip Arms: Early Experience From a Multicenter Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Kar, S.; Spargias, K.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.C.; Fam, N.P.; Walters, D.L.; Webb, J.G.; Smith, R.L.; et al. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc. Interv. 2019, 12, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefevre, T.; Piot, C.; Rouleau, F.; Carrie, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–22306. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Deharo, P.; Obadia, J.F.; Guerin, P.; Cuisset, T.; Avierinos, J.F.; Habib, G.; Torras, O.; Bisson, A.; Vigny, P.; Etienne, C.S.; et al. Mitral transcatheter edge to edge repair versus isolated mitral surgery for severe mitral regurgitation: A French nationwide study. Eur. Heart J. 2024, ehae046. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Genereux, P.; Vranckx, P.; Mehran, R.; Kuck, K.H.; Leon, M.B.; Piazza, N.; et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 2: Endpoint definitions: A consensus document from the Mitral Valve Academic Research Consortium. Eur. Heart J. 2015, 36, 1878–1891. [Google Scholar] [CrossRef]

| Baseline Parameters | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| M-TEER (n = 723) | MIC-MVS (n = 123) | p-Value | M-TEER (n = 49) | MIC-MVS (n = 49) | p-Value | |
| Age, years | 78.3 (77.7–78.8) | 61.5 (59.0–63.9) | <0.01 | 71.7 (69.3–74.1) | 70.0 (67.9–72.1) | 0.30 |
| Sex (female), n (%) | 305 (42.2) | 52 (42.3) | 0.98 | 21 (42.9) | 25 (51.0) | 0.42 |
| BMI, kg/m2 | 26.1 (25.6–26.6) | 26.3 (25.5–27.2) | 0.59 | 25.5 (23.9–27.1) | 26.7 (25.3–28.1) | 0.27 |
| Logistic EuroSCORE II, % | 5.5 (5.2–5.9) | 1.3 (1.1–1.5) | <0.01 | 2.3 (1.9–2.7) | 1.8 (1.4–2.2) | 0.08 |
| LVEF, % | 48.4 (47.3–49.6) | 60.4 (58.8–62.0) | <0.01 | 60.0 (57.7–62.2) | 60.5 (58.4–62.4) | 0.73 |
| Glomerular filtration rate, mL/min | 45.9 (44.3–47.5) | 93.5 (86.8–100.1) | <0.01 | 60.6 (52.7–68.6) | 76.3 (67.4–85.3) | 0.01 |
| Previous Cardiac Surgery, n (%) | 183 (25.3) | 2 (1.6) | <0.01 | 4 (8.2) | 0 | 0.04 |
| COPD, n (%) | 132 (18.2) | 11 (8.9) | <0.01 | 12 (24.5) | 6 (12.2) | 0.12 |
| Previous Stroke/TIA, n (%) | 70 (9.7) | 7 (5.7) | <0.01 | 4 (8.2) | 2 (4.1) | 0.34 |
| Coronary artery disease, n (%) | 403 (55.7) | 16 (13.0) | <0.01 | 7 (14.3) | 4 (8.2) | <0.01 |
| Previous PCI, n (%) | 284 (39.3) | 4 (3.3) | <0.01 | 17 (34.7) | 3 (6.1) | <0.01 |
| Permanent Pacemaker/internal Defibrillator present, n (%) | 251 (34.7) | 6 (4.9) | <0.01 | 6 (12.2) | 1 (2.0) | 0.05 |
| Creatinine, mg/dL | 1.5 (1.4–1.6) | 0.9 (0.9–1.0) | <0.01 | 1.2 (1.0–1.5) | 1.0 (0.9–1.1) | 0.07 |
| History of atrial fibrillation/atrial flutter, n (%) | 546 (75.5) | 40 (32.5) | <0.01 | 34 (69.4) | 20 (40.8) | <0.01 |
| Systolic PAP, mmHg | 42.2 (41.1–43.4) | 45.6 (41.9–49.4) | 0.02 | 42.9 (37.7–48.2) | 45.6 (41.1–50.1) | 0.47 |
| TAPSE, mm | 18.4 (17.9–18.8) | 23.7 (22.3–25.2) | <0.01 | 20.2 (18.6–21.8) | 25.4 (23.7–27.1) | <0.01 |
| Vena contracta MV, mm | 0.7 (0.6–0.7) | 0.7 (0.6–0.7) | 1.00 | 0.7 (0.7–0.8) | 0.7 (0.6–0.8) | 0.95 |
| LVEDD, mm | 52.7 (51.8–53.7) | 51.1 (49.3–53.0) | 0.12 | 51.0 (48.7–53.3) | 50.4 (47.7–53.2) | 0.37 |
| TR Grade, n (%) None Mild Moderate Severe Massive Torrential | <0.01 | <0.01 | ||||
| 9/709 (1.3) 200/709 (28.2) 259/709 (36.5) 171/709 (24.1) 58/709 (8.2) 12/709 (1.7) | 36 (29.3) 56 (45.5) 15 (12.2) 13 (10.6) 3 (2.4) 0 | 1 (2.0) 19 (38.8) 19 (38.8) 8 (16.3) 2 (4.1) - | 9 (18.4) 29 (59.1) 9 (18.4) 2 (4.1) 0 - | <0.01 | ||
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Procedural Data and 30-day Results | M-TEER (n = 723) | MIC-MVS (n = 123) | p-Value | M-TEER (n = 49) | MIC-MVS (n = 49) | p-Value |
| Procedure time, minutes | 78.3 (77.7–78.8) | 61.5 (59.0–63.9) | <0.01 | 72.8 (60.5–85.1) | 266.8 (247.4–286.3) | <0.01 |
| Time on ECC, minutes Cross Clamp time, minutes | n/a | 192.5 (182–202) | n/a | |||
| Time on ECC, minutes Cross Clamp time, minutes | n/a | 127.1 (120–134) | n/a | |||
| Mitral valve repair (surgical), n (%) Mitral valve replacement (surgical), n (%) Method of TEER, n (%) MitraClip Pascal Number of clips placed, n (%): 1 Clip 2 Clips 3 Clips | n/a n/a 691 (95.6) 32 (4.4) 380 (52.6) 299 (41.3) 44 (6.1) | 48 (98.0) 1 (2.0) 48 (98.0) 1 (2.0) 24 (48.9) 21 (42.9) 4 (8.2) | n/a | |||
| Concomitant procedures, n (%) Pacemaker lead extraction Rhythm ablation therapy LAA closure PFO/ASD closure Tricuspid valve surgery | n/a | 0 21 (17.1) 18 (14.6) 16 (13.0) 17 (13.8) | n/a | 0 9 (18.4) 5 (10.2) 6 (12.2) 0 | 0.27 | |
| Conversion to sternotomy, n (%) | 5 (0.7) | 6 (4.9) | <0.01 | 0 | 3 (6.1) | 0.34 |
| 30-day mortality, n (%) | 26 (3.6) | 3 (2.4) | 0.78 | 3 (6.1) | 2 (4.1) | 1.00 |
| TIA, n (%) Stroke, n (%) | 0 6 (0.8) | 0 1 (0.8) | 1.00 1.00 | 0 0 | 0 1 (2.0) | 1.00 1.00 |
| Major bleeding (BARC 3 or 4), n (%) | 24 (3.3) | 23 (18.7) | <0.01 | 2 (4.1) | 8 (16.3) | 0.09 |
| LCOS with, n (%) VA ECMO left heart Impella right heart Impella | 2 (0.3) 1 (0.1) 0 | 5 (4.1) 1 (0.8) 0 | <0.01 0.27 1.00 | 1(2.0) 0 0 | 3 (6.1) 0 0 | 0.62 |
| Renal failure requiring dialysis, n (%) | 4 (0.5) | 7 (5.7) | <0.01 | 1 (2.0) | 4 (8.2) | 0.36 |
| Hospital stay, days | 7.0 (6.6–7.5) | 18.1 (14.9–21.3) | <0.01 | 5.7 (4.6–6.9) | 23.5 (16.4–30.5) | <0.01 |
| MR severity at discharge, n (%) None Mild Moderate Moderate-Severe Severe | 46/696 (6.6) 479/696 (68.8) 131/696 (18.8) 28/696 (4.0) 12/696 (1.7) | 38/107 (35.5) 61/107 (57.0) 6/107 (5.6) 2/107 (1.9) 0 | <0.01 | 1/48 (2.1) 33/48 (68.8) 8/48 (16.7) 4/48 (8.3) 2/48 (4.1) | 21/47 (44.7) 20/47 (42.5) 6/47 (12.8) 0 0 | <0.01 |
| Mitral mean gradient at discharge, mmHg | 3.8 (3.6–3.9) | 3.6 (3.3–3.9) | 0.32 | 4.1 (3.6–4.6) | 3.4 (3.0–3.8) | 0.04 |
| Parameters | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| M-TEER (n = 254) | MIC-MVS (n = 102) | p-Value | M-TEER (n = 40) | MIC-MVS (n = 40) | p-Value | |
| Age, years | 80.3 (79.3–81.2) | 60.0 (57.2–62.8) | <0.01 | 72.0 (69.1–74.9) | 70.1 (67.6–72.7) | 0.33 |
| Logistic EuroSCOREII, % | 5.4 (4.8–5.9) | 1.2 (1.0–1.3) | <0.01 | 2.1 (1.8–2.4) | 1.6 (1.3–1.9) | <0.01 |
| 30-day mortality, n (%) | 8 (3.1) | 2 (1.9) | 0.73 | 3 (7.5) | 1 (2.5) | 0.62 |
| MR severity at discharge, n (%) None Mild Moderate Moderate-Severe Severe | 28/244 (11.5) 148/244 (60.6) 51/244 (20.9) 11/244 (4.5) 6/244 (2.5) | 30/89 (33.7) 51/89 (57.3) 8/89 (9.0) 0 0 | <0.01 | 5/38 (13.2) 20/38 (52.5) 9/38 (23.7) 2/38 (5.3) 2/38 (5.3) | 16/38 (42.1) 16/38 (42.1) 6/38 (15.8) 0 0 | 0.03 |
| Mitral mean gradient at discharge, mmHg | 4.0 (3.8–4.3) | 3.5 (3.2–3.8) | 0.01 | 4.1 (3.5–4.7) | 3.2 (2.8–3.6) | 0.02 |
| 1-year mortality, n (%) | 21 (8.3) | 2 (1.9) | 0.03 | 5 (12.5) | 1 (2.5) | 0.20 |
| Cardiac rehospitalization at 1 year, n (%) | 20 (7.9) | 4 (3.9) | 0.24 | 2 (5.0) | 2 (5.0) | 1.00 |
| Re-intervention or operation on MV at 1 year, n (%) | 7 (2.8) | 0 | 0.20 | 1 (2.5) | 0 | 1.00 |
| Re-intervention or operation on MV total, n (%) | 19 (7.5) | 0 | <0.01 | 5 (12.5) | 0 | 0.06 |
| Mean follow-up duration, days | 728 (639–817) | 528 (463–593) | <0.01 | 846 (596–1097) | 580 (483–678) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silaschi, M.; Cattelaens, F.; Alirezaei, H.; Vogelhuber, J.; Sommer, S.; Sugiura, A.; Schulz, M.; Tanaka, T.; Sudo, M.; Zimmer, S.; et al. Transcatheter Edge-to-Edge Mitral Valve Repair versus Minimally Invasive Mitral Valve Surgery: An Observational Study. J. Clin. Med. 2024, 13, 1372. https://doi.org/10.3390/jcm13051372
Silaschi M, Cattelaens F, Alirezaei H, Vogelhuber J, Sommer S, Sugiura A, Schulz M, Tanaka T, Sudo M, Zimmer S, et al. Transcatheter Edge-to-Edge Mitral Valve Repair versus Minimally Invasive Mitral Valve Surgery: An Observational Study. Journal of Clinical Medicine. 2024; 13(5):1372. https://doi.org/10.3390/jcm13051372
Chicago/Turabian StyleSilaschi, Miriam, Franca Cattelaens, Hossien Alirezaei, Johanna Vogelhuber, Susanne Sommer, Atsushi Sugiura, Max Schulz, Tetsu Tanaka, Mitsumasa Sudo, Sebastian Zimmer, and et al. 2024. "Transcatheter Edge-to-Edge Mitral Valve Repair versus Minimally Invasive Mitral Valve Surgery: An Observational Study" Journal of Clinical Medicine 13, no. 5: 1372. https://doi.org/10.3390/jcm13051372
APA StyleSilaschi, M., Cattelaens, F., Alirezaei, H., Vogelhuber, J., Sommer, S., Sugiura, A., Schulz, M., Tanaka, T., Sudo, M., Zimmer, S., Nickenig, G., Weber, M., Bakhtiary, F., & Wilde, N. (2024). Transcatheter Edge-to-Edge Mitral Valve Repair versus Minimally Invasive Mitral Valve Surgery: An Observational Study. Journal of Clinical Medicine, 13(5), 1372. https://doi.org/10.3390/jcm13051372







