Abstract
(1) Background: infective endocarditis (IE) is a significant health concern associated with important morbidity and mortality. Only limited, often monocentric, retrospective data on IE in Belgium are available. This prospective study sought to assess the clinical characteristics and outcomes of Belgian IE patients in the ESC EORP European endocarditis (EURO-ENDO) registry; (2) Methods: 132 IE patients were identified based on the ESC 2015 criteria and included in six tertiary hospitals in Belgium; (3) Results: The average Belgian IE patient was male and 62.8 ± 14.9 years old. The native valve was most affected (56.8%), but prosthetic/repaired valves (34.1%) and intracardiac device-related (5.3%) IE are increasing. The most frequently identified microorganisms were S. aureus (37.2%), enterococci (15.5%), and S. viridans (15.5%). The most frequent complications were acute renal failure (36.2%) and embolic events (23.6%). Cardiac surgery was effectively performed when indicated in 71.7% of the cases. In-hospital mortality occurred in 15.7% of patients. Predictors of mortality in the multivariate analysis were S. aureus (HR = 2.99 [1.07–8.33], p = 0.036) and unperformed cardiac surgery when indicated (HR = 19.54 [1.91–200.17], p = 0.012). (4) Conclusion: This prospective EURO-ENDO ancillary analysis provides valuable contemporary insights into the profile, treatment, and clinical outcomes of IE patients in Belgium.
1. Introduction
Despite advancements in infective endocarditis (IE) management, it remains a significant health concern associated with important morbidity and mortality [1,2]. Annually, there are an estimated 13.8 IE cases per 100,000 individuals, resulting in 66,300 global deaths attributed to IE [2,3]. In the past decades, the epidemiologic profile of IE has considerably evolved, with alterations in affected populations, risk factors, and causative microorganisms. Moreover, developments in imaging modalities have been transformative in IE diagnostics [4]. Recently, ESC guidelines on the management of IE have been updated to reflect these insights [2]. Currently, only limited, often monocentric, retrospective data about IE in Belgium are available, and contemporary national epidemiological data are lacking [5,6,7,8]. The European Society of Cardiology (ESC) EURObservational Registry Programme (EORP) European Endocarditis registry (EURO-ENDO) is a multicentre, prospective, observational cohort study investigating the care and outcomes of IE patients at hospitals in Europe and ESC-affiliated/non-affiliated countries [9]. The EORP has been established to enhance the understanding of medical practice through observational studies with rigorous methodological procedures. This ancillary, prospective EORP sub-analysis of IE in Belgium aimed to provide an overview of the contemporary clinical characteristics, microbiological profile, diagnostic workup, in-hospital complications, surgical intervention, and mortality in a nation at the crossroads of Europe.
2. Materials and Methods
2.1. Study Design and Data Collection
The methodology of the ESC EORP EURO-ENDO registry has been previously reported [9]. From 1 January 2016 to 31 March 2018, 3116 IE patients aged at least 18 years were included from 156 centres across 40 countries. Follow-up over a one-year period lasted until April 2019. Centers taking part in the study were categorized into high-level IE centres, characterized by a substantial patient load (≥20 patients per year) and expertise in IE diagnosis, management, imaging, and surgical therapy; or low-volume centres (<20 patients per year), without surgical facilities. In Belgium, six tertiary high-volume hospitals actively participated in the registry: Algemeen Ziekenhuis (AZ) Maria Middelares Gent, Centre Hospitalier Universitaire (CHU) de Liège, CHU Saint-Pierre, Cliniques universitaires Saint-Luc, Universitair Ziekenhuis (UZ) Antwerpen and UZ Brussel. Potential participants were identified from both echocardiographic laboratories and hospitals that cater to patients with IE. Inclusion criteria were a diagnosis of definite IE (or possible IE, but considered and treated as IE) based on the ESC 2015 IE guidelines [4]. Definitions of the terms used in the ESC 2015 modified criteria for the diagnosis of IE are provided in Supplementary Table S1. IE was considered definite if any of the following criteria were met: two major clinical criteria, one major clinical criterion along with three minor criteria, or five minor criteria. A diagnosis of possible IE was considered when one major and one minor criterion were present or when three minor criteria were present. The study exclusion criteria were a patient age of less than 18 years and participation in other interventional clinical studies that would interfere with the patient’s usual care. Data collected at inclusion and during hospitalization comprised demographics (age, sex) and patient history (cardiovascular and other co-morbidities), clinical characteristics at presentation (signs and symptoms), previous invasive intervention within the last 6 months (dental, colonoscopy), microbiology (blood cultures, serology), imaging and its results (echocardiography, cardiac multislice Computed Tomography (CT), 18F-FDG positron emission tomography/computed tomography (18F-FDG PET/CT), cardiac magnetic resonance imaging (MRI)), complications (embolic events, haemodynamic), surgery, and in-hospital mortality. Data were pseudonymized using a unique patient study code [9]. This study complied with the Declaration of Helsinki. The locally appointed ethics committee approved the research protocol, and informed consent was obtained from all subjects (or their legally authorized representative).
2.2. Data Management and Statistical Analysis
Gathering officers at the participating sites were responsible for data collection, which was entered into a comprehensive online electronic case report form (CRF) encompassing 430 distinct data fields. ESC EORP management teams oversaw data quality. The first author, having full access to all study data, assumed responsibility for its integrity and the current data analysis. Continuous variables are presented as mean ± standard deviation. For group comparisons, the Kruskal–Wallis H test for non-parametric data was used. Categorical variables are expressed as frequency and percentages, with 2 × 2 among-group comparisons conducted using Pearson’s chi-squared χ2 test or Fisher’s exact test when expected cell counts were <5. In other instances, the Monte Carlo estimate of the exact p-value was applied. Univariable analysis was performed for both continuous and categorical variables. Prior to advancing to the backward multivariable Cox regression analysis to identify predictors of mortality, pairwise correlations between all candidate variables (with p < 0.10 in univariable analysis) were tested. Survival and event-free survival were also assessed using Kaplan–Meier curves. Several measures of the model of fit, including concordance and the goodness of fit test proposed by May and Hosmer, were considered. Proportional hazard ratio assumptions were graphically verified using the Schoenfeld residuals test. A value of p < 0.050 was considered significant. All analyses were performed using IBM SPSS Statistics software version 29.0.1.0 (171) (Chicago, IL, USA).
3. Results
132 Belgian IE patients were prospectively included. IE was definite in 120/132 (90.9%) patients and possible in 12/132 (9.1%) patients. 51/132 (38.6%) of IE patients were referred from regional centres to one of the participating, tertiary hospitals.
3.1. Patient Demographics and Characteristics
The demographics and characteristics of the patients are displayed in Table 1. Among the 132 IE patients, 47/132 (35.6%) underwent a previous heart valve intervention and 16/132 (12.1%) underwent a preceding intracardiac device implantation. Seventy-five (56.8%) patients were diagnosed with native valve IE (NVE, group 1), 45 (34.1%) with prosthetic or repaired valve IE (PRVE, group 2), and 7 (5.3%) with intracardiac device-related IE (CDRIE, group 3). The remaining five patients, with IE corresponding either to another or an undetermined location, were not included in the group comparison analysis. The valvular IE location was aortic in 76 (57.6%), mitral in 55 (41.7%), tricuspid in 6 (4.5%), and pulmonary in 3 (2.3%) IE patients. IE affected two or more valvular locations in 14.2% of the patients. There were no transcatheter aortic valve replacement (TAVR) or transcatheter mitral valve procedure (TMVP) IE cases in this study.

Table 1.
Demographics and clinical characteristics of Belgian infective endocarditis patients.
3.2. Clinical and Biological Features
The clinical features can be found in Supplementary Table S2. Fever (70.1%), cardiac murmur (49.6%), and embolic events (26.0%) were the most frequent symptoms at initial presentation.
Blood cultures were positive in 90% of Belgian IE patients. The microbiological results are shown in Supplementary Table S3. The most frequently identified microorganisms were S. aureus (37.2%), Enterococci (15.5%) and S. viridans (15.5%). Gram-negative HACEK infections were rare (2.7%). There were no C. burnetii infections and no significant differences in microbiological profiles between the groups.
3.3. Imaging
Echocardiography was performed at least once in 100.0%, transthoracic echocardiography (TTE) in 81.9%, and transoesophageal echocardiography (TOE) in 94.5% of Belgian IE patients. There were no significant differences in usage between the groups, nor in the echocardiographically assessed vegetation size or local IE complications.
18F-FDG PET/CT was performed in 46/127 (36.2%) and positive in 28/46 (60.1%) IE patients. There was 75% cardiac and 53.6% extra-cardiac uptake, without significant differences in utilization or findings between the groups. Cardiac CT (5.5%), leukocyte scintigraphy (3.1%) and cardiac MRI (0.0%) were rarely used, likewise without statistically significant differences between groups. Extra-cardiac CT was performed in 63.0% and revealed extra-cardiac lesions in 46.3% of IE patients.
3.4. In-Hospital Complications under Treatment
The main in-hospital complications are shown in Supplementary Table S4. Acute renal failure (36.2%) was the most frequent in-hospital complication in Belgian IE patients, followed by embolic events (23.6%, of which 46.7% were cerebral embolic events) and positive blood cultures after 48 h (20.5%). There were no significant differences between the groups.
3.5. Cardiac Surgery and Mortality
Following the ESC guidelines [4], the theoretical indication for cardiac surgery (103/127, 81.1%) was not significantly different between the groups (p = 0.092). The most frequent indication in all groups was infection (67.0%, p = 0.956). Surgery was effectively performed when indicated in 91/127 (71.7%) patients: 70.1% in NVE, 75.5% in PRVE, and 57.1% in CDRIE (p = 0.722). The most common reason for not performing surgery despite a theoretical indication was a high surgical risk (83.3%, p = 1.000), followed by death before surgery (8.3%) and neurological complications (8.3%). When operated on, bioprostheses were most often implanted (aortic valve position: 42.4% vs. 22.0% mechanical; mitral valve position: 47.1% vs. 26.5% mechanical). Mitral valve repair was only performed in 23.5% of patients with mitral valve IE.
In-hospital death occurred in 20/127 (15.7%) IE patients. The causes of mortality are shown in Table 2. Independent predictors of mortality by multivariable analysis in IE patients are shown in Table 3 and by univariate analysis in Supplementary Table S5.

Table 2.
In-hospital mortality of Belgian infective endocarditis patients.

Table 3.
Multivariate Cox regression analysis for in-hospital all-cause mortality in Belgian infective endocarditis patients.
Kaplan-Meier survival curves are provided for all-cause mortality according to group (Figure 1) and according to the performance of surgery when indicated (Figure 2).
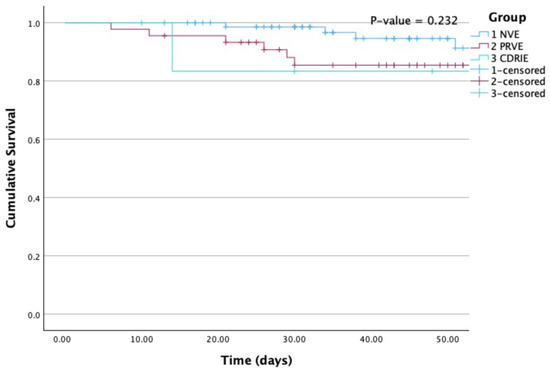
Figure 1.
Kaplan-Meier curves for all-cause mortality according to group. NVE, native valve infective endocarditis; PRVE, prosthetic + repaired valve infective endocarditis; CDRIE, cardiac device-related infective endocarditis. P (Log Rank) = 0.232.
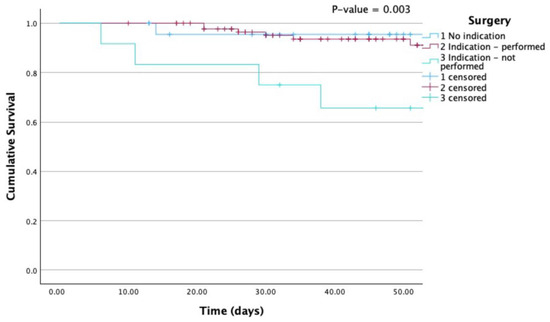
Figure 2.
Kaplan-Meier curves for all-cause mortality according to surgery. Mortality was particularly elevated when surgery was indicated but not performed. P (Log Rank) 0.003.
After follow-up, an additional four patients died, resulting in a total one-year mortality rate of 18.9%.
4. Discussion
This is the first prospective, multicentric study of IE in Belgium. The following findings arise from this EURO-ENDO ancillary analysis: first, the average Belgian IE patient was male and approximately 63 years old. Second, the native aortic valve is most commonly affected, but PRVE and CDRIE are markedly increasing. Third, cancer is a frequent comorbidity in Belgian IE patients. Fourth, the most frequently identified microorganism was S. aureus, followed by S. viridans and enterococci. Fifth, when indicated, surgery was often performed. Mainly bioprostheses are used. Sixth, notwithstanding, IE remains a deadly disease.
4.1. Demographics, Clinical and Microbiological Characteristics
With a mean age of 62.8 ± 14.9 years, Belgian IE patients are notably older than the global IE population in EURO-ENDO (around 59 years old) [10]. This finding is in accordance with previous retrospective Belgian IE studies [5,8], but in stark contrast to the more commonly affected younger age groups outside of Europe (between 35 and 55 years old). This underscores regional variations in the epidemiology of this disease, with varying predisposing conditions, such as rheumatic heart disease and injection drug use [11,12]. Nevertheless, the mean age of IE patients has been gradually increasing globally since the 1990s [13,14], presenting treatment challenges due to additional comorbidities and frailty. This study further confirms a previously asserted 2:1 male-to-female ratio in IE [2].
The frequency of PRVE (34.1%) and CDRIE (5.3%) in Belgium is comparable to the global IE population, and the proportion of valvular IE locations is equally similar, with the aortic (57.6%) and mitral valves (41.7%) being most commonly affected [10]. In many European countries, the share of PVRE is steadily increasing since the 2000s due to older age [15]. In contrast, in countries with a younger IE population due to injection drug use, NVE remains much more common (90%), and mitral and tricuspid valve infections often exceed aortic valve IE [11,12].
Belgian IE patients commonly retain a history of arterial hypertension (53.5%), atrial fibrillation (29.9%), cancer (23.6%), and ischemic heart disease (19.7%), particularly in the slightly older PRVE and CDRIE groups. A previous EURO-ENDO ancillary study showed a cancer prevalence of 11.6% in the global IE population [16]. In Belgium, combined cardiovascular disease and cancer cause > 50% of all deaths, emphasizing the importance of investigating the shared pathophysiological mechanisms [17]. Cancer patients may be at an increased risk of developing IE, mainly after invasive procedures or due to compromised immunity [16]. In Belgian IE patients, immunosuppressive treatments (3.9%) and colonoscopy procedures (5.5%) were comparable to those in the global IE population [10]. The current study’s IE patients had distinctly less heart failure compared to the global EURO-ENDO IE population (11.8% vs. 23.3%), and less frequently presented with congestion (18.1% vs. 27.2%) and cardiogenic shock (1.6% vs. 2.3%) [10], possibly due to a referral bias, as patients with severe heart failure might have been deemed unfit to be transferred to a tertiary cardiac surgery centre.
There were only 10% blood culture-negative Belgian IE cases, compared to 20% in the global EURO-ENDO population [10], possibly testifying to improved diagnostic strategies, such as culturing on specialized media, systematic serological testing, polymerase chain reaction assays, and ribosomal ribonucleic acid sequencing [2]. A recent EURO-ENDO ancillary study showed increased mortality in blood culture-negative IE [18], but there was no significant difference in mortality between blood culture-positive and-negative IE patients in the current study (p = 0.307). The most frequently identified microorganism remains S. aureus (37.2%), which is a persisting concern given its potential for severe complications and high mortality rate [19]. On the other hand, the prevalence of enterococci (15.5%) has become equal to that of S. viridans IE in Belgium (15.5%), while these former infections were rarer in the past (around 10%) [14,15], but have been steadily increasing in Belgium since the 2000s [5,8]. This might be explained by the aging population and a higher cancer prevalence [20,21]. In contrast, outside Europe, S. aureus remains much more prevalent (45%), compared to streptococci (7%) and enterococci (6%) [11,12]. Together with S. gallolyticus, intestinal bacteria accounted for 23.7% of IE cases in this study. This microbiological shift was also observed in the global EURO-ENDO data, where Enterococcus overtook S. viridans IE (15.8% vs. 12.4%) [10]. As such, opportunities for IE prevention may arise during invasive urogenital and gastrointestinal procedures, particularly in older individuals or those with a cancer history [2,16,20].
4.2. Imaging
The transformative application of imaging techniques shortly after the publication of the 2015 ESC guidelines was similarly observed in Belgian IE patients, as TEE was performed in 94.5% (vs. 58.1% in the EURO-ENDO global population) and 18F-FDG PET/CT in 36.2% (vs. 16.6%) [4,10]. 18F-FDG PET/CT has proven useful in the diagnosis of PRVE and CDRIE [2]. However, in Belgian IE patients, there was only a marginally higher application in CDRIE (57.1%) and PRVE (40.0%) compared to NVE (32.0%, p = 0.463). In NVE, 18F-FDG PET/CT mainly has value in detecting peripheral lesions or adding minor diagnostic criteria [2]. In Belgium, there might still be room for improvement in the application of leukocyte scintigraphy (3.1%), cardiac MRI (0.0%), and especially cardiac CT (5.5%) for the detection of perivalvular infection in PRVE [2]. However, given the elapsed time since the EURO-ENDO registry and the currently more prevalent availability of these modern imaging methods, a more contemporary study might already show increased utilization.
4.3. Management and Outcome of IE in Belgium
4.3.1. In-Hospital Complications
Acute renal failure is the most common complication and is remarkably more frequent than in the global EURO-ENDO population (36.2% vs. 17.7%) [10], possibly due to the older Belgian IE population with regularly underlying chronic renal failure (17.3%). In contrast, Belgian IE patients developed less congestive heart failure during hospitalisation (8.7%), possibly due to a previously mentioned referral bias. At admission, embolic events were already present in 26.0% of IE patients, and despite the initiation of antibiotic therapy, additional (especially cerebral) embolism was detected in 23.6%. These are frequent and life-threatening complications, with a high global incidence ranging from 13 to 49% in IE [5,19]. However, in this study (cerebral) embolism accounted for only 5% of all-cause mortality. In comparison, outside of Europe, pulmonary embolism is more frequently seen (up to 35%) and is typically related to right-sided IE due to injection drug use [22].
4.3.2. Surgery
Surgery was performed in 71.7% of IE patients, which is high compared to the global IE-related interventions of about 50% since the 2000s [10,23] and a sometimes low cardiac surgery rate (13%) outside Europe [11]. Improved identification of patients eligible for surgery may have contributed to this number, as well as a referral bias to tertiary centres with cardiac surgery [24]. The theoretical indication for surgery following the ESC 2015 guidelines in Belgian IE patients was high (81.1%), and when indicated, cardiac surgery was often effectively performed. When denied surgery, the main reason was a high surgical risk, possibly related to old age, frailty, and comorbidities. Bioprosthetic valves were most often used in Belgian IE patients, more frequently than observed in the Euro heart survey of 2003, when mechanical prostheses were still more prominent (74%) [25]. This change might be related to older age and the possible need for further surgical procedures with a risk of bleeding under anticoagulant therapy [26]. Mitral valve repair is underused in Belgian (23.5%), as in global IE patients, perhaps due to excessive valvular destruction [10]. In contrast, several studies have shown that valve repair, when feasible, is associated with better outcomes [13,27].
4.3.3. In-Hospital Mortality
In-hospital all-cause mortality was comparable to that of the global EURO-ENDO population (15.7% vs. 17.1%) and remains substantial [10]. There was a trend towards higher all-cause death in the CDRIE and PRVE groups (p = 0.051). The lower mortality compared to previous registries might once again be due to a referral bias to tertiary centres. The main driver of all-cause mortality in Belgian patients was non-cardiovascular disease, particularly sepsis (60.0%). By univariate Cox regression analysis, predictors of mortality were the Charlson index (HR = 1.18 [1.04–1.34], p = 0.011), creatinine > 2 mg/dL (HR = 3.49 [1.34–9.08], p = 0.011), and unperformed cardiac surgery when indicated (HR = 13.72 [1.56–120.61], p = 0.018). Given the significant impact of cancer on the Charlson index, it might be worthwhile to conduct a sub-analysis using a modified Charlson score that excludes cancer [16,28]. By multivariate analysis, adjusted for age, S. aureus (HR = 2.99 [1.07–8.33], p = 0.036) and especially unperformed cardiac surgery when indicated (HR = 19.54 [1.91–200.17], p = 0.012) remained significant, independent predictors of outcome. This further emphasizes the need for early discussion with surgeons within the IE team, as recently reemphasized by the 2023 ESC guidelines [2].
4.4. Study Limitations
This ancillary study has the same limitations as the EURO-ENDO registry, especially selection and referral bias, as all patients were recruited in high-level centres and inclusion was based on voluntary participation [10]. Therefore, estimating the true incidence of IE in Belgium is unfeasible based on the current dataset, patient characteristics might have been influenced, and the effect on mortality is unclear [24]. Additionally, while the present dataset dating back to 2016–2018 does not include TAVR or TMVP IE, these would be more frequently present in contemporary data collection and warrant future exploration. Furthermore, comprehensive data concerning the pivotal role of the IE team, highlighted in the latest guidelines [2], were missing in the current study. Future studies should elucidate its impact on clinical outcomes. The reasons for denial of surgery should also be investigated in future studies of IE patients, as well as the development of scores to predict the futility of surgical therapy [2]. This IE study had a restricted follow-up period of one year. However, given potential late relapse, complications, and mortality [8], future investigations should incorporate an extended follow-up. Finally, this was an ancillary analysis of a registry that was not dedicated to the Belgian population. The limited number of Belgian IE patients in the EURO-ENDO underscores the necessity for a future prospective, observational cohort study of IE in Belgium, proportionally including non-tertiary regional centers and with an extended inclusion period to improve recruitment and increase the study’s power. This might provide a more comprehensive understanding of this rare but deadly disease. These limitations were counterbalanced by the active participation of many tertiary centers and the quality of CRF completion in EURO-ENDO [9].
5. Conclusions
This prospective EURO-ENDO ancillary analysis provides valuable contemporary insights into the profile, diagnostics, treatment, and clinical outcomes of IE patients in Belgium, in light of recent updates in IE management recommendations. Belgian IE patients are older and often have cancer. The native aortic valve is frequently affected; however, PRVE and CDRIE are markedly increasing. S. aureus, followed by S. viridans and Enterococci are the primary micro-organisms. When indicated, surgery is often performed. Multivariate analysis revealed that S. aureus and unperformed cardiac surgery, when indicated, were independent predictors of outcome. Despite important study limitations, these data could nevertheless guide future research endeavors to enhance the management and prognosis of IE in Belgian healthcare.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13051371/s1, Table S1: Definitions of the terms used in the European Society of Cardiology 2015 modified criteria for the diagnosis of infective endocarditis; Table S2: Clinical presentation of Belgian infective endocarditis patients; Table S3: Microbiology in Belgian infective endocarditis patients; Table S4: In-hospital complications under therapy in Belgian infective endocarditis patients; Table S5: Univariate Cox regression analysis for all causes of death in hospital in Belgian IE patients. Complete list of the EURO-ENDO Investigators Group and EURO-ENDO National Coordinators.
Author Contributions
Conceptualization, G.H., B.I. and B.C.; methodology, G.H., C.L. and C.S.-S.; validation, B.C., P.L., B.I. and G.H.; formal analysis, B.R.; investigation, B.R., B.C., P.L., A.P., J.D.S., P.U., B.P., P.V., A.M., X.G., B.I. and G.H.; data curation, B.R. and C.L.; writing—original draft preparation, B.R.; writing—review and editing, B.R., B.C., C.L., C.S.-S., P.L., A.P., J.D.S., P.U., B.P., P.V., A.M., X.G., B.I. and G.H; supervision, B.C., C.L. and G.H.; funding acquisition, G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Abbott Vascular Int. (2011–2021), Amgen Cardiovascular (2009–2018), AstraZeneca (2014–2021), Bayer AG (2009–2018), Boehringer Ingelheim (2009–2019), Boston Scientific (2009–2012), The Bristol Myers Squibb and Pfizer Alliance (2011–2019), Daiichi Sankyo Europe GmbH (2011–2020), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–2017), Edwards (2016–2019), and Gedeon Richter Plc. (2014–2016); Menarini, Int. Op. (2009–2012), MSD-Merck & Co. (2011–2014), Novartis Pharma AG (2014–2020), ResMed (2014–2016), Sanofi (2009–2011), SERVIER (2009–2021), Vifor (2019–2022).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Institutional Review Boards (or Ethics Committees).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
EORP Oversight Committee, Registry Executive, and Steering Committees. The Data collection was conducted by the EORP department of the ESC: Emanuela Fiorucci, as Project Officer; Viviane Missiamenou, Florian Larras and Rachid Mir Hassaine, as Data Managers. Statistical analyses were performed using the Cécile Laroche of the EURObservational Research Programme, European Society of Cardiology, Sophia-Antipolis, France. Overall activities were coordinated and supervised by Doctor Aldo P. Maggioni (EORP Scientific Coordinator). Special thanks to the European Association of CardioVascular Imaging (EACVI) and the ESC Working Group on Valvular Heart Disease for their support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Habib, G. Management of Infective Endocarditis. Heart 2006, 92, 124–130. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; De Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the Management of Endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Momtazmanesh, S.; Moghaddam, S.S.; Rad, E.M.; Azadnajafabad, S.; Ebrahimi, N.; Mohammadi, E.; Rouhifard, M.; Rezaei, N.; Masinaei, M.; Rezaei, N.; et al. Global, Regional, and National Burden and Quality of Care Index of Endocarditis: The Global Burden of Disease Study 1990–2019. Eur. J. Prev. Cardiol. 2022, 29, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis. Eur. Heart J. 2015, 36, 3075–3123. [Google Scholar] [CrossRef]
- Motoc, A.; Kessels, J.; Roosens, B.; Lacor, P.; Van de Veire, N.; De Sutter, J.; Magne, J.; Droogmans, S.; Cosyns, B. Impact of the Initial Clinical Presentation on the Outcome of Patients with Infective Endocarditis. Cardiol. J. 2023, 30, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Poesen, K.; Pottel, H.; Colaert, J.; De Niel, C. Epidemiology of Infective Endocarditis in a Large Belgian Non-Referral Hospital. Acta Clin. Belg. 2014, 69, 183–190. [Google Scholar] [CrossRef]
- Yombi, J.C.; Yuma, S.N.; Pasquet, A.; Astarci, P.; Robert, A.; Rodriguez, H.V. Staphylococcal versus Streptococcal Infective Endocarditis in a Tertiary Hospital in Belgium: Epidemiology, Clinical Characteristics and Outcome. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2017, 72, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Tahon, J.; Geselle, P.J.; Vandenberk, B.; Hill, E.E.; Peetermans, W.E.; Herijgers, P.; Janssens, S.; Herregods, M.C. Long-Term Follow-up of Patients with Infective Endocarditis in a Tertiary Referral Center. Int. J. Cardiol. 2021, 331, 176–182. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Erba, P.A.; Sadeghpour, A.; Meshaal, M.; Sambola, A.; Furnaz, S.; Citro, R.; Ternacle, J.; Donal, E.; et al. The ESC-EORP EURO-ENDO (European Infective Endocarditis) Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 202–207. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical Presentation, Aetiology and Outcome of Infective Endocarditis. Results of the ESC-EORP EURO-ENDO (European Infective Endocarditis) Registry: A Prospective Cohort Study. Eur. Heart J. 2019, 40, 3222–3232B. [Google Scholar] [CrossRef]
- Arora, N.; Panda, P.K.; CR, P.; Uppal, L.; Saroch, A.; Angrup, A.; Sharma, N.; Sharma, Y.P.; Vijayvergiya, R.; Rohit, M.K.; et al. Changing Spectrum of Infective Endocarditis in India: An 11-Year Experience from an Academic Hospital in North India. Indian Heart J. 2021, 73, 711–717. [Google Scholar] [CrossRef]
- Liaqat, W.; Palaiodimos, L.; Li, W.; Karamanis, D.; Tahir, A.; Tzoumas, A.; Nagraj, S.; Tiwari, N.; Grushko, M.; Kokkinidis, D.; et al. Epidemiologic and Clinical Characteristics of Infective Endocarditis: A Single-Center Retrospective Study in the Bronx, New York. Infection 2022, 50, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Solari, S.; Tamer, S.; Aphram, G.; Mastrobuoni, S.; Navarra, E.; Noirhomme, P.; Poncelet, A.; Astarci, P.; Rubay, J.; El Khoury, G.; et al. Aortic Valve Repair in Endocarditis: Scope and Results. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 104–112. [Google Scholar] [CrossRef]
- Hoen, B.; Alla, F.; Selton-Suty, C.; Béguinot, I.; Bouvet, A.; Briançon, S.; Casalta, J.P.; Danchin, N.; Delahaye, F.; Etienne, J.; et al. Changing Profile of Infective Endocarditis: Results of a 1-Year Survey in France. JAMA 2002, 288, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, R.G.; Hoen, B.; Miró, M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century the International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Cosyns, B.; Roosens, B.; Lancellotti, P.; Laroche, C.; Dulgheru, R.; Scheggi, V.; Vilacosta, I.; Pasquet, A.; Piper, C.; Reyes, G.; et al. Cancer and Infective Endocarditis: Characteristics and Prognostic Impact. Front. Cardiovasc. Med. 2022, 8, 766996. [Google Scholar] [CrossRef]
- von Kemp, B. Belgian Guidelines on Supportive Care: Cardiotoxicity of Cancer Treatments. Belgian J. Med. Oncol. 2021, 15, 367–373. [Google Scholar]
- Kong, W.K.F.; Salsano, A.; Giacobbe, D.R.; Popescu, B.A.; Laroche, C.; Duval, X.; Schueler, R.; Moreo, A.; Colonna, P.; Piper, C.; et al. Outcomes of Culture-Negative vs. Culture-Positive Infective Endocarditis: The ESC-EORP EURO-ENDO Registry. Eur. Heart J. 2022, 43, 2770–2780. [Google Scholar] [CrossRef]
- Sambola, A.; Lozano-Torres, J.; Boersma, E.; Olmos, C.; Ternacle, J.; Calvo, F.; Tribouilloy, C.; Reskovic-Luksic, V.; Separovic-Hanzevacki, J.; Park, S.-W.; et al. Predictors of Embolism and Death in Left-Sided Infective Endocarditis: The European Society of Cardiology EURObservational Research Programme European Infective Endocarditis Registry. Eur. Heart J. 2023, 44, 4566–4575. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Lee, S.E.; Cho, I.J.; Shim, C.Y.; Hong, G.R.; Ha, J.W. Infective Endocarditis in Cancer Patients: Causative Organisms, Predisposing Procedures, and Prognosis Differ from Infective Endocarditis in Non-Cancer Patients. Circ. J. 2019, 83, 452–460. [Google Scholar] [CrossRef]
- Oliver, L.; Lavoute, C.; Giorgi, R.; Salaun, E.; Hubert, S.; Casalta, J.P.; Gouriet, F.; Renard, S.; Saby, L.; Avierinos, J.F.; et al. Infective Endocarditis in Octogenarians. Heart 2017, 103, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, A.; Muñoz, P.; Sandoval, C.; Fariñas-Álvarez, M.C.; Gutiérrez-Cuadra, M.; Pericás Pulido, J.M.; Miró, J.M.; Goenaga-Sánchez, M.; De Alarcón, A.; Bonache-Bernal, F.; et al. Infective Endocarditis in Patients with Cancer: A Consequence of Invasive Procedures or a Harbinger of Neoplasm? A Prospective, Multicenter Cohort. Medicine 2017, 96, e7913. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the Practice of Echocardiography in Infective Endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef] [PubMed]
- El Kadi, S.; van den Buijs, D.M.F.; Meijers, T.; Gilbers, M.D.; Bekkers, S.C.A.M.; van Melle, J.P.; Riezebos, R.K.; Blok, W.L.; Tanis, W.; Wahadat, A.R.; et al. Infective Endocarditis in the Netherlands: Current Epidemiological Profile and Mortality: An Analysis Based on Partial ESC EORP Collected Data. Netherlands Heart J. 2020, 28, 526–536. [Google Scholar] [CrossRef]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A Prospective Survey of Patients with Valvular Heart Disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Tornos, P.; Iung, B.; Permanyer-Miralda, G.; Baron, G.; Delahaye, F.; Gohlke-Bärwolf, C.; Butchart, E.G.; Ravaud, P.; Vahanian, A. Infective Endocarditis in Europe: Lessons from the Euro Heart Survey. Heart 2005, 91, 571–575. [Google Scholar] [CrossRef]
- Toyoda, N.; Itagaki, S.; Egorova, N.N.; Tannous, H.; Anyanwu, A.C.; El-Eshmawi, A.; Adams, D.H.; Chikwe, J. Real-World Outcomes of Surgery for Native Mitral Valve Endocarditis. J. Thorac. Cardiovasc. Surg. 2017, 154, 1906–1912.e9. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a Combined Comorbidity Index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).