Expanding the Armamentarium of Donor Sites in Microvascular Head and Neck Reconstruction
Abstract
1. Introduction
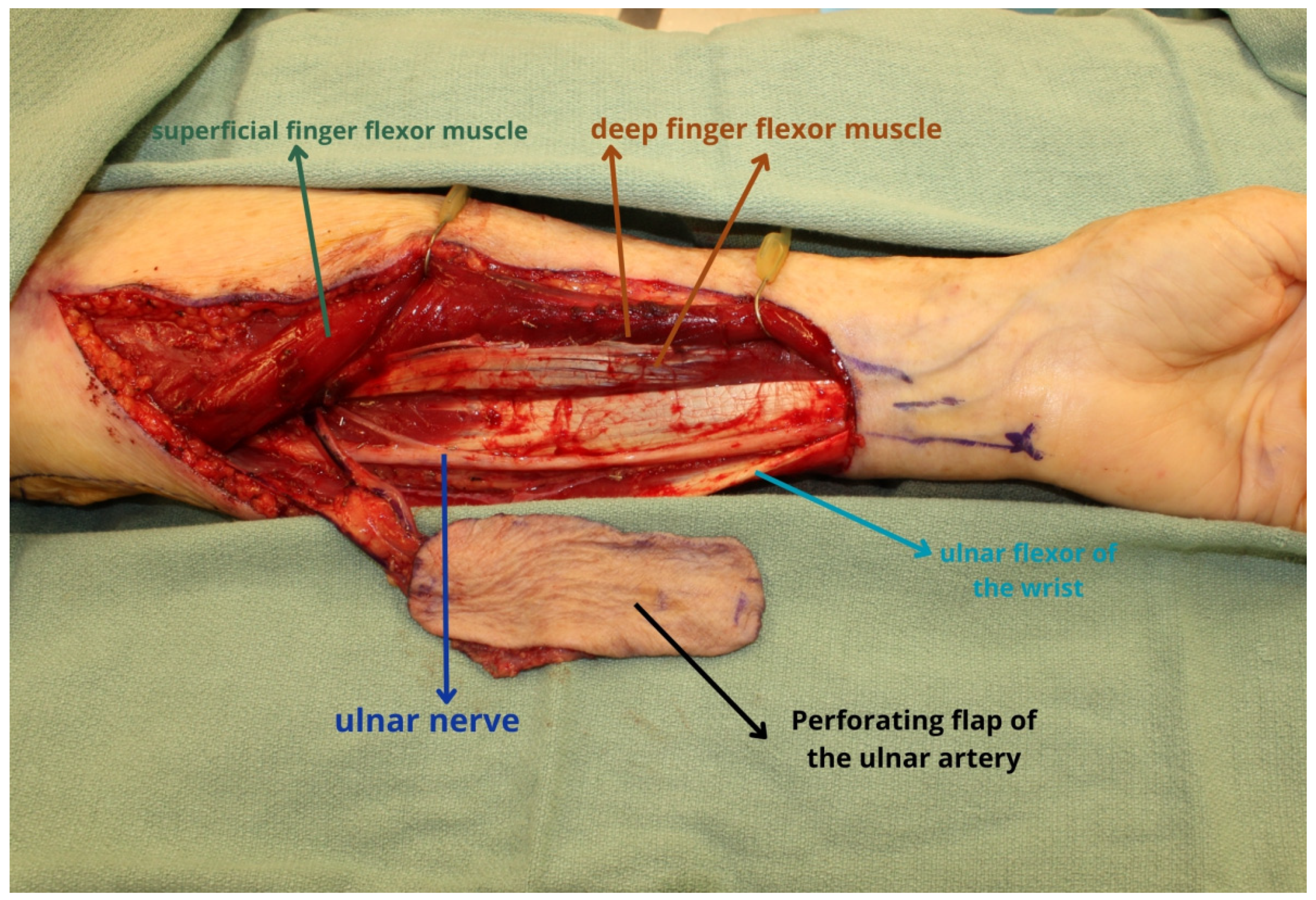
2. Ulnar Forearm Flap
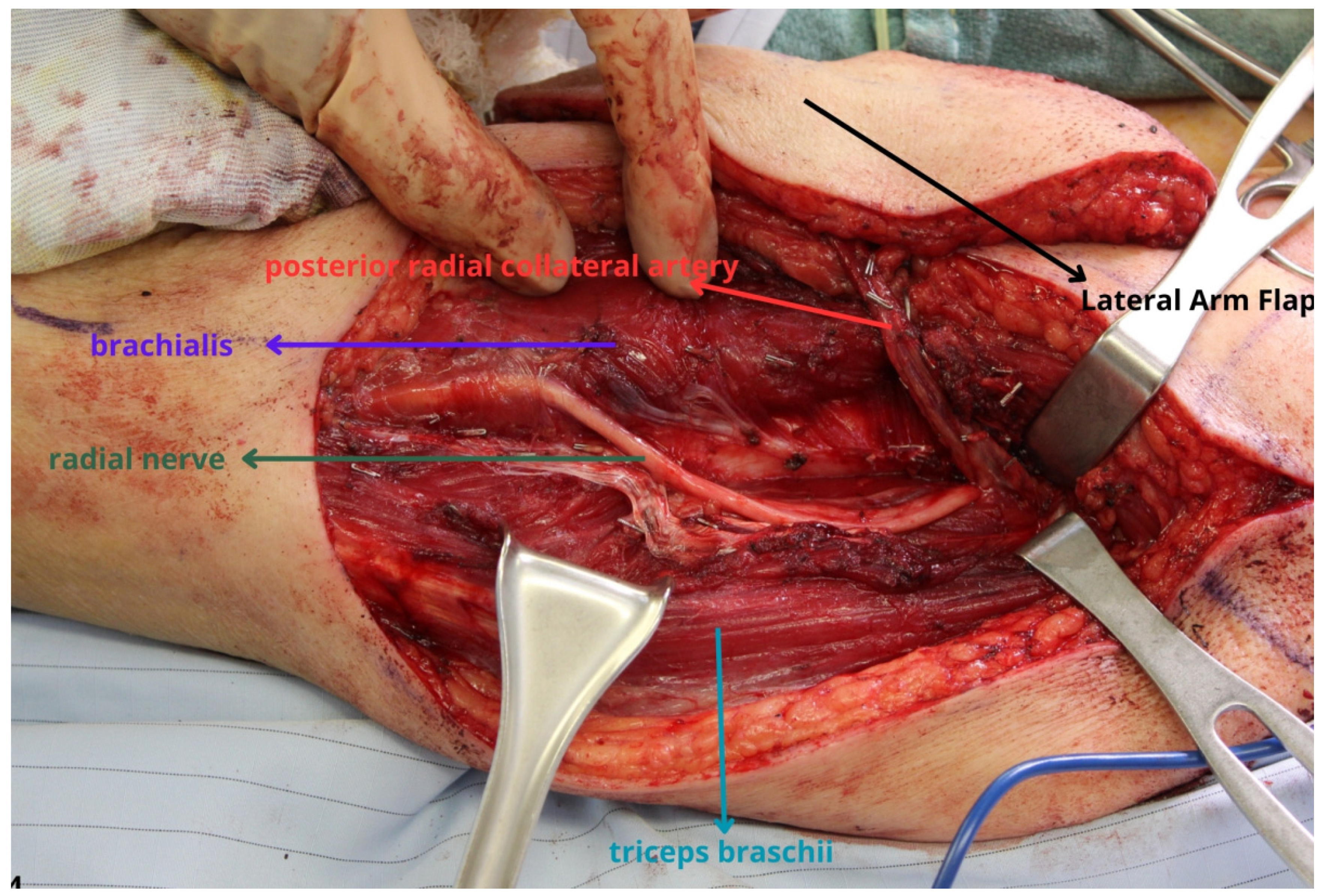
3. Lateral Arm and Lateral Forearm Flap
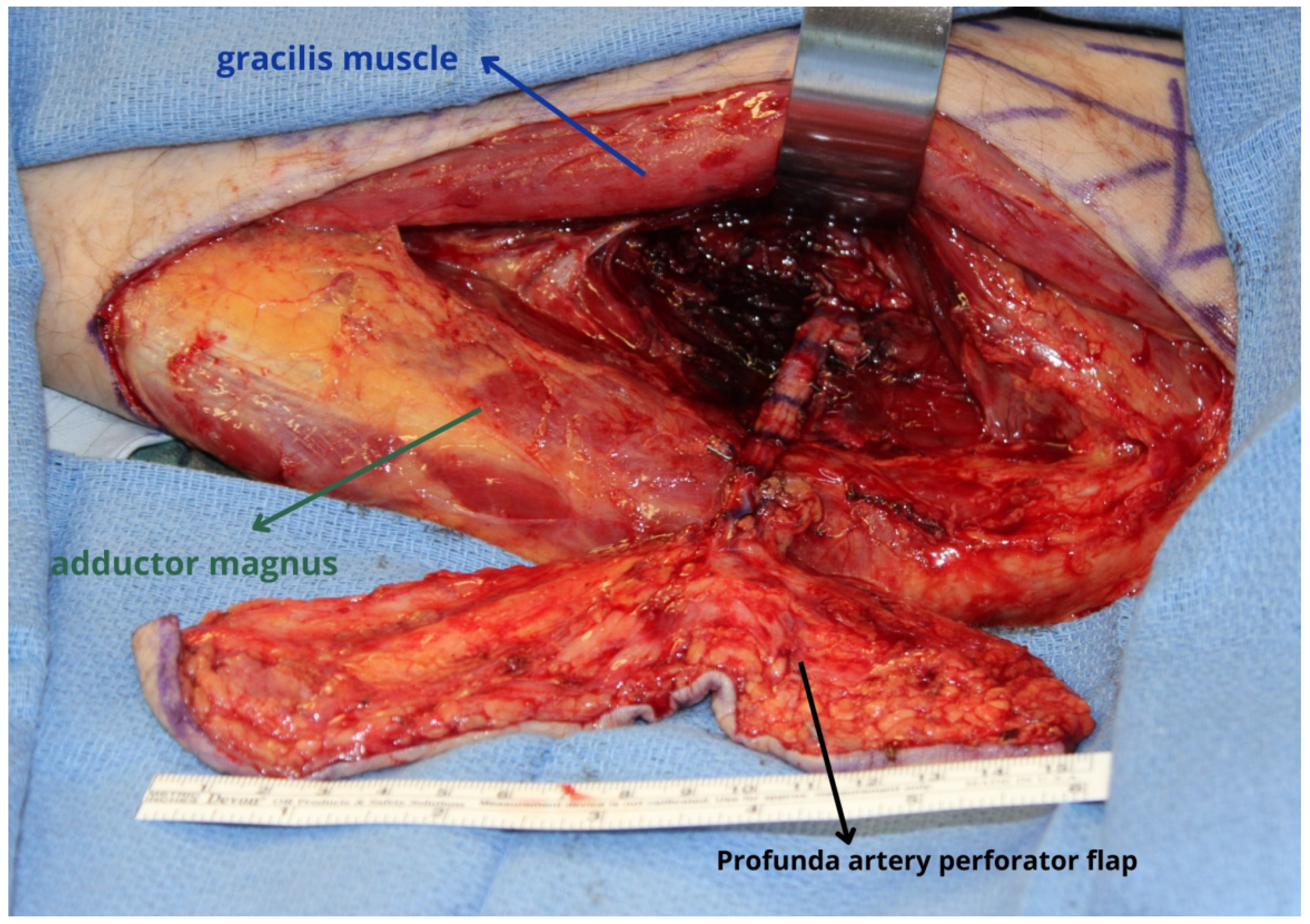
4. Profunda Artery Perforator Flap
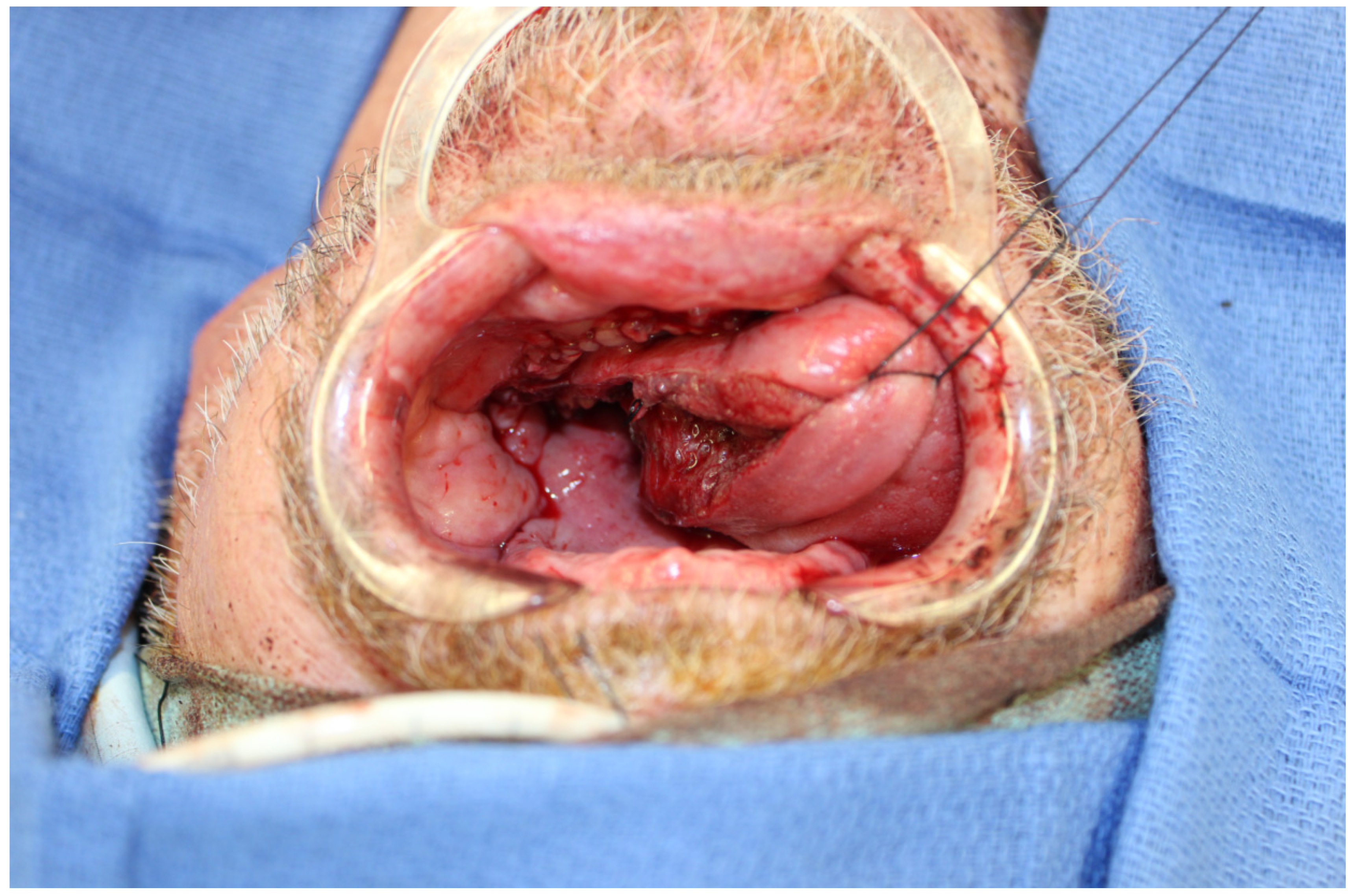
5. Medial Sural Artery Perforator Flap
6. Superficial Circumflex Iliac Perforator Flap
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, C.H.; Wei, F.C. Microsurgical free flap in head and neck reconstruction. Head Neck 2010, 32, 1236–1245. [Google Scholar] [CrossRef]
- Mücke, T.; Ritschl, L.M.; Roth, M.; Güll, F.D.; Rau, A.; Grill, S.; Kesting, M.R.; Wolff, K.D.; Loeffelbein, D.J. Predictors of free flap loss in the head and neck region: A four-year retrospective study with 451 microvascular transplants at a single centre. J. Craniomaxillofac. Surg. 2016, 44, 1292–1298. [Google Scholar] [CrossRef]
- Pellini, R.; De Virgilio, A.; Mercante, G.; Pichi, B.; Manciocco, V.; Marchesi, P.; Ferreli, F.; Spriano, G. Vastus lateralis myofascial free flap in tongue reconstruction. Acta Otorhinolaryngol. Ital. 2016, 36, 321–325. [Google Scholar] [CrossRef]
- Chang, E.I.; Hanasono, M.M.; Butler, C.E. Management of Unfavorable Outcomes in Head and Neck Free Flap Reconstruction: Experience-Based Lessons from the MD Anderson Cancer Center. Clin. Plast. Surg. 2016, 43, 653–667. [Google Scholar] [CrossRef]
- Chang, E.I. Alternate Soft-Tissue Free Flaps for Head and Neck Reconstruction: The Next Generation of Workhorse Flaps. Plast. Reconstr. Surg. 2023, 152, 184–193. [Google Scholar] [CrossRef]
- Wood, J.W.; Broussard, K.C.; Burkey, B. Preoperative testing for radial forearm free flaps to reduce donor site morbidity. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 183–186. [Google Scholar] [CrossRef]
- Kantar, R.S.; Rifkin, W.J.; Cammarata, M.J.; Jacoby, A.; Farber, S.J.; Diaz-Siso, J.R.; Ceradini, D.J.; Rodriguez, E.D. Appraisal of the Free Ulnar Flap Versatility in Craniofacial Soft-tissue Reconstruction. Plast. Reconstr. Surg. Glob. Open. 2018, 6, e1863. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Chang, E.I.; Selber, J.C.; Hanasono, M.M. Perforator patterns of the ulnar artery perforator flap. Plast. Reconstr. Surg. 2012, 129, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ishiko, M.; Yano, K.; Onode, E.; Takamatsu, K. Identification of Ulnar Artery Perforators Using Color Doppler Ultrasonography. J. Reconstr. Microsurg. 2020, 36, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.K.; Hootnick, J.L.; Antony, A.K. Ulnar forearm free flaps in head and neck reconstruction: Systematic review of the literature and a case report. Microsurgery 2014, 34, 68–75. [Google Scholar] [CrossRef]
- Hekner, D.D.; Abbink, J.H.; van Es, R.J.; Rosenberg, A.; Koole, R.; Van Cann, E.M. Donor-site morbidity of the radial forearm free flap versus the ulnar forearm free flap. Plast. Reconstr. Surg. 2013, 132, 387–393. [Google Scholar] [CrossRef]
- Chang, E.I.; Liu, J. Prospective Comparison of Donor-Site Morbidity following Radial Forearm and Ulnar Artery Perforator Flap Harvest. Plast. Reconstr. Surg. 2020, 145, 1267–1274. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Siegberg, F.; Römer, P.; Blatt, S.; Pabst, A.; Heimes, D.; Al-Nawas, B.; Kämmerer, P.W. Long-Term Donor Site Morbidity and Flap Perfusion Following Radial versus Ulnar Forearm Free Flap-A Randomized Controlled Prospective Clinical Trial. J. Clin. Med. 2022, 11, 3601. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, P.L.; Liu, Y.H.; Wang, S.M.; Xu, Z.F.; Feng, C.J. Comparing donor site morbidity between radial and ulnar forearm free flaps: A meta-analysis. Br. J. Oral. Maxillofac. Surg. 2022, 60, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Marques Faria, J.C.; Rodrigues, M.L.; Scopel, G.P.; Kowalski, L.P.; Ferreira, M.C. The versatility of the free lateral arm flap in head and neck soft tissue reconstruction: Clinical experience of 210 cases. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Ibrahim, A.; Papazian, N.; Jurgus, A.; Nguyen, A.T.; Suami, H.; Yu, P. Perforator Mapping and Optimizing Design of the Lateral Arm Flap: Anatomy Revisited and Clinical Experience. Plast. Reconstr. Surg. 2016, 138, 300e–306e. [Google Scholar] [CrossRef] [PubMed]
- Busnardo, F.F.; Coltro, P.S.; Olivan, M.V.; Faes, J.C.; Lavor, E.; Ferreira, M.C.; Rodrigues, A.J., Jr.; Gemperli, R. Anatomical comparison among the anterolateral thigh, the parascapular, and the lateral arm flaps. Microsurgery 2015, 35, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Shuck, J.; Chang, E.I.; Mericli, A.F.; Gross, N.D.; Hanasono, M.M.; Garvey, P.B.; Yu, P.; Largo, R.D. Free Lateral Forearm Flap in Head and Neck Reconstruction: An Attractive Alternative to the Radial Forearm Flap. Plast. Reconstr. Surg. 2020, 146, 446e–450e. [Google Scholar] [CrossRef]
- Danker, S.; Shuck, J.W.; Taher, A.; Mujtaba, B.; Chang, E.I.; Chu, C.K.; Liu, J.; Garvey, P.B.; Hanna, E.; Yu, P.; et al. The lateral forearm flap versus traditional upper extremity flaps: A comparison of donor site morbidity and flap thickness. Head Neck 2023, 45, 2413–2423. [Google Scholar] [CrossRef]
- Contrera, K.J.; Hassan, A.M.; Shuck, J.W.; Bobian, M.; Ha, A.Y.; Chang, E.I.; Garvey, P.B.; Roubaud, M.S.; Lee, Z.H.; Hanasono, M.M.; et al. Outcomes for 160 Consecutive Lateral Arm Free Flaps for Head and Neck Reconstruction. Otolaryngol. Head Neck Surg. 2023. [Google Scholar] [CrossRef]
- Chu, C.K.; Largo, R.D.; Lee, Z.H.; Adelman, D.M.; Egro, F.; Winocour, S.; Reece, E.M.; Selber, J.C.; Butler, C.E. Introduction of the L-PAP Flap: Bipedicled, Conjoined, and Stacked Thigh-Based Flaps for Autologous Breast Reconstruction. Plast. Reconstr. Surg. 2023, 152, 1005e–1010e. [Google Scholar] [CrossRef]
- Cohen, Z.; Azoury, S.C.; Matros, E.; Nelson, J.A.; Allen, R.J., Jr. Modern Approaches to Alternative Flap-Based Breast Reconstruction: Profunda Artery Perforator Flap. Clin. Plast. Surg. 2023, 50, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.D.; Bhadkamkar, M.A.; Asaad, M.; Chu, C.K.; Garvey, P.B.; Butler, C.E.; Yu, P.; Hanasono, M.M.; Chang, E.I. The Profunda Artery Perforator Flap: A Versatile Option for Head and Neck Reconstruction. Plast. Reconstr. Surg. 2021, 147, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Padilla, P.; Kurlander, D.E.; Corkum, J.P.; Hanasono, M.M.; Garvey, P.B.; Chang, E.I.; Yu, P.; Largo, R.D. Profunda Artery Perforator Flap Tongue Reconstruction: An Effective and Safe Alternative to the Anterolateral Thigh Flap. Plast. Reconstr. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Hanick, A.L.; Meleca, J.B.; Roskies, M.; Hadford, S.P.; Genther, D.J.; Ciolek, P.J.; Lamarre, E.D.; Ku, J.A. The profunda artery perforator flap for head and neck reconstruction. Am. J. Otolaryngol. 2023, 44, 103772. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.M.K.; Jozaghi, Y.; Danker, S.; Karami, R.; Asaad, M.; Lai, S.Y.; Hanna, E.Y.; Esmaeli, B.; Gidley, P.W.; Chang, E.I. The combined profunda artery perforator-gracilis flap for immediate facial reanimation and resurfacing of the radical parotidectomy defect. Microsurgery 2023, 43, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.D.; Chu, C.K.; Chang, E.I.; Liu, J.; Abu-Ghname, A.; Wang, H.; Schaverien, M.V.; Mericli, A.F.; Hanasono, M.M.; Yu, P. Perforator Mapping of the Profunda Artery Perforator Flap: Anatomy and Clinical Experience. Plast. Reconstr. Surg. 2020, 146, 1135–1145. [Google Scholar] [CrossRef]
- Deek, N.F.A.; Hsiao, J.C.; Do, N.T.; Kao, H.K.; Hsu, C.C.; Lin, C.H.; Lin, C.H. The Medial Sural Artery Perforator Flap: Lessons Learned from 200 Consecutive Cases. Plast. Reconstr. Surg. 2020, 146, 630e–641e. [Google Scholar] [CrossRef] [PubMed]
- Daar, D.A.; Abdou, S.A.; Cohen, J.M.; Wilson, S.C.; Levine, J.P. Is the Medial Sural Artery Perforator Flap a New Workhorse Flap? A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2019, 143, 393e–403e. [Google Scholar] [CrossRef]
- Danielian, A.; Cheng, M.Y.; Han, P.S.; Blackwell, K.E.; Kerr, R.P.R. Medial Sural Artery Perforator Flap: A Middle Ground Between Anterolateral Thigh and Radial Forearm Flaps. Otolaryngol. Head Neck Surg. 2023, 169, 852–857. [Google Scholar] [CrossRef]
- Al Omran, Y.; Evans, E.; Jordan, C.; Borg, T.M.; AlOmran, S.; Sepehripour, S.; Akhavani, M.A. The Medial Sural Artery Perforator Flap versus Other Free Flaps in Head and Neck Reconstruction: A Systematic Review. Arch. Plast. Surg. 2023, 50, 264–273. [Google Scholar] [CrossRef]
- Ng, M.J.M.; Goh, C.S.L.; Tan, N.C.; Song, D.H.; Ooi, A.S.H. A Head-to-Head Comparison of the Medial Sural Artery Perforator versus Radial Forearm Flap for Tongue Reconstruction. J. Reconstr. Microsurg. 2021, 37, 445–452. [Google Scholar] [CrossRef]
- Molina, A.R.; Citron, I.; Chinaka, F.; Cascarini, L.; Townley, W.A. Calf Perforator Flaps: A Freestyle Solution for Oral Cavity Reconstruction. Plast. Reconstr. Surg. 2017, 139, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Pease, N.L.; Ong, J.; Townley, W.A. Fixed reference points in mapping medial sural artery perforator location. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Dusseldorp, J.R.; Pham, Q.J.; Ngo, Q.; Gianoutsos, M.; Moradi, P. Vascular anatomy of the medial sural artery perforator flap: A new classification system of intra-muscular branching patterns. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Q.; Lv, Y.; Shen, H.; Lu, H.; Wu, S.C.; Lin, X.J. Endoscope-assisted medial sural artery perforator flap for head and neck reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Taufique, Z.M.; Daar, D.A.; Cohen, L.E.; Thanik, V.D.; Levine, J.P.; Jacobson, A.S. The medial sural artery perforator flap: A better option in complex head and neck reconstruction? Laryngoscope 2019, 129, 1330–1336. [Google Scholar] [CrossRef]
- Altiparmak, M.; Cha, H.G.; Hong, J.P.; Suh, H.P. Superficial Circumflex Iliac Artery Perforator Flap as a Workhorse Flap: Systematic Review and Meta-analysis. J. Reconstr. Microsurg. 2020, 36, 600–605. [Google Scholar] [CrossRef]
- Hong, J.P. The Superficial Circumflex Iliac Artery Perforator Flap in Lower Extremity Reconstruction. Clin. Plast. Surg. 2021, 48, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Lopez, C.; Higueras Suñe, C.; Vila-Poyatos, J.; Garcia Senosian, O.; Priego, D.; Del Rio, M.; Malagon, P.; Huesa, L.; Reina-de-la-Torre, F.; Carrera-Burgaya, A. SCIP flap in high-risk extremity reconstruction: Anatomical study of additional superficial venous patterns and implications in caucasian patients. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1174–1205. [Google Scholar] [CrossRef]
- Iida, T.; Mihara, M.; Yoshimatsu, H.; Narushima, M.; Koshima, I. Versatility of the superficial circumflex iliac artery perforator flap in head and neck reconstruction. Ann. Plast. Surg. 2014, 72, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Rahman, K.M.; Owen, S.; Paleri, V.; Adams, J.; Ahmed, O.A.; Ragbir, M. The superficial circumflex iliac artery perforator flap in intra-oral reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, M.F.; Meroni, M.; Fritsche, E.; Rajan, G. Superficial Circumflex Iliac Artery Perforator Flap in Advanced Head and Neck Reconstruction: From Simple to Its Chimeric Patterns and Clinical Experience with 22 Cases. Plast. Reconstr. Surg. 2022, 149, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, M.J.L.; Clark, J.R.; Ch’ng, S.; Hubert Low, T.H.; Nguyen, K.M.; Elliott, M.S.; Palme, C.E.; Wykes, J. Comparison between the radial forearm and superficial circumflex iliac artery perforator free flaps for oral soft tissue reconstruction. Int. J. Oral. Maxillofac. Surg. 2023, 52, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zubler, C.; Lese, I.; Pastor, T.; Attinger, M.; Constantinescu, M.A.; Olariu, R. The osteocutaneous SCIP flap: A detailed description of the surgical technique and retrospective cohort study of consecutive cases in a tertiary European centre. J. Plast. Reconstr. Aesthet. Surg. 2023, 77, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Narushima, M.; Yoshimatsu, H.; Yamamoto, T.; Araki, J.; Koshima, I. A free vascularised iliac bone flap based on superficial circumflex iliac perforators for head and neck reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1596–1599. [Google Scholar] [CrossRef]
- Fernandez-Garrido, M.; Nunez-Villaveiran, T.; Zamora, P.; Masia, J.; Leon, X. The extended SCIP flap: An anatomical and clinical study of a new SCIP flap design. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 3217–3225. [Google Scholar] [CrossRef]
- Yoshimatsu, H.; Yamamoto, T.; Hayashi, A.; Fuse, Y.; Karakawa, R.; Iida, T.; Narushima, M.; Tanakura, K.; Weninger, W.J.; Tzou, C.H.J. Use of the transverse branch of the superficial circumflex iliac artery as a landmark facilitating identification and dissection of the deep branch of the superficial circumflex iliac artery for free flap pedicle: Anatomical study and clinical applications. Microsurgery 2019, 39, 721–729. [Google Scholar] [CrossRef]
- Moratin, J.; Horn, D.; Heinemann, M.; Metzger, K.; Mrosek, J.; Ristow, O.; Engel, M.; Freudlsperger, C.; Freier, K.; Hoffmann, J. Multiple Sequential Free Flap Reconstructions of the Head and Neck: A Single-Center Experience. Plast. Reconstr. Surg. 2021, 148, 791e–799e. [Google Scholar] [CrossRef]
- Vamadeva, S.V.; Henry, F.P.; Mace, A.; Clarke, P.M.; Wood, S.H.; Jallali, N. Secondary free tissue transfer in head and neck reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1129–1134. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Corbitt, C.A.; Yu, P.; Skoracki, R.J. Success of sequential free flaps in head and neck reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Maricevich, M.; Lin, L.O.; Liu, J.; Chang, E.I.; Hanasono, M.M. Interposition Vein Grafting in Head and Neck Free Flap Reconstruction. Plast. Reconstr. Surg. 2018, 142, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, G.; Chen, S.H.; Elia, R.; Sitpahul, N.; Chan, J.C.Y.; Losco, L.; Cigna, E.; Ribuffo, D.; Chen, H.C. Outcomes following head neck free flap reconstruction requiring interposition vein graft or vascular bridge flap. Head Neck 2019, 41, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, A.C.; Manfro, G.; Teixeira, G.V.; Cernea, C.R.; Dias, F.L.; Marco, M.; Polo, R.; Abu-Ghname, A.; Maricevich, M. Use of Single Chimeric Free Flaps or Double Free Flaps for Complex Head and Neck Reconstruction. J. Reconstr. Microsurg. 2021, 37, 791–798. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Z.-H.; Canzi, A.; Yu, J.; Chang, E.I. Expanding the Armamentarium of Donor Sites in Microvascular Head and Neck Reconstruction. J. Clin. Med. 2024, 13, 1311. https://doi.org/10.3390/jcm13051311
Lee Z-H, Canzi A, Yu J, Chang EI. Expanding the Armamentarium of Donor Sites in Microvascular Head and Neck Reconstruction. Journal of Clinical Medicine. 2024; 13(5):1311. https://doi.org/10.3390/jcm13051311
Chicago/Turabian StyleLee, Z-Hye, Ana Canzi, Jessie Yu, and Edward I. Chang. 2024. "Expanding the Armamentarium of Donor Sites in Microvascular Head and Neck Reconstruction" Journal of Clinical Medicine 13, no. 5: 1311. https://doi.org/10.3390/jcm13051311
APA StyleLee, Z.-H., Canzi, A., Yu, J., & Chang, E. I. (2024). Expanding the Armamentarium of Donor Sites in Microvascular Head and Neck Reconstruction. Journal of Clinical Medicine, 13(5), 1311. https://doi.org/10.3390/jcm13051311





