A Systematic Review of the Advances in the Study of T Lymphocyte Suppressor Receptors in HBV Infection: Potential Therapeutic Targets
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Exclusion Criteria
2.3. Data Sources and Search Strategy
2.4. Record Screening Process
2.5. Synthesis of Results
3. Results
3.1. Programmed Cell Death-1 (PD-1)
3.1.1. PD-1 Expression on Normal T Cells
3.1.2. PD-1 Expression on HBV-Specific T Cells
3.1.3. Blocking the PD-1/PD-L1 Pathway in the Treatment of HBV Infection
3.2. Cytotoxic T Lymphocyte Antigen-4 (CTLA-4)
3.2.1. CTLA-4 Expression on Normal T Cells
3.2.2. CTLA-4 Expression on HBV-Specific T Cells
3.2.3. Blocking CTLA-4 in the Treatment of HBV Infection
3.3. T Cell Immunoglobulin and Mucin Structural Domain-3 (Tim-3)
3.3.1. Tim-3 Expression on Normal T Cells
3.3.2. Tim-3 Expression on HBV-Specific T Cells
3.3.3. The Promise of Blocking Tim-3/Tim-3L Pathway in the Treatment of HBV Infection
3.4. Lymphocyte Activation Gene 3 (LAG3)
3.4.1. LAG3 Expression on Normal T Cells
3.4.2. LAG3 Expression on HBV-Specific T Cells
3.4.3. The Promise of Blocking LAG3 in the Treatment of HBV Infection
3.5. CD244/2B4 (SLAMF4)
3.5.1. CD244/2B4 Expressed on Normal T Cells
3.5.2. CD244/2B4 Expression on HBV-Specific T Cells
3.5.3. The Promise of Blocking CD244/2B4 in the Treatment of HBV Infection
3.6. CD160
3.6.1. CD160 Expression on Normal T Cells
3.6.2. CD160 Expression on HBV-Specific T Cells
3.6.3. The Promise of Blocking CD160 in the Treatment of HBV Infection
3.7. B and T Lymphocyte Attenuator (BTLA)
3.8. T Cell Immunoreceptor with Ig and ITIM Domains (TIGIT)
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef]
- Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [CrossRef]
- Guidotti, L.G.; Isogawa, M.; Chisari, F.V. Host-virus interactions in hepatitis B virus infection. Curr. Opin. Immunol. 2015, 36, 61–66. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zhang, X.; Schwartz, J.C.; Guo, X.; Bhatia, S.; Cao, E.; Lorenz, M.; Cammer, M.; Chen, L.; Zhang, Z.Y.; Edidin, M.A.; et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 2004, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, D.; Shimizu, K.; Maruhashi, T.; Okazaki, I.M.; Okazaki, T. T-cell-intrinsic and -extrinsic regulation of PD-1 function. Int. Immunol. 2021, 33, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion during Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Kuchroo, J.R.; Sage, P.T.; Liang, D.; Francisco, L.M.; Buck, J.; Thaker, Y.R.; Zhang, Q.; McArdel, S.L.; Juneja, V.R.; et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J. Exp. Med. 2021, 218, e20182232. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.M.; Wu, X.J.; Wang, Y.; Zhao, H.; Shen, B.; Wang, G.Q. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm. Res. 2011, 60, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.Y.; Wherry, E.J.; Jin, B.; Xu, B.; Zou, Z.S.; Zhang, S.Y.; Li, B.S.; Wang, H.F.; Wu, H.; et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology 2008, 134, 1938–1949.e19493. [Google Scholar] [CrossRef]
- Ye, P.; Weng, Z.H.; Zhang, S.L.; Zhang, J.A.; Zhao, L.; Dong, J.H.; Jie, S.H.; Pang, R.; Wei, R.H. Programmed death-1 expression is associated with the disease status in hepatitis B virus infection. World J. Gastroenterol. 2008, 14, 4551–4557. [Google Scholar] [CrossRef]
- He, M.K.; Peng, C.; Zhao, Y.; Liang, R.B.; Lai, Z.C.; Kan, A.; Li, Q.J.; Wei, W.; Zhang, Y.J.; Chen, M.S.; et al. Comparison of HBV reactivation between patients with high HBV-DNA and low HBV-DNA loads undergoing PD-1 inhibitor and concurrent antiviral prophylaxis. Cancer Immunol. Immunother. 2021, 70, 3207–3216. [Google Scholar] [CrossRef]
- Ferrando-Martinez, S.; Snell Bennett, A.; Lino, E.; Gehring, A.J.; Feld, J.; Janssen, H.L.A.; Robbins, S.H. Functional Exhaustion of HBV-Specific CD8 T Cells Impedes PD-L1 Blockade Efficacy in Chronic HBV Infection. Front. Immunol. 2021, 12, 648420. [Google Scholar] [CrossRef]
- Zhen, S.; Qiang, R.; Lu, J.; Tuo, X.; Yang, X.; Li, X. Enhanced antiviral benefit of combination therapy with anti-HBV and anti-PD1 gRNA/cas9 produces a synergistic antiviral effect in HBV infection. Mol. Immunol. 2021, 130, 7–13. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Guo, J.; Li, M.; Lu, Y.; Zhang, L.; Shen, G.; Wu, S.; Chang, M.; Hu, L.; et al. The reduction in CD8(+)PD-1(+) T cells in liver histological tissue is related to Pegylated IFN-α therapy outcomes in chronic hepatitis B patients. BMC Infect. Dis. 2020, 20, 590. [Google Scholar] [CrossRef]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef]
- Lingel, H.; Brunner-Weinzierl, M.C. CTLA-4 (CD152): A versatile receptor for immune-based therapy. Semin. Immunol. 2019, 42, 101298. [Google Scholar] [CrossRef] [PubMed]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef] [PubMed]
- Schurich, A.; Khanna, P.; Lopes, A.R.; Han, K.J.; Peppa, D.; Micco, L.; Nebbia, G.; Kennedy, P.T.; Geretti, A.M.; Dusheiko, G.; et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011, 53, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.T.F.; Sandalova, E.; Jo, J.; Gill, U.; Ushiro-Lumb, I.; Tan, A.T.; Naik, S.; Foster, G.R.; Bertoletti, A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012, 143, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, N.; Fan, X.; Wang, X.; Zhang, X.; Zhang, K.; Han, Q.; Lv, Y.; Liu, Z. Circulating CTLA-4 levels and CTLA4 polymorphisms associate with disease condition and progression and hepatocellular carcinoma patients’ survival in chronic hepatitis B virus infection. Int. Immunopharmacol. 2020, 82, 106377. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, H.; Tang, Z.; Zang, G. CTLA4 silencing with siRNA promotes deviation of Th1/Th2 in chronic hepatitis B patients. Cell Mol. Immunol. 2009, 6, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 2022, 21, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.K.; Qin, S.; Tai, D.W.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients with Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef]
- Keam, S.J. Tremelimumab: First Approval. Drugs 2023, 83, 93–102. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, P. Preserving the CTLA-4 Checkpoint for Safer and More Effective Cancer Immunotherapy. Trends Pharmacol. Sci. 2020, 41, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manzanet, R.; DeKruyff, R.; Kuchroo, V.K.; Umetsu, D.T. The costimulatory role of TIM molecules. Immunol. Rev. 2009, 229, 259–270. [Google Scholar] [CrossRef]
- Dolina, J.S.; Braciale, T.J.; Hahn, Y.S. Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice. Hepatology 2014, 59, 1351–1365. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.; Dougan, S.K.; Petersen, B.S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, Y.; Li, S.; Zhang, Y.; Liu, Y.; Wu, Y.; Chen, Z. Blockade of Tim-3 signaling restores the virus-specific CD8+ T-cell response in patients with chronic hepatitis B. Eur. J. Immunol. 2012, 42, 1180–1191. [Google Scholar] [CrossRef]
- Dong, J.; Yang, X.F.; Wang, L.X.; Wei, X.; Wang, A.H.; Hao, C.Q.; Shen, H.J.; Huang, C.X.; Zhang, Y.; Lian, J.Q. Modulation of Tim-3 Expression by Antigen-Dependent and -Independent Factors on T Cells from Patients with Chronic Hepatitis B Virus Infection. Front. Cell Infect. Microbiol. 2017, 7, 98. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Fu, J.; Wang, X.; Feng, X.; Pan, X. Tim-3 regulates inflammatory cytokine expression and Th17 cell response induced by monocytes from patients with chronic hepatitis B. Scand. J. Immunol. 2019, 89, e12755. [Google Scholar] [CrossRef]
- Liu, S.; Xu, C.; Yang, F.; Zong, L.; Qin, Y.; Gao, Y.; Su, Q.; Li, T.; Li, Y.; Xu, Y.; et al. Natural Killer Cells Induce CD8(+) T Cell Dysfunction via Galectin-9/TIM-3 in Chronic Hepatitis B Virus Infection. Front. Immunol. 2022, 13, 884290. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Wu, H.; Zhang, B.; Zhang, X.; Wu, X. Blockade of TIM-3 and PD-1 enhances the antitumor effects of MAGE-A11 antigen-specific cytotoxic T lymphocytes. Bull. Cancer 2022, 109, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Zhao, B.; Chen, H.; Cai, Z.; Zheng, Y.; Zeng, Y.; Zhang, D.; Liu, X. Neoantigen Immunotherapeutic-Gel Combined with TIM-3 Blockade Effectively Restrains Orthotopic Hepatocellular Carcinoma Progression. Nano Lett. 2022, 22, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Sabatos-Peyton, C.; Anderson, A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer 2020, 8, e000911. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Du, X.; Liu, Y.; Song, X.; Wang, T.; Tan, S.; Liang, X.; Gao, L.; Ma, C. Tim-3 blockade promotes iNKT cell function to inhibit HBV replication. J. Cell Mol. Med. 2018, 22, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Okazaki, I.M.; Wang, J.; Sugiura, D.; Nakaki, F.; Yoshida, T.; Kato, Y.; Fagarasan, S.; Muramatsu, M.; Eto, T.; et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 2011, 208, 395–407. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, A.; Qiu, C.; Wang, W.; Yang, Y.; Qiu, C.; Liu, A.; Zhu, L.; Yuan, S.; Hu, H.; et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J. Immunol. 2015, 194, 3873–3882. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Okazaki, I.M.; Sugiura, D.; Takahashi, S.; Maeda, T.K.; Shimizu, K.; Okazaki, T. LAG-3 inhibits the activation of CD4(+) T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 2018, 19, 1415–1426. [Google Scholar] [CrossRef]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e312. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, X.; Zhang, L.; Zhu, Q.; Chen, C.; Bao, J.; Chen, Y. CD4(+) T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol. 2019, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Bocanegra, A.; Blanco, E.; Fernández-Rubio, L.; Arasanz, H.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro-Hermida, S.; Vera, R.; et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells 2022, 11, 2351. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Sierro, S.; Romero, P.; Speiser, D.E. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert. Opin. Ther. Targets 2011, 15, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Brignone, C.; Grygar, C.; Marcu, M.; Perrin, G.; Triebel, F. IMP321 (sLAG-3), an immunopotentiator for T cell responses against a HBsAg antigen in healthy adults: A single blind randomised controlled phase I study. J. Immune Based Ther. Vaccines 2007, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Georgoudaki, A.M.; Khodabandeh, S.; Puiac, S.; Persson, C.M.; Larsson, M.K.; Lind, M.; Hammarfjord, O.; Nabatti, T.H.; Wallin, R.P.; Yrlid, U.; et al. CD244 is expressed on dendritic cells and regulates their functions. Immunol. Cell Biol. 2015, 93, 581–590. [Google Scholar] [CrossRef]
- McArdel, S.L.; Terhorst, C.; Sharpe, A.H. Roles of CD48 in regulating immunity and tolerance. Clin. Immunol. 2016, 164, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gang, X.; Li, Z.; Zhao, X.; Zhou, T.; Zhang, S.; Wang, G. Advances in Understanding the Roles of CD244 (SLAMF4) in Immune Regulation and Associated Diseases. Front. Immunol. 2021, 12, 648182. [Google Scholar] [CrossRef]
- Raziorrouh, B.; Schraut, W.; Gerlach, T.; Nowack, D.; Grüner, N.H.; Ulsenheimer, A.; Zachoval, R.; Wächtler, M.; Spannagl, M.; Haas, J.; et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 2010, 52, 1934–1947. [Google Scholar] [CrossRef]
- Cooksley, H.; Riva, A.; Katzarov, K.; Hadzhiolova-Lebeau, T.; Pavlova, S.; Simonova, M.; Williams, R.; Chokshi, S. Differential Expression of Immune Inhibitory Checkpoint Signatures on Antiviral and Inflammatory T Cell Populations in Chronic Hepatitis B. J. Interferon Cytokine Res. 2018, 38, 273–282. [Google Scholar] [CrossRef]
- Xie, C.; Wang, S.; Zhang, H.; Zhu, Y.; Jiang, P.; Shi, S.; Si, Y.; Chen, J. Lnc-AIFM2-1 promotes HBV immune escape by acting as a ceRNA for miR-330-3p to regulate CD244 expression. Front. Immunol. 2023, 14, 1121795. [Google Scholar] [CrossRef]
- Cai, G.; Freeman, G.J. The CD160, BTLA, LIGHT/HVEM pathway: A bidirectional switch regulating T-cell activation. Immunol. Rev. 2009, 229, 244–258. [Google Scholar] [CrossRef]
- Cai, G.; Anumanthan, A.; Brown, J.A.; Greenfield, E.A.; Zhu, B.; Freeman, G.J. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 2008, 9, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Kajikawa, M.; Shiroishi, M.; Kuroki, K.; Maenaka, K. Molecular basis for herpesvirus entry mediator recognition by the human immune inhibitory receptor CD160 and its relationship to the cosignaling molecules BTLA and LIGHT. J. Mol. Biol. 2011, 413, 762–772. [Google Scholar] [CrossRef]
- Yu, L.; Guan, Y.; Li, L.; Lu, N.; Zhang, C. The transcription factor Eomes promotes expression of inhibitory receptors on hepatic CD8(+) T cells during HBV persistence. FEBS J. 2022, 289, 3241–3261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, Q.; Yuan, J.; Xu, X.; Cao, L. lncRNA-CD160 decreases the immunity of CD8(+) T cells through epigenetic mechanisms in hepatitis B virus infection. Oncol. Lett. 2020, 20, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Spodzieja, M.; Kuncewicz, K.; Rodziewicz-Motowidło, S.; Orlikowska, M. CD160 protein as a new therapeutic target in a battle against autoimmune, infectious and lifestyle diseases. Analysis of the structure, interactions and functions. Eur. J. Med. Chem. 2021, 224, 113694. [Google Scholar] [CrossRef] [PubMed]
- del Rio, M.L.; Kaye, J.; Rodriguez-Barbosa, J.I. Detection of protein on BTLAlow cells and in vivo antibody-mediated down-modulation of BTLA on lymphoid and myeloid cells of C57BL/6 and BALB/c BTLA allelic variants. Immunobiology 2010, 215, 570–578. [Google Scholar] [CrossRef]
- Xu, X.; Hou, B.; Fulzele, A.; Masubuchi, T.; Zhao, Y.; Wu, Z.; Hu, Y.; Jiang, Y.; Ma, Y.; Wang, H.; et al. PD-1 and BTLA regulate T cell signaling differentially and only partially through SHP1 and SHP2. J. Cell Biol. 2020, 219, e201905085. [Google Scholar] [CrossRef]
- Wang, H.; Wu, B.; Li, L.; Hu, L.; Lin, J.; Jiang, C.; Cai, G.; Shen, Q. Hepatic expansion of virus-specific CD8(+)BTLA(+) T cells with regulatory properties in chronic hepatitis B virus infection. Cell. Immunol. 2017, 311, 36–45. [Google Scholar] [CrossRef]
- Cai, G.; Nie, X.; Li, L.; Hu, L.; Wu, B.; Lin, J.; Jiang, C.; Wang, H.; Wang, X.; Shen, Q. B and T lymphocyte attenuator is highly expressed on intrahepatic T cells during chronic HBV infection and regulates their function. J. Gastroenterol. 2013, 48, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Fan, J.; Shan, M.X.; Yin, D.D.; Wang, L.L.; Ye, W.; Zhao, W. TIGIT marks exhausted T cells and serves as a target for immune restoration in patients with chronic HBV infection. Am. J. Transl. Res. 2022, 14, 942–954. [Google Scholar]
- Liu, X.; Li, M.; Wang, X.; Dang, Z.; Jiang, Y.; Wang, X.; Kong, Y.; Yang, Z. PD-1(+) TIGIT(+) CD8(+) T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 2041–2054. [Google Scholar] [CrossRef]

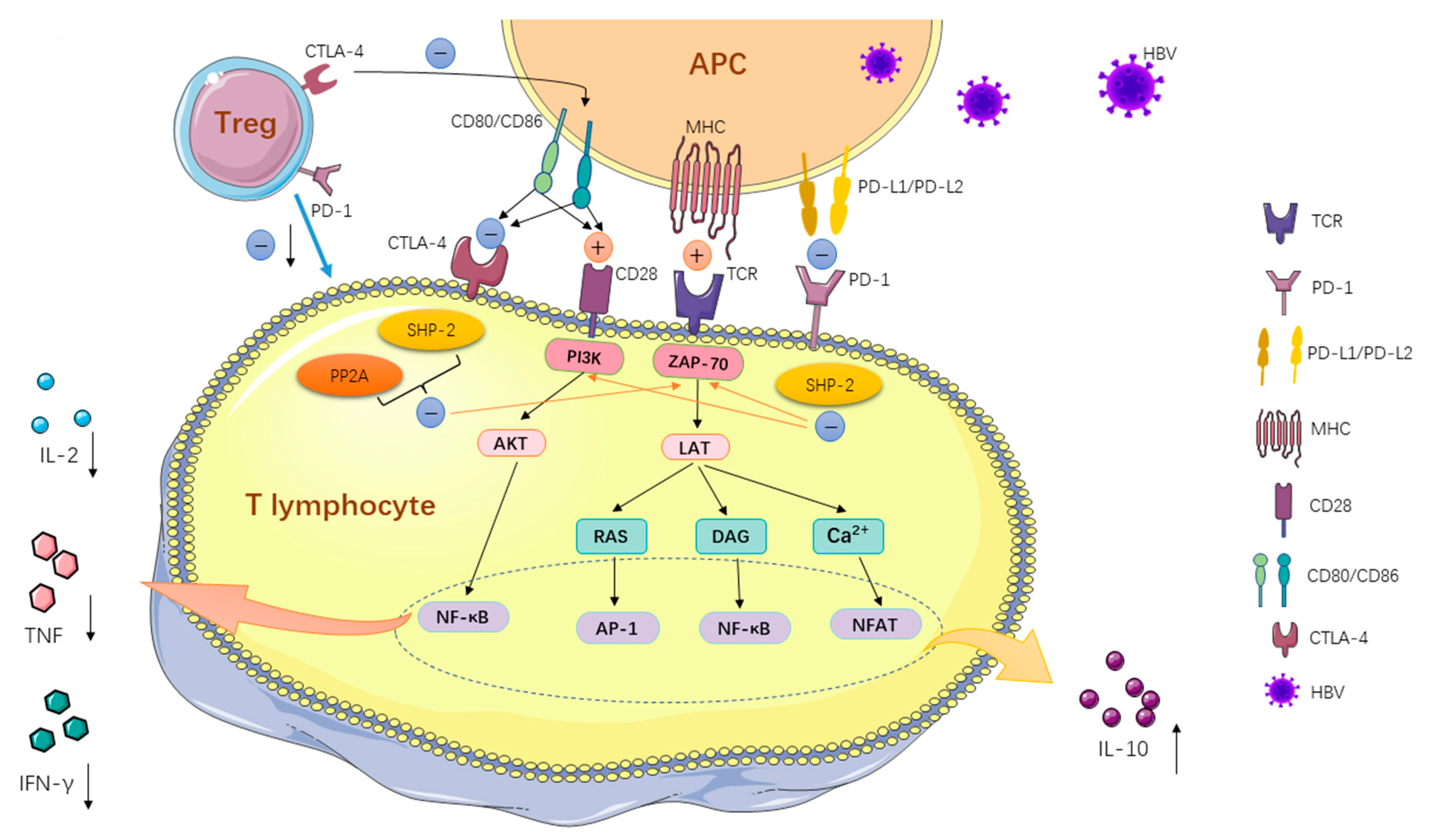
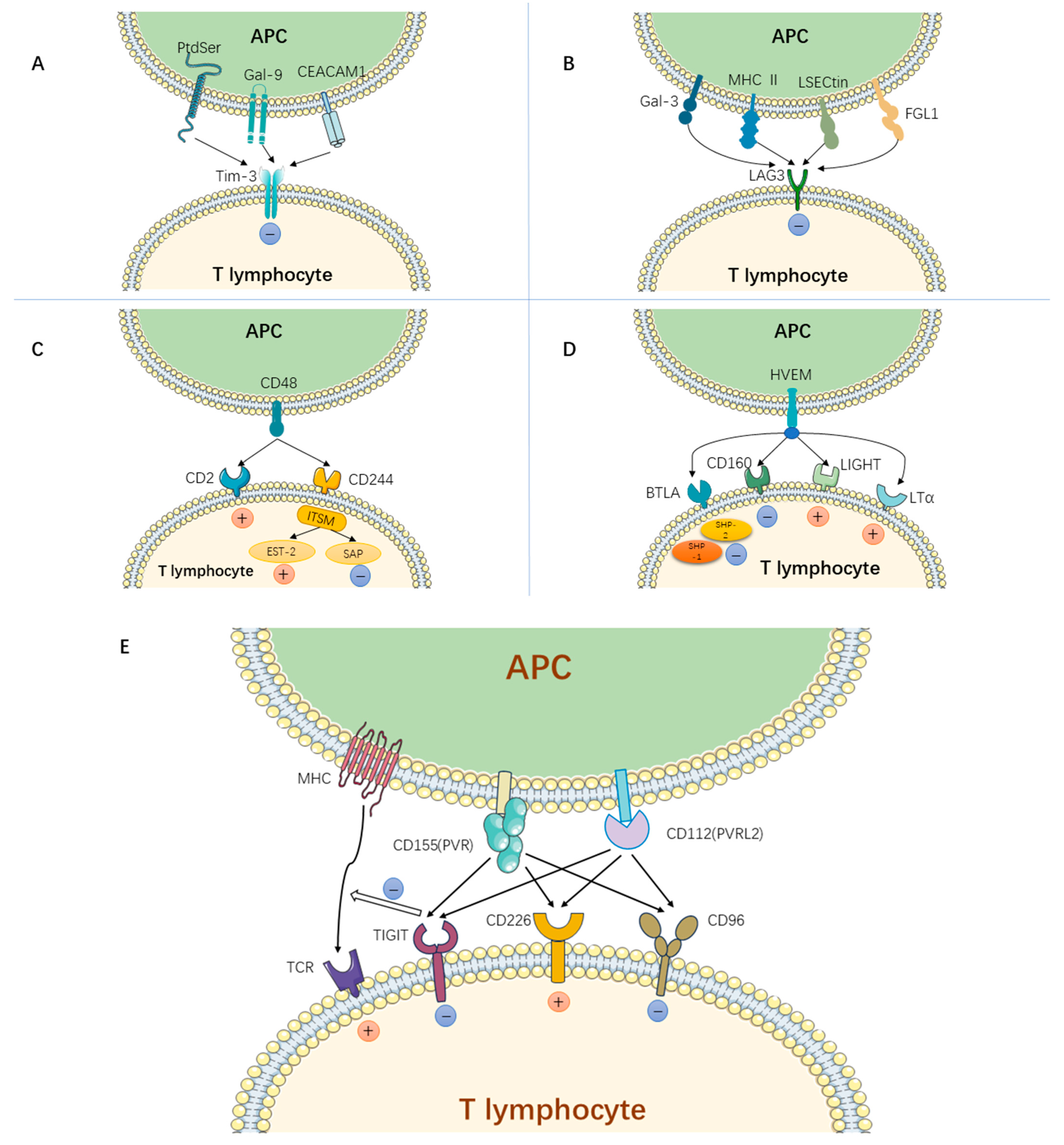
| Immune Checkpoint Pathway | NCT Number | Phase | Study Start Date | Status | Inclusion Criteria | Mechanism | Drug | Primary Outcome Measures | Results | Locations |
|---|---|---|---|---|---|---|---|---|---|---|
| PD-1/PD-L | NCT04638439 | Phase 2 | December 2021 | Recruiting | CHB patients; HBsAg < 1500 IU/mL; HBV DNA (−) | Anti-PD-1 | P1101 Nivolumab Entecavir | Safety | N/A | Taiwan, China |
| NCT05769816 | Phase 4 | June 2023 | Not yet recruiting | CHB patients | Anti-PD-1 | Anti-PD-1 antibody NAs | Serum HBsAg level | N/A | Chongqing, China | |
| NCT05771402 | Phase 4 | June 2023 | Not yet recruiting | CHB patients | Anti-PD-1 | Anti-PD-1 antibody NAs Peg-IFNα | Serum HBsAg level | N/A | Chongqing, China | |
| NCT05275023 | Phase 2 | June 2022 | Active, not recruiting | CHB patients; LSM ≤ 9 KPa/ metavir F0-F2 | Anti-PD-1 | JNJ-73763989 PD-1 Inhibitor NAs | HBsAg seroclearance | N/A | Toronto, Canada | |
| NCT04133259 | Phase 2 | December 2019 | Unknown | CHB patients; HBsAg > 100 IU/mL; HBV DNA < 2000 IU/mL | Anti-PD-1 | HLX10 NAs | Levels of HBsAg decline | N/A | Taiwan, China | |
| NCT05242445 | Phase 1 | April 2022 | Active, not recruiting | CHB patients; LSM ≤ 9 KPa/ metavir F0-F2 | Anti-PD-1 | Cetrelimab (JNJ-63723283) Placebo | Pharmacokinetics, Pharmacodynamics, and safety of single doses of Cetrelimab (JNJ 63723283), | N/A | Belgium; France; Germany; Poland; Spain | |
| NCT04294498 | Phase 2 | November 2020 | Recruiting | HBeAg (−); HBsAg (+); HBV DNA ≥ 2000 IU/mL; Barcelona Clinic Liver Cancer (BCLC) Stage C disease or BCLC Stage B disease not amenable to locoregional therapy (Advanced Hepatocellular Carcinoma) | Anti-PD-1 | Durvalumab | The rate of HBV reactivation during durvalumab treatment | N/A | Taiwan, China | |
| NCT04044651 | Phase 3 Phase 2 | October 2019 | Withdrawn | HBsAg (+); BCLC (C stage) | Anti-PD-1 | Nivolumab Lenvatinib | Overall survival (OS). Time frame: 18 months | N/A | Guangdong, China | |
| NCT04465890 | Phase 2 | July 2020 | Recruiting | HBV DNA (−); HBeAg (−); HBsAg ≤ 10,000 IU/mL | Anti-PD-L1 | PD-L1 Antibody (ASC22) | Levels of HBsAg decline | N/A | Beijing, China | |
| NCT04225715 | Phase 2 | July 2020 | Recruiting | HBsAg (+) ≥ 6 months; HBV DNA below the lower LLOQ or <20 IU/mL for >6 months | Anti-PD-L1 | NUCs CpAM (RO7049389) TLR7 (RO7020531) siRNA (RO7445482) PEG-IFN PD-L1 LNA (RO7191863) | HBsAg seroclearance | N/A | 54 study locations including United States, Canada et al. | |
| NCT04680598 | N/A | December 2020 | Recruiting | HBsAg (+); HCC (for EASL) | Anti-PD-1/anti-PD-L1 | ICI (including PD-1 inhibitor or PD-L1 inhibitor); NAs | HBV reactivation rate | N/A | Guangdong, China | |
| NCT03419481 | Phase 2 | April 2018 | Recruiting | HBsAg (+); HBV DNA < 100 IU/mL; HCC (for AASLD) | Anti-PD-L1 | pembrolizumab | Response rate (RR) according to RECIST1.1. Time frame: 2 years | N/A | Hong Kong, China | |
| LAG3 | NCT00354861 | Phase 1 | May 2005 | Completed | Healthy | hLAG-3Ig | IMP321 (AG-3) | Safety and tolerability profiles. Time frame: 3 months | IMP321 was very well tolerated | Paris, France |
| PubMed | Search query: (“Hepatitis B”MeSH Terms OR “HBV”All Fields) AND (“Costimulatory and Inhibitory T-Cell Receptors”MeSH Terms OR (“inhibitory”All Fields AND (“cell cycle checkpoints”MeSH Terms OR (“cell”All Fields AND “cycle”All Fields AND “checkpoints”All Fields) OR “cell cycle checkpoints”All Fields OR “checkpoint”All Fields OR “checkpoints”All Fields)) OR ((“immune”All Fields OR “immuned”All Fields OR “immunes”All Fields OR “immunisation”All Fields OR “vaccination”MeSH Terms OR “vaccination”All Fields OR “immunization”All Fields OR “immunization”MeSH Terms OR “immunisations”All Fields OR “immunizations”All Fields OR “immunise”All Fields OR “immunised”All Fields OR “immuniser”All Fields OR “immunisers”All Fields OR “immunising”All Fields OR “immunities”All Fields OR “immunity”MeSH Terms OR “immunity”All Fields OR “immunization s”All Fields OR “immunize”All Fields OR “immunized”All Fields OR “immunizer”All Fields OR “immunizers”All Fields OR “immunizes”All Fields OR “immunizing”All Fields) AND (“cell cycle checkpoints”MeSH Terms OR (“cell”All Fields AND “cycle”All Fields AND “checkpoints”All Fields) OR “cell cycle checkpoints”All Fields OR “checkpoint”All Fields OR “checkpoints”All Fields)) OR (“PD-1”All Fields OR (“ctla 4 antigen”MeSH Terms OR (“ctla 4”All Fields AND “antigen”All Fields) OR “ctla 4 antigen”All Fields OR “ctla 4”All Fields) OR “LAG3”All Fields OR “Tim-3”All Fields OR “cd244”All Fields OR “CD160”All Fields OR “TIGIT”All Fields OR “BTLA”All Fields)) AND (“T-Lymphocytes”MeSH Terms OR (“T-Lymphocytes”MeSH Terms OR “T-Lymphocytes”All Fields OR “t cell”All Fields)) |
| Results: 396 | |
| Web of Science | ((hepatitis B (Topic) or HBV (Topic) and Preprint Citation Index (Exclude—Database)) AND inhibitory receptors (Topic) or inhibitory checkpoints (Topic) or immunization checkpoints (Topic) and Preprint Citation Index (Exclude—Database)) AND T lymphocyte (Topic) or T cell (Topic) and Preprint Citation Index (Exclude—Database) and Preprint Citation Index (Exclude—Database) |
| Result: 492 | |
| Scopus | (((ALL(hepatitis B) OR ALL(HBV))) AND ((ALL(inhibitory AND receptors) OR ALL(inhibitory AND checkpoints) OR ALL(immunization AND checkpoints))) AND ((ALL(T lymphocyte) OR ALL(T cell))) AND (LIMIT-TO (LANGUAGE,”English”)) AND (LIMIT-TO (DOCTYPE,”ar”)) AND (LIMIT-TO (EXACTKEYWORD,”Hepatitis B”) OR LIMIT-TO (EXACTKEYWORD,”T-Lymphocytes”) OR LIMIT-TO (EXACTKEYWORD,”Hepatitis B Virus”) OR LIMIT-TO (EXACTKEYWORD,”T Lymphocyte”))) |
| Results: 3310 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Liu, L.; Liu, J.; Li, H.; Zhang, J.; Cao, Z. A Systematic Review of the Advances in the Study of T Lymphocyte Suppressor Receptors in HBV Infection: Potential Therapeutic Targets. J. Clin. Med. 2024, 13, 1210. https://doi.org/10.3390/jcm13051210
Zhou D, Liu L, Liu J, Li H, Zhang J, Cao Z. A Systematic Review of the Advances in the Study of T Lymphocyte Suppressor Receptors in HBV Infection: Potential Therapeutic Targets. Journal of Clinical Medicine. 2024; 13(5):1210. https://doi.org/10.3390/jcm13051210
Chicago/Turabian StyleZhou, Daqiong, Lili Liu, Jiangyu Liu, Hong Li, Jing Zhang, and Zhenhuan Cao. 2024. "A Systematic Review of the Advances in the Study of T Lymphocyte Suppressor Receptors in HBV Infection: Potential Therapeutic Targets" Journal of Clinical Medicine 13, no. 5: 1210. https://doi.org/10.3390/jcm13051210
APA StyleZhou, D., Liu, L., Liu, J., Li, H., Zhang, J., & Cao, Z. (2024). A Systematic Review of the Advances in the Study of T Lymphocyte Suppressor Receptors in HBV Infection: Potential Therapeutic Targets. Journal of Clinical Medicine, 13(5), 1210. https://doi.org/10.3390/jcm13051210






