The Impact of Mechanical Bowel Preparation and Oral Antibiotics in Colorectal Cancer Surgery (MECCA Study): A Prospective Randomized Clinical Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Ethical Approvals
2.3. Study Parameters and Outcomes
2.4. Statistical Analysis
3. Results
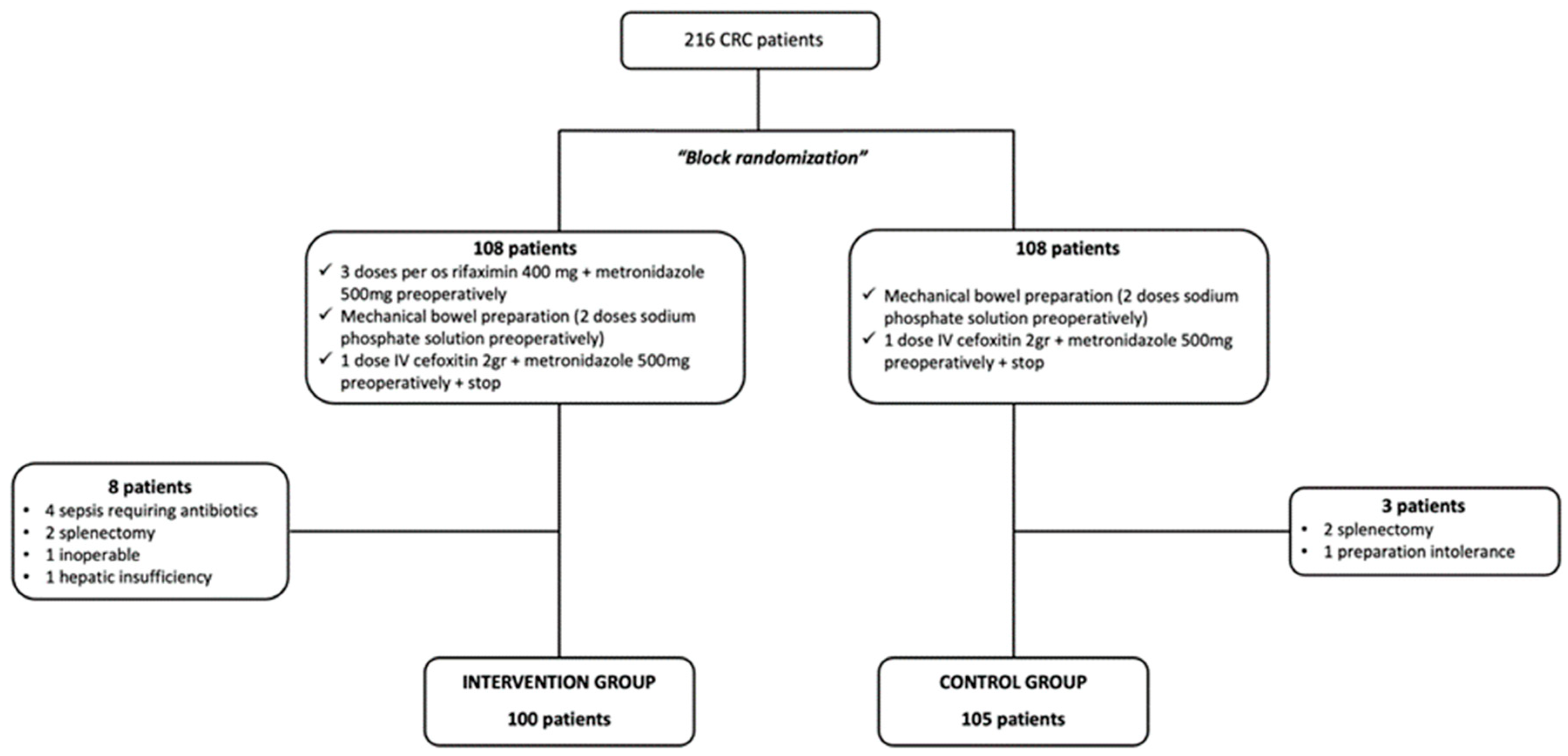
3.1. Included Patients
3.2. Patient Characteristics and Postoperative Outcomes
3.3. Univariable Analysis
3.4. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon. Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef]
- Willis, M.A.; Toews, I.; Soltau, S.L.; Kalff, J.C.; Meerpohl, J.J.; Vilz, T.O. Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database Syst. Rev. 2023, 2, CD014909. [Google Scholar] [PubMed]
- Chiarello, M.M.; Fransvea, P.; Cariati, M.; Adams, N.J.; Bianchi, V.; Brisinda, G. Anastomotic leakage in colorectal cancer surgery. Surg. Oncol. 2022, 40, 101708. [Google Scholar] [CrossRef] [PubMed]
- Fuglestad, M.A.; Tracey, E.L.; Leinicke, J.A. Evidence-based Prevention of Surgical Site Infection. Surg. Clin. N. Am. 2021, 101, 951–966. [Google Scholar] [CrossRef]
- Saito, Y.; Takakura, Y.; Hinoi, T.; Egi, H.; Tashiro, H.; Ohdan, H. Body mass index as a predictor of postoperative complications in loop ileostomy closure after rectal resection in Japanese patients. Hiroshima J. Med. Sci. 2014, 63, 33–38. [Google Scholar]
- Chu, D.I.; Schlieve, C.R.; Colibaseanu, D.T.; Simpson, P.J.; Wagie, A.E.; Cima, R.R.; Habermann, E.B. Surgical site infections (SSIs) after stoma reversal (SR): Risk factors, implications, and protective strategies. J. Gastrointest. Surg. 2015, 19, 327–334. [Google Scholar] [CrossRef]
- Mik, M.; Berut, M.; Trzcinski, R.; Dziki, L.; Buczynski, J.; Dziki, A. Preoperative oral antibiotics reduce infections after colorectal cancer surgery. Langenbeck’s Arch. Surg. 2016, 401, 1153–1162. [Google Scholar] [CrossRef]
- Migaly, J.; Bafford, A.C.; Francone, T.D.; Gaertner, W.B.; Eskicioglu, C.; Bordeianou, L.; Feingold, D.L.; Steele, S.R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Dis. Colon. Rectum 2019, 62, 3–8. [Google Scholar] [CrossRef]
- Patel, P.H.; Hashmi, M.F. Macrolides. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Meyer, J.; Naiken, S.; Christou, N.; Liot, E.; Toso, C.; Buchs, N.C.; Ris, F. Reducing anastomotic leak in colorectal surgery: The old dogmas and the new challenges. World J. Gastroenterol. 2019, 25, 5017–5025. [Google Scholar] [CrossRef]
- Woodfield, J.A.-O.; Clifford, K.; Schmidt, B.; Thompson-Fawcett, M. Has network meta-analysis resolved the controversies related to bowel preparation in elective colorectal surgery? Color. Dis. 2022, 24, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Veziant, J.; Joris, J. Bowel preparation for colorectal surgery: Questions to answer. Surgery 2022, 171, 1700–1701. [Google Scholar] [CrossRef] [PubMed]
- Berger, V.W.; Bour, L.J.; Carter, K.; Chipman, J.J.; Everett, C.C.; Heussen, N.; Hewitt, C.; Hilgers, R.D.; Luo, Y.A.; Renteria, J.; et al. A roadmap to using randomization in clinical trials. BMC Med. Res. Methodol. 2021, 21, 168. [Google Scholar] [CrossRef] [PubMed]
- Efird, J. Blocked randomization with randomly selected block sizes. Int. J. Env. Res. Public. Health 2011, 8, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, R.A.; Tzizik, D. Update on surgical site infections: The new CDC guidelines. Jaapa 2018, 31, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sadahiro, S.; Tanaka, A.; Okada, K.; Saito, G.; Miyakita, H.; Ogimi, T. Usefulness of Preoperative Mechanical Bowel Preparation in Patients with Colon Cancer who Undergo Elective Surgery: A Prospective Randomized Trial Using Oral Antibiotics. Dig. Surg. 2019, 37, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Vadhwana, B.; Pouzi, A.; Kaneta, G.S.; Reid, V.; Claxton, D.; Pyne, L.; Chalmers, R.; Malik, A.; Bowers, D.; Groot-Wassink, T. Preoperative oral antibiotic bowel preparation in elective resectional colorectal surgery reduces rates of surgical site infections: A single-centre experience with a cost-effectiveness analysis. Ind. Mark. Manag. 2020, 102, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Papp, G.; Saftics, G.; Szabó, B.E.; Baracs, J.; Vereczkei, A.; Kollár, D.; Oláh, A.; Mészáros, P.; Dubóczki, Z.; Bursics, A. Systemic versus Oral and Systemic Antibiotic Prophylaxis (SOAP) study in colorectal surgery: Prospective randomized multicentre trial. Br. J. Surg. 2021, 108, 271–276. [Google Scholar] [CrossRef]
- Morris, M.S.; Graham, L.A.; Chu, D.I.; Cannon, J.A.; Hawn, M.T. Oral Antibiotic Bowel Preparation Significantly Reduces Surgical Site Infection Rates and Readmission Rates in Elective Colorectal Surgery. Ann. Surg. 2015, 261, 1034–1040. [Google Scholar] [CrossRef]
- Futier, E.; Jaber, S.; Garot, M.; Vignaud, M.; Panis, Y.; Slim, K.; Lucet, J.C.; Lebuffe, G.; Ouattara, A.; El Amine, Y.; et al. Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: Multicentre, randomised, double blind, placebo controlled trial. BMJ 2022, 379, e071476. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, B.K.; Ryu, J.; Lee, K.H. Mechanical bowel preparation combined with oral antibiotics in colorectal cancer surgery: A nationwide population-based study. Int. J. Color. Dis. 2021, 36, 1929–1935. [Google Scholar] [CrossRef]
- Scarborough, J.E.; Mantyh, C.R.; Sun, Z.; Migaly, J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates After Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP. Ann. Surg. 2015, 262, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kiran, R.P.; Murray, A.C.; Chiuzan, C.; Estrada, D.; Forde, K. Combined Preoperative Mechanical Bowel Preparation with Oral Antibiotics Significantly Reduces Surgical Site Infection, Anastomotic Leak, and Ileus After Colorectal Surgery. Ann. Surg. 2015, 262, 416–425. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Phan, K.; Hitos, K.; Pathma-Nathan, N.; El-Khoury, T.; Richardson, A.J.; Morgan, G.; Engel, A.; Ctercteko, G. Association of Mechanical Bowel Preparation and Oral Antibiotics Before Elective Colorectal Surgery With Surgical Site Infection: A Network Meta-analysis. JAMA Netw. Open 2018, 1, e183226. [Google Scholar] [CrossRef]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Acheson, A.G.; Lobo, D.N. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery: A Meta-analysis. Ann. Surg. 2019, 270, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, M.A.-O.; Akid, A.; Kbir, G.H.; Mabrouk, A.; Selmi, M.; Ben Dhaou, A.; Daldoul, S.; Haouet, K.; Ben Moussa, M. Is There a Role for Mechanical and Oral Antibiotic Bowel Preparation for Patients Undergoing Minimally Invasive Colorectal Surgery? A Systematic Review and Meta-analysis. J. Gastrointest. Surg. 2023, 27, 1011–1025. [Google Scholar] [CrossRef]
- Hansen, R.A.-O.; Balachandran, R.; Valsamidis, T.N.; Iversen, L.H. The role of preoperative mechanical bowel preparation and oral antibiotics in prevention of anastomotic leakage following restorative resection for primary rectal cancer—A systematic review and meta-analysis. Int. J. Color. Dis. 2023, 38, 129. [Google Scholar] [CrossRef] [PubMed]
- Ambe, P.C.; Zarras, K.; Stodolski, M.; Wirjawan, I.; Zirngibl, H. Routine preoperative mechanical bowel preparation with additive oral antibiotics is associated with a reduced risk of anastomotic leakage in patients undergoing elective oncologic resection for colorectal cancer. World J. Surg. Oncol. 2019, 17, 20. [Google Scholar] [CrossRef]
- Lei, P.; Jia, G.; Yang, X.; Ruan, Y.; Wei, B.; Chen, T. Region-specific protection effect of preoperative oral antibiotics combined with mechanical bowel preparation before laparoscopic colorectal resection: A prospective randomized controlled trial. Int. J. Surg. 2023, 109, 3042–3051. [Google Scholar] [CrossRef]
- Koskenvuo, L.; Lehtonen, T.; Koskensalo, S.; Rasilainen, S.; Klintrup, K.; Ehrlich, A.; Pinta, T.; Scheinin, T.; Sallinen, V. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): A multicentre, randomised, parallel, single-blinded trial. Lancet 2019, 394, 840–848. [Google Scholar] [CrossRef]
- Güenaga, K.F.; Matos, D.; Wille-Jørgensen, P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst. Rev. 2011, 2011, CD001544. [Google Scholar] [CrossRef] [PubMed]
- Frontali, A.; Panis, Y.A.-O. Bowel preparation in colorectal surgery: Back to the future? Updates Surg. 2019, 71, 205–207. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y. Understanding perioperative bowel preparation for colorectal surgery. Dig. Med. Res. 2020, 3, 3. [Google Scholar] [CrossRef]
- Contant, C.M.; Hop, W.C.; van’t Sant, H.P.; Oostvogel, H.J.; Smeets, H.J.; Stassen, L.P.; Neijenhuis, P.A.; Idenburg, F.J.; Dijkhuis, C.M.; Heres, P.; et al. Mechanical bowel preparation for elective colorectal surgery: A multicentre randomised trial. Lancet 2007, 370, 2112–2117. [Google Scholar] [CrossRef]
- Cao, F.; Li, F.; Fau, J. Mechanical bowel preparation for elective colorectal surgery: Updated systematic review and meta-analysis. Int. J. Color. Dis. 2012, 27, 803–810. [Google Scholar] [CrossRef]
- Ram, E.; Sherman, Y.; Weil, R.; Vishne, T.; Kravarusic, D.; Dreznik, Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch. Surg. 2005, 140, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.D.; Zhang, Q.Y.; Zeng, Q.Q.; Yu, Z.P.; Tao, C.L.; Yang, W.J. Efficacy of mechanical bowel preparation with polyethylene glycol in prevention of postoperative complications in elective colorectal surgery: A meta-analysis. Int. J. Color. Dis. 2009, 25, 267–275. [Google Scholar] [CrossRef]
- Leenen, J.P.L.; Hentzen, J.; Ockhuijsen, H.D.L. Effectiveness of mechanical bowel preparation versus no preparation on anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Updates Surg. 2018, 71, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Lobo, D.N. Impact of mechanical bowel preparation in elective colorectal surgery: A meta-analysis. World J. Gastroenterol. 2018, 24, 519–536. [Google Scholar] [CrossRef]
- Lewis, J.; Kinross, J. Mechanical bowel preparation for elective colorectal surgery. Tech. Coloproctology 2019, 23, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ryan, J.; Davey, M.G.; McHugh, F.T.; Creavin, B.; Whelan, M.C.; E Kelly, M.; Neary, P.C.; O Kavanagh, D.; O’riordan, J.M. Mechanical bowel preparation and antibiotics in elective colorectal surgery: Network meta-analysis. BJS Open 2023, 7, zrad040. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.A.-O.; Hassan, M.; Grant, M.D. Antibiotic prophylaxis in colorectal surgery: Are oral, intravenous or both best and is mechanical bowel preparation necessary? Tech. Coloproctology 2020, 24, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Basany, E.E.; Solís-Peña, A.; Pellino, G.; Kreisler, E.; Fraccalvieri, D.; Muinelo-Lorenzo, M.; Maseda-Díaz, O.; García-González, J.M.; Santamaría-Olabarrieta, M.; Codina-Cazador, A.; et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): A multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2020, 5, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Abd El Aziz, M.A.; Grass, F.; Calini, G.; Behm, K.T.; D’Angelo, A.L.; Kelley, S.R.; Mathis, K.L.; Larson, D.W. Oral Antibiotics Bowel Preparation Without Mechanical Preparation for Minimally Invasive Colorectal Surgeries: Current Practice and Future Prospects. Dis. Colon Rectum 2022, 65, e897–e906. [Google Scholar] [CrossRef]
- Arezzo, A.A.-O.; Mistrangelo, M.; Bonino, M.A.; Salusso, P.; Forcignanò, E.; Vettoretto, N.; Botteri, E.; Cillara, N.; Ottonello, R.; Testa, V.; et al. Oral neomycin and bacitracin are effective in preventing surgical site infections in elective colorectal surgery: A multicentre, randomized, parallel, single-blinded trial (COLORAL-1). Updates Surg. 2021, 73, 1775–1786. [Google Scholar] [CrossRef]
- Moroz, N.; Sitarz, R.; Mruk, A.; Bakalarz, R.; Maciąg, E.; Litwiński, J.; Wierzbicki, R. The use of rifaximin in pre-operative period of patients with tumors of the gastrointestinal tract—A retrospective study (2013–2016). Pol. Przegl. Chir. 2018, 90, 35–40. [Google Scholar] [CrossRef]
- Koo, C.H.; Chok, A.Y.; Wee, I.J.Y.; Seow-En, I.; Zhao, Y.; Tan, E. Effect of preoperative oral antibiotics and mechanical bowel preparation on the prevention of surgical site infection in elective colorectal surgery, and does oral antibiotic regime matter? a bayesian network meta-analysis. Int. J. Colorectal. Dis. 2023, 38, 151. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Zocco, M.A.; D’aversa, F.; Pompili, M.; Gasbarrini, A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J. Gastroenterol. 2017, 23, 4491–4499. [Google Scholar] [CrossRef]
- Ikeda, A.; Konishi, T.; Ueno, M.; Fukunaga, Y.; Nagayama, S.; Fujimoto, Y.; Akiyoshi, T.; Yamaguchi, T. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br. J. Surg. 2016, 103, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Yamaguchi, T.; Hasegawa, S.; Nomura, A.; Hida, K.; Nishitai, R.; Yamanokuchi, S.; Yamanaka, T.; Sakai, Y. Oral and Parenteral Versus Parenteral Antibiotic Prophylaxis in Elective Laparoscopic Colorectal Surgery (JMTO PREV 07–01): A Phase 3, Multicenter, Open-label, Randomized Trial. Ann. Surg. 2016, 263, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- McSorley, S.T.; Steele, C.W.; McMahon, A.J. Meta-analysis of oral antibiotics, in combination with preoperative intravenous antibiotics and mechanical bowel preparation the day before surgery, compared with intravenous antibiotics and mechanical bowel preparation alone to reduce surgical-site infections in elective colorectal surgery. BJS Open 2018, 2, 185–194. [Google Scholar]
- Hoffman, T.; Shitrit, P.; Chowers, M. Risk factors for surgical site infections following open versus laparoscopic colectomies: A cohort study. BMC Surg. 2021, 21, 376. [Google Scholar] [CrossRef] [PubMed]
- Caroff, D.A.; Chan, C.; Kleinman, K.; Calderwood, M.S.; Wolf, R.; Wick, E.C.; Platt, R.; Huang, S. Association of Open Approach vs Laparoscopic Approach With Risk of Surgical Site Infection After Colon Surgery. JAMA Netw. Open 2019, 2, e1913570. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | |
|---|---|
| Age | 70 ± 11 years |
| Gender | |
| Male | 118 (58%) |
| Female | 85 (42%) |
| Tumor location | |
| Ascending colon | 36 (18%) |
| Transverse colon | 46 (22%) |
| Descending colon | 25 (12%) |
| Sigmoid colon | 56 (27%) |
| Rectum | 42 (21%) |
| Recurrence | 24 (12%) |
| Hypertension | 100 (49%) |
| Diabetes | 42 (21%) |
| Heart arrhythmia | 15 (7%) |
| Ischemic heart disease | 25 (12%) |
| Renal dysfunction | 3 (2%) |
| Chronic obstructive pulmonary disease | 17 (8%) |
| Other | 32 (16%) |
| Past surgery | 103 (50%) |
| Postoperative outcomes | |
| Surgical site infection | 24 (12%) |
| Anastomotic leakage | 11 (5%) |
| Overall complications | 46 (22%) |
| Readmission | 15 (7%) |
| Length of stay (>7 days) | 155 (76%) |
| Group | p-Value | ||
|---|---|---|---|
| Intervention | Control | ||
| Surgical Site Infection | 7 (7%) | 17 (16%) | 0.049 |
| Anastomotic Leak | 4 (4%) | 7 (7%) | 0.447 |
| Overall complications | 17 (18%) | 29 (28%) | 0.095 |
| Readmission | 5 (5%) | 10 (10%) | 0.237 |
| Length of stay (>7 days) | 75 (77%) | 80 (76%) | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frountzas, M.; Michalopoulou, V.; Georgiou, G.; Kanata, D.; Matiatou, M.; Kimpizi, D.; Matthaiou, G.; Spiliotopoulos, S.; Vouros, D.; Toutouzas, K.G.; et al. The Impact of Mechanical Bowel Preparation and Oral Antibiotics in Colorectal Cancer Surgery (MECCA Study): A Prospective Randomized Clinical Trial. J. Clin. Med. 2024, 13, 1162. https://doi.org/10.3390/jcm13041162
Frountzas M, Michalopoulou V, Georgiou G, Kanata D, Matiatou M, Kimpizi D, Matthaiou G, Spiliotopoulos S, Vouros D, Toutouzas KG, et al. The Impact of Mechanical Bowel Preparation and Oral Antibiotics in Colorectal Cancer Surgery (MECCA Study): A Prospective Randomized Clinical Trial. Journal of Clinical Medicine. 2024; 13(4):1162. https://doi.org/10.3390/jcm13041162
Chicago/Turabian StyleFrountzas, Maximos, Victoria Michalopoulou, Georgia Georgiou, Despoina Kanata, Maria Matiatou, Despina Kimpizi, Georgia Matthaiou, Spilios Spiliotopoulos, Dimitrios Vouros, Konstantinos G. Toutouzas, and et al. 2024. "The Impact of Mechanical Bowel Preparation and Oral Antibiotics in Colorectal Cancer Surgery (MECCA Study): A Prospective Randomized Clinical Trial" Journal of Clinical Medicine 13, no. 4: 1162. https://doi.org/10.3390/jcm13041162
APA StyleFrountzas, M., Michalopoulou, V., Georgiou, G., Kanata, D., Matiatou, M., Kimpizi, D., Matthaiou, G., Spiliotopoulos, S., Vouros, D., Toutouzas, K. G., & Theodoropoulos, G. E. (2024). The Impact of Mechanical Bowel Preparation and Oral Antibiotics in Colorectal Cancer Surgery (MECCA Study): A Prospective Randomized Clinical Trial. Journal of Clinical Medicine, 13(4), 1162. https://doi.org/10.3390/jcm13041162









