The DizzyQuest Combined with Accelerometry: Daily Physical Activities and Limitations among Patients with Bilateral Vestibulopathy Due to DFNA9
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Procedures
2.2. Vestibular and Audiometric Testing
2.3. DizzyQuest
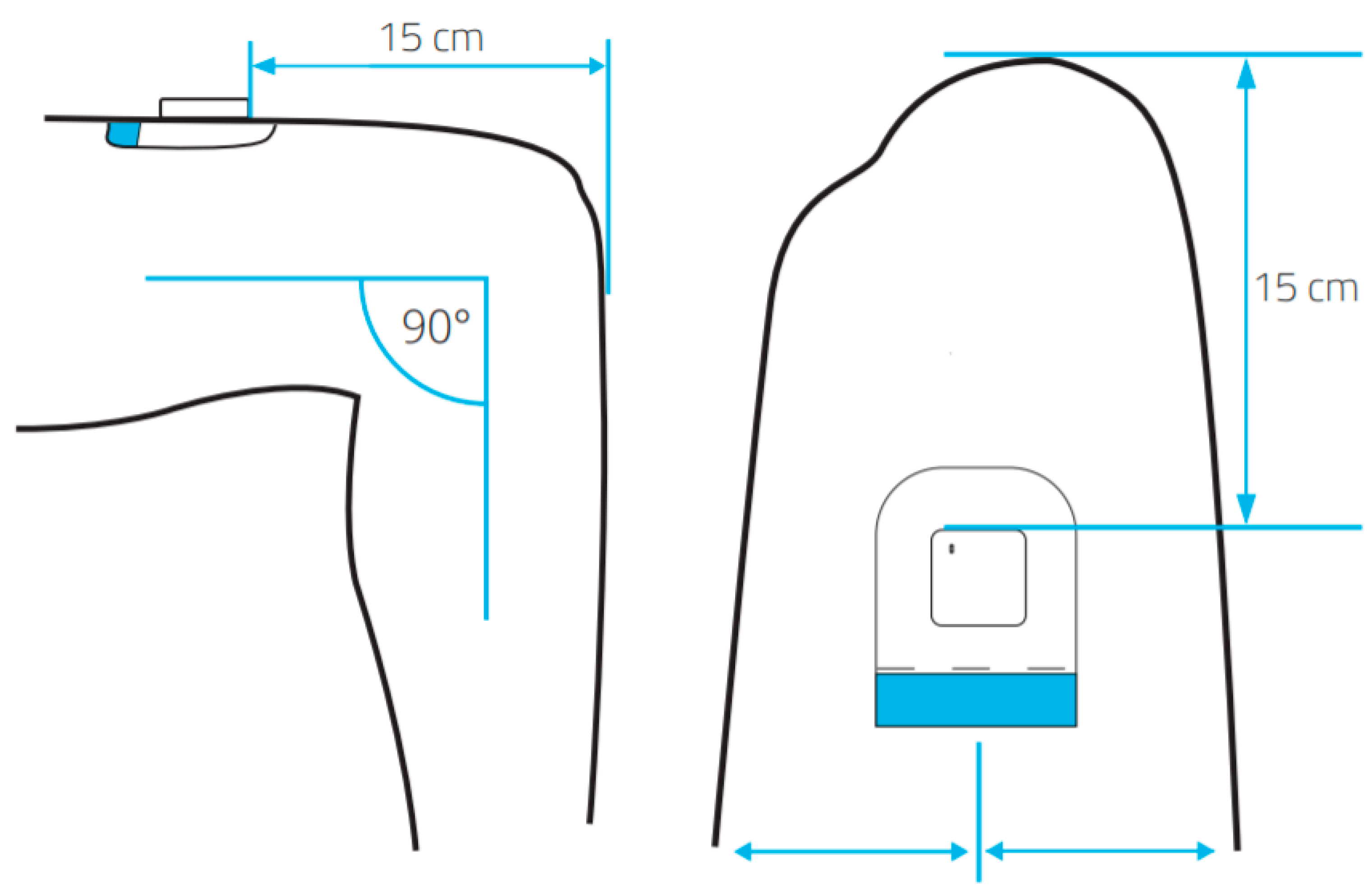
2.4. The MOX Accelerometer
2.5. Study Design
2.6. Data Selection
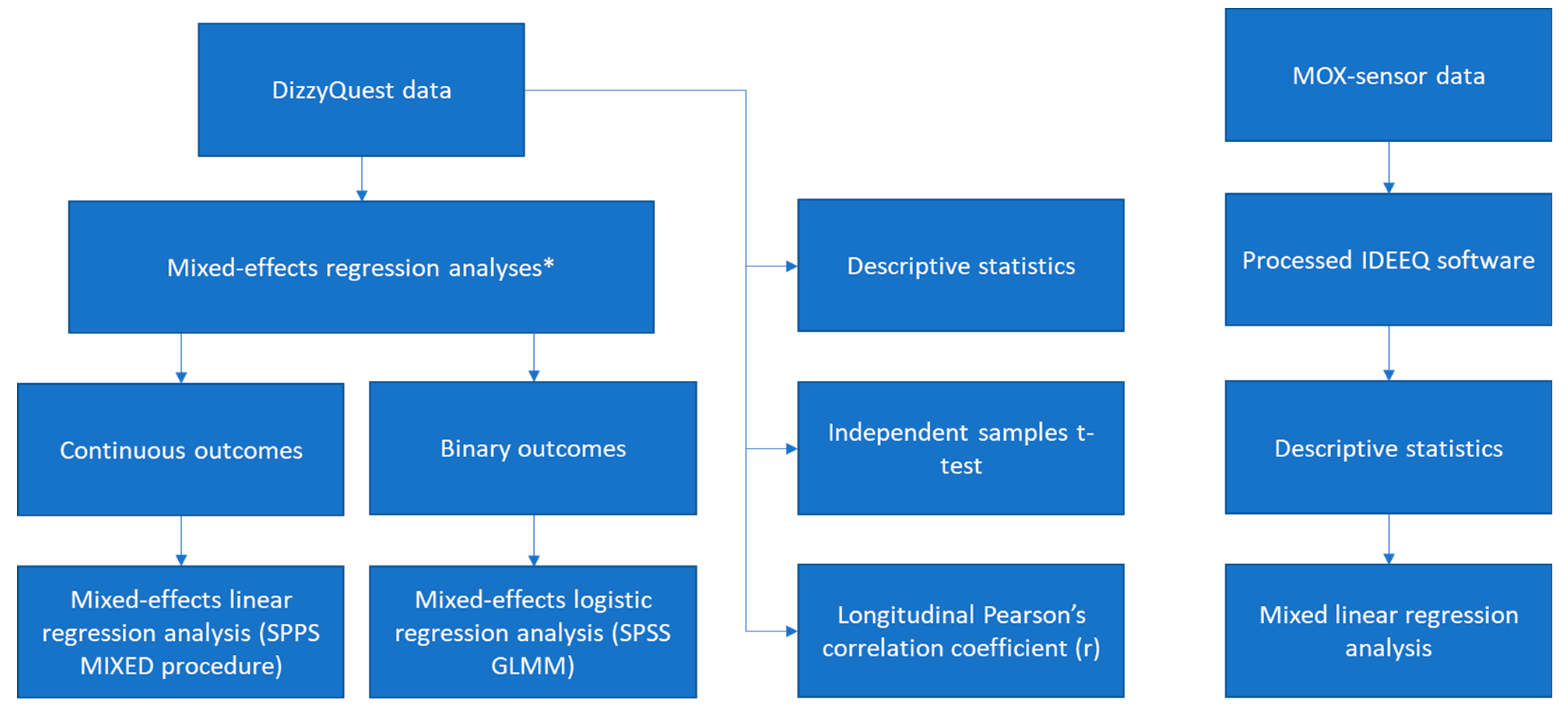
2.7. Data Analysis
2.8. Ethical Considerations
3. Results
3.1. Patient Characteristics and Response Rates
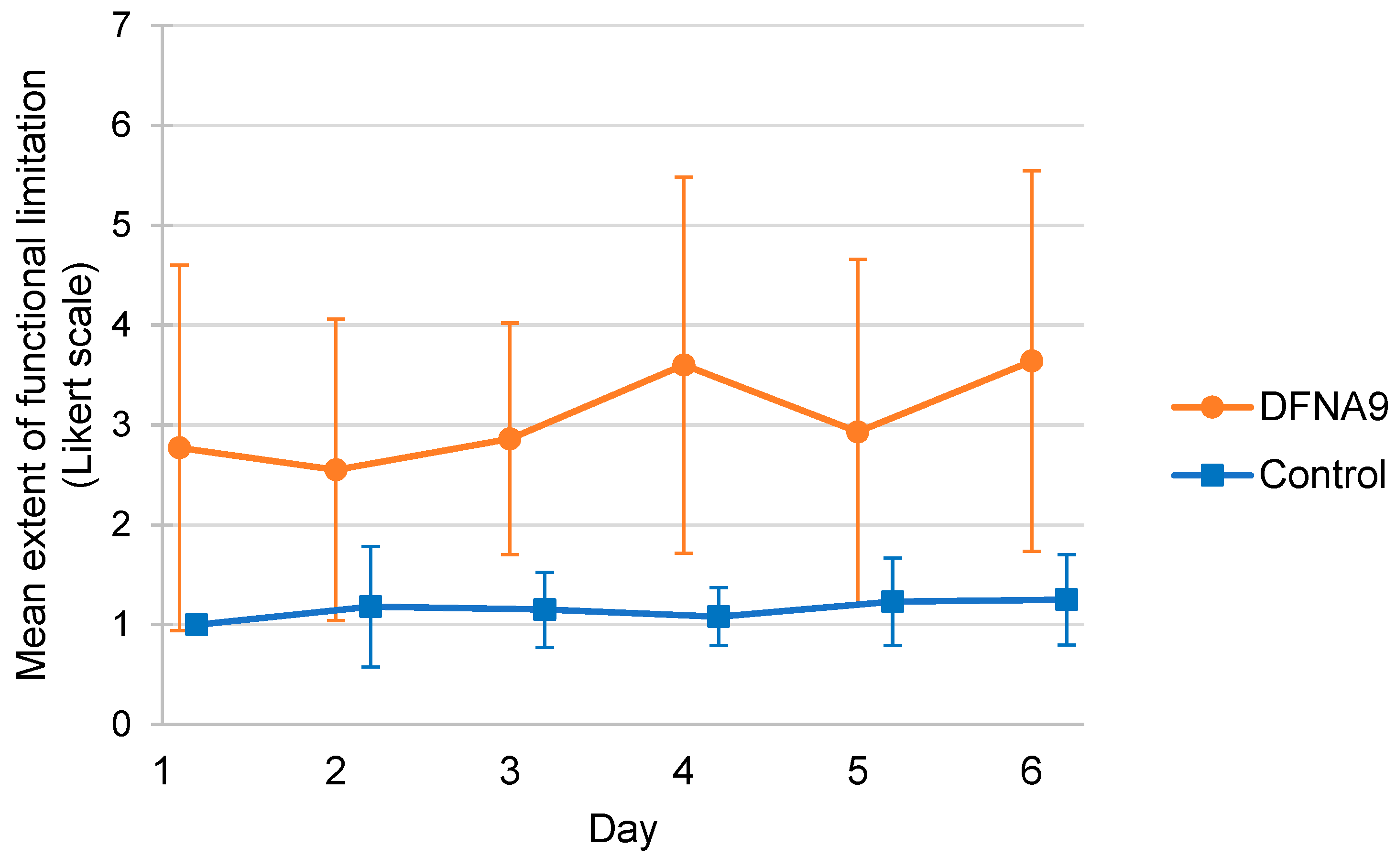
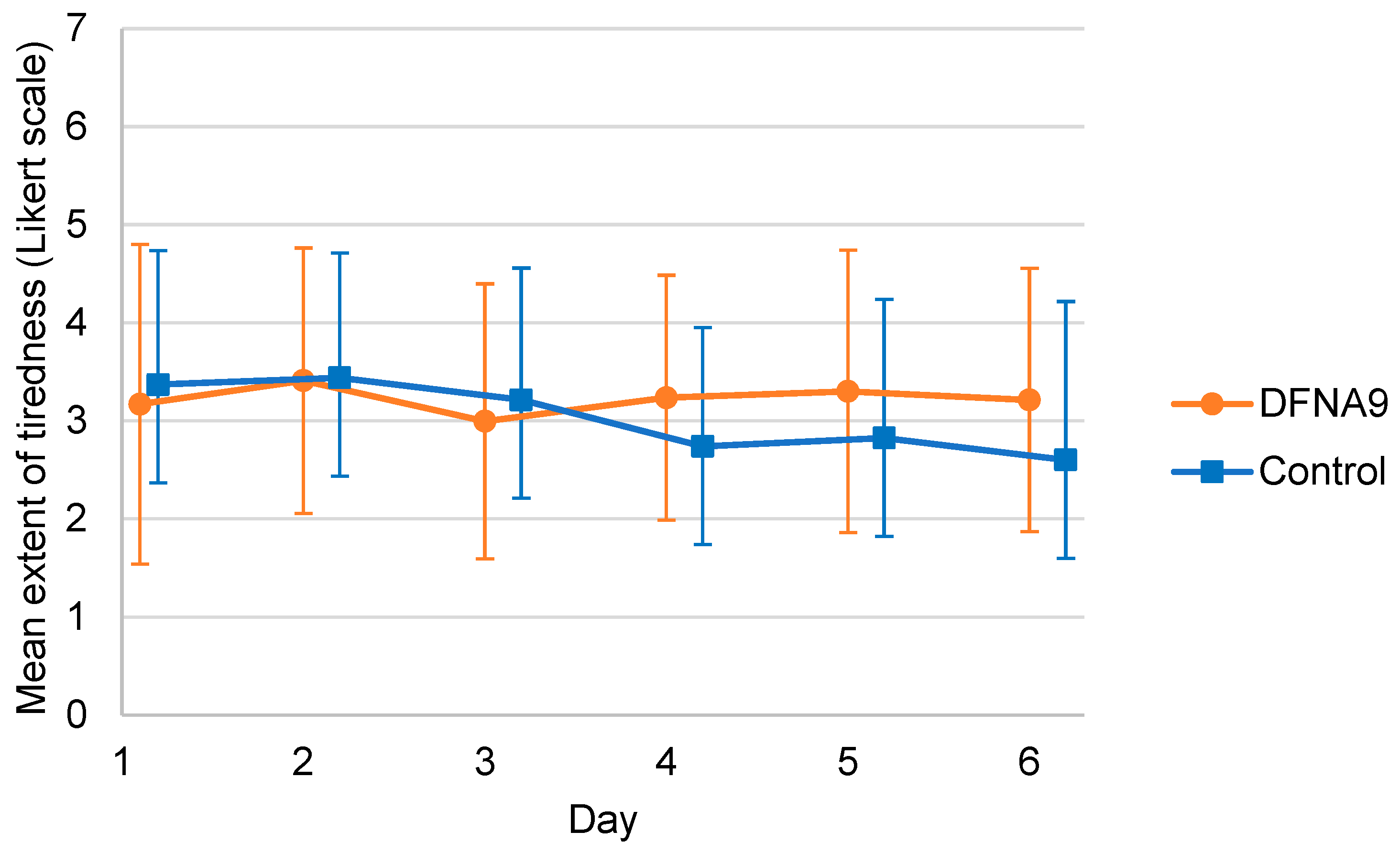
3.2. Subjective Measurements of Functional Limitations and Tiredness
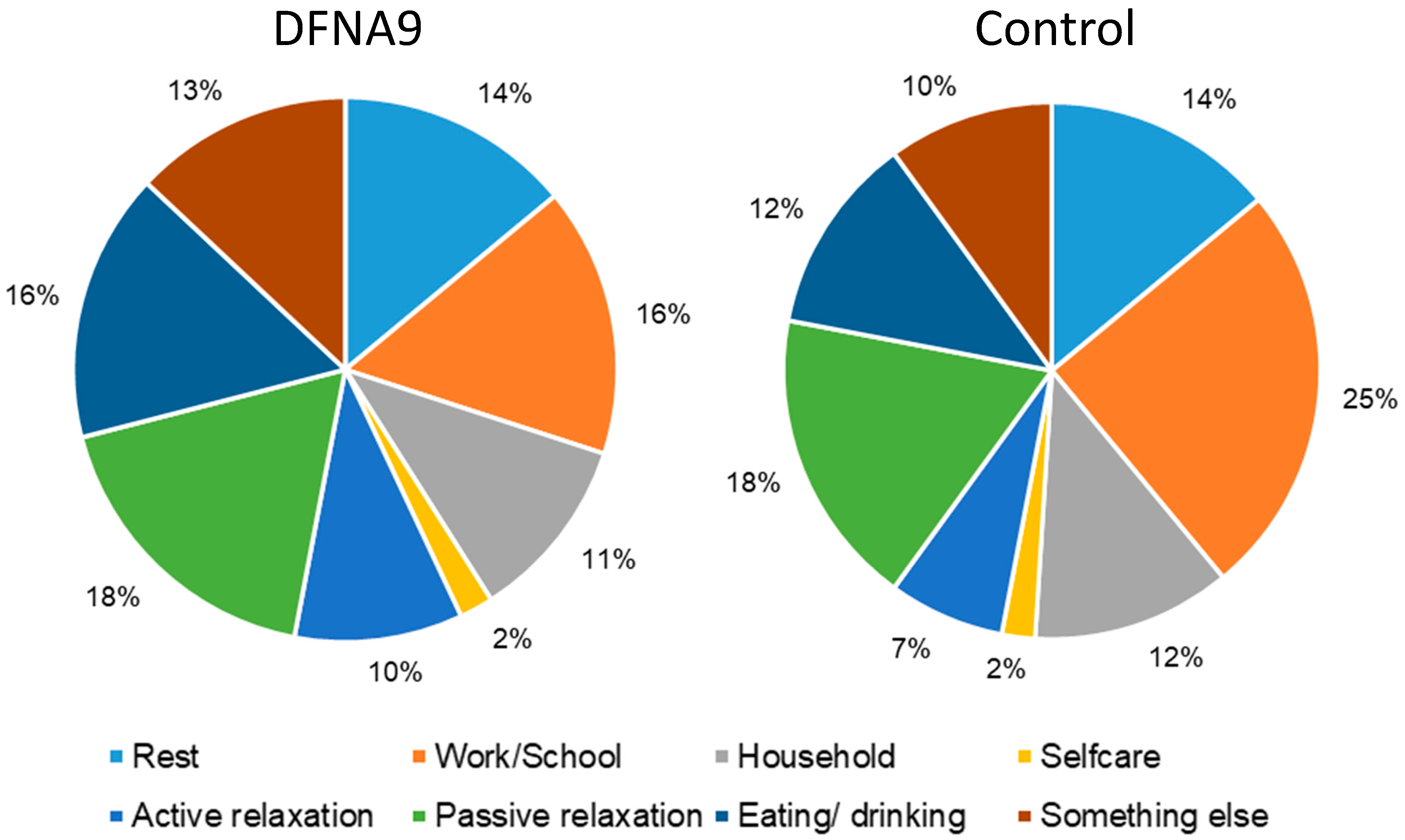
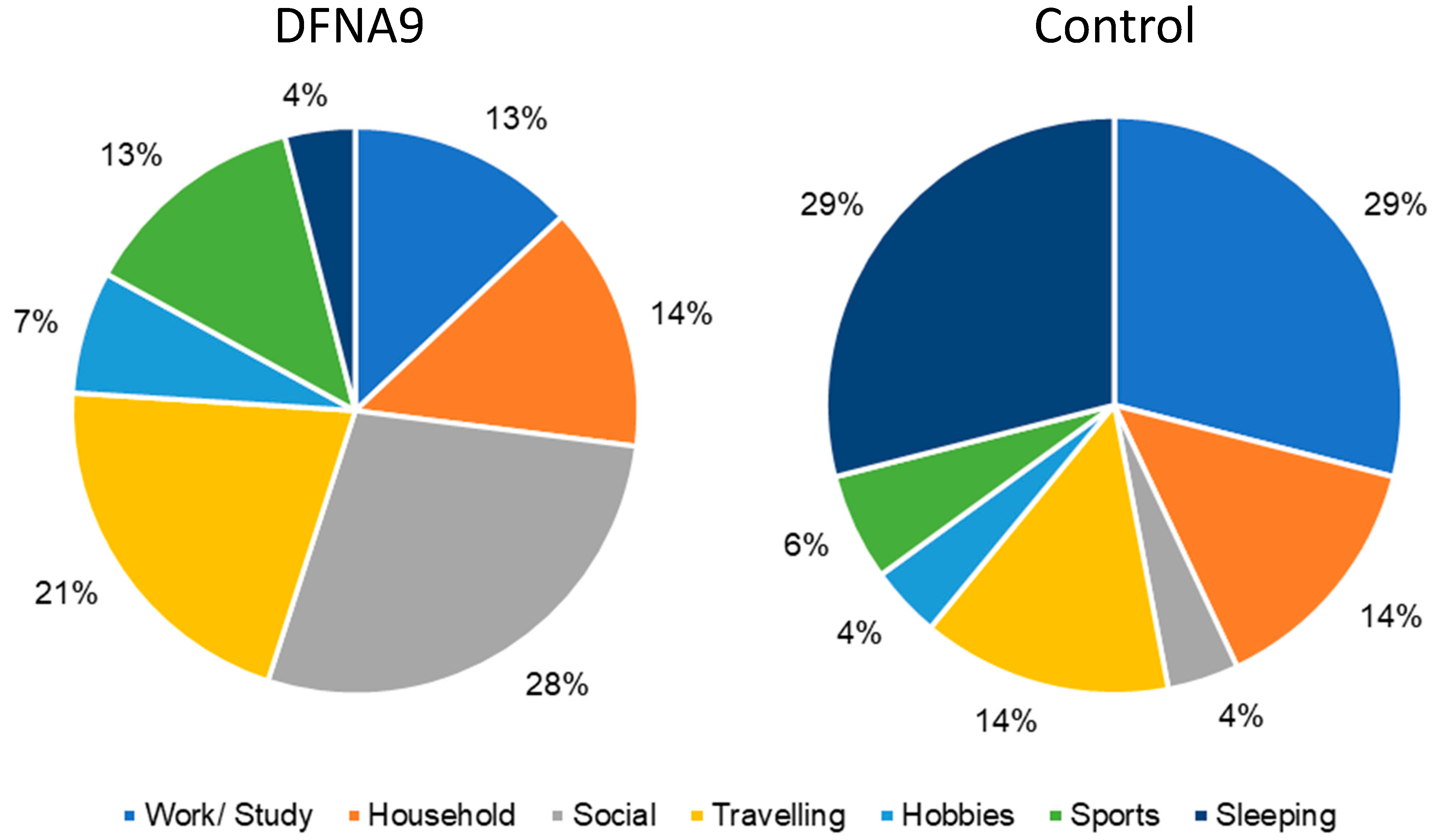
3.3. Subjective Measurements of Performed Activities and Activities during Which Participants Felt Limited
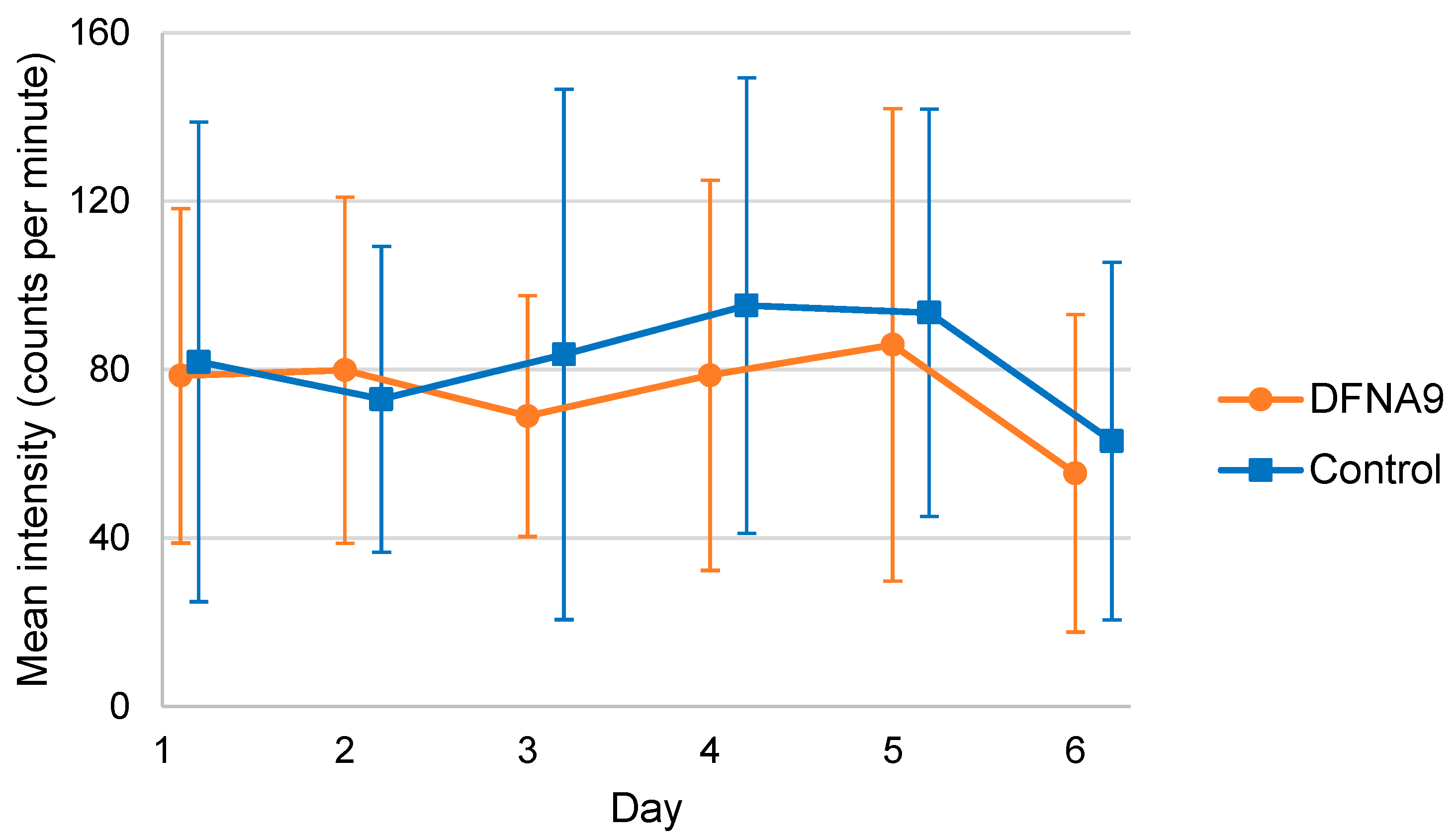
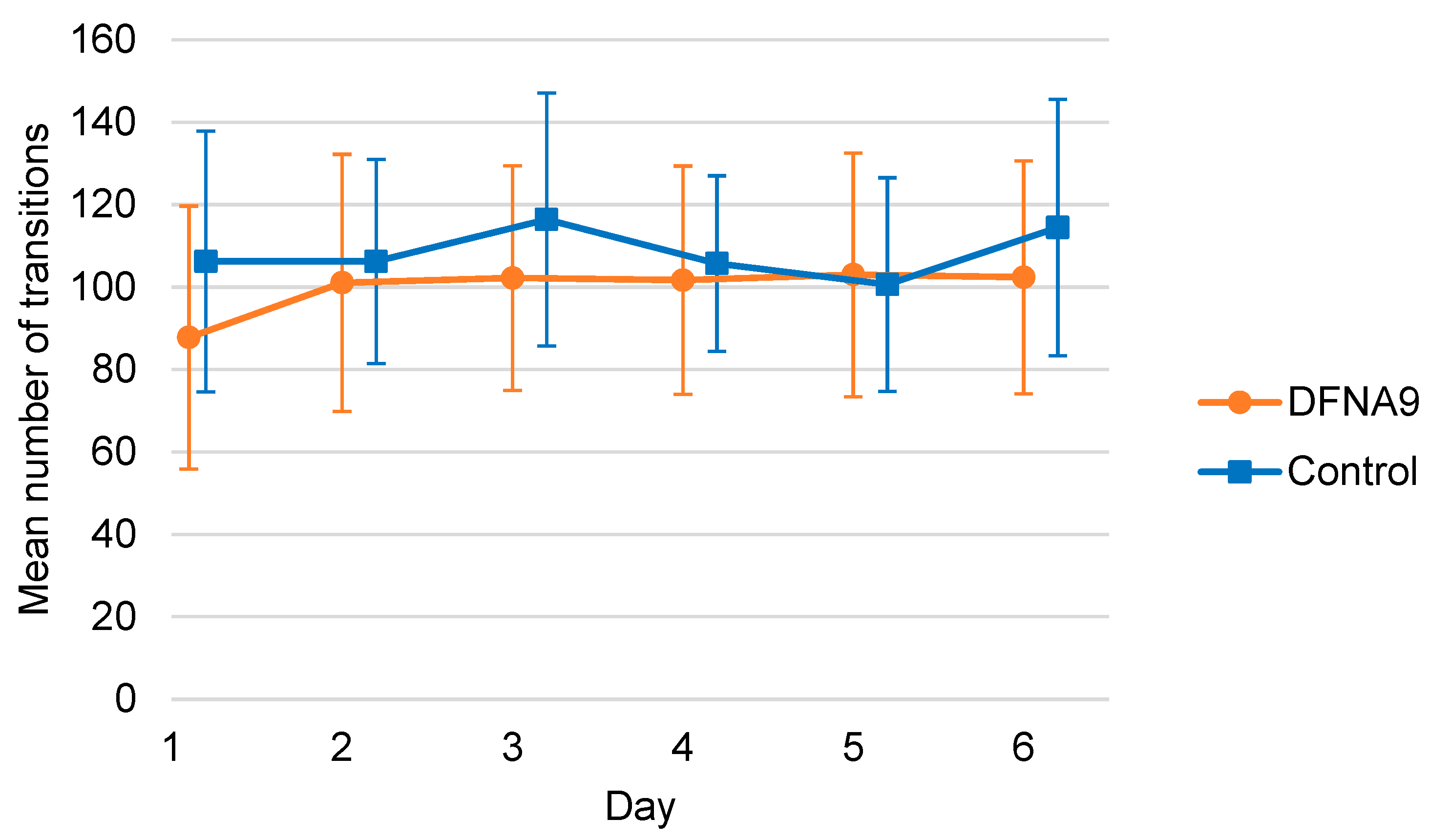
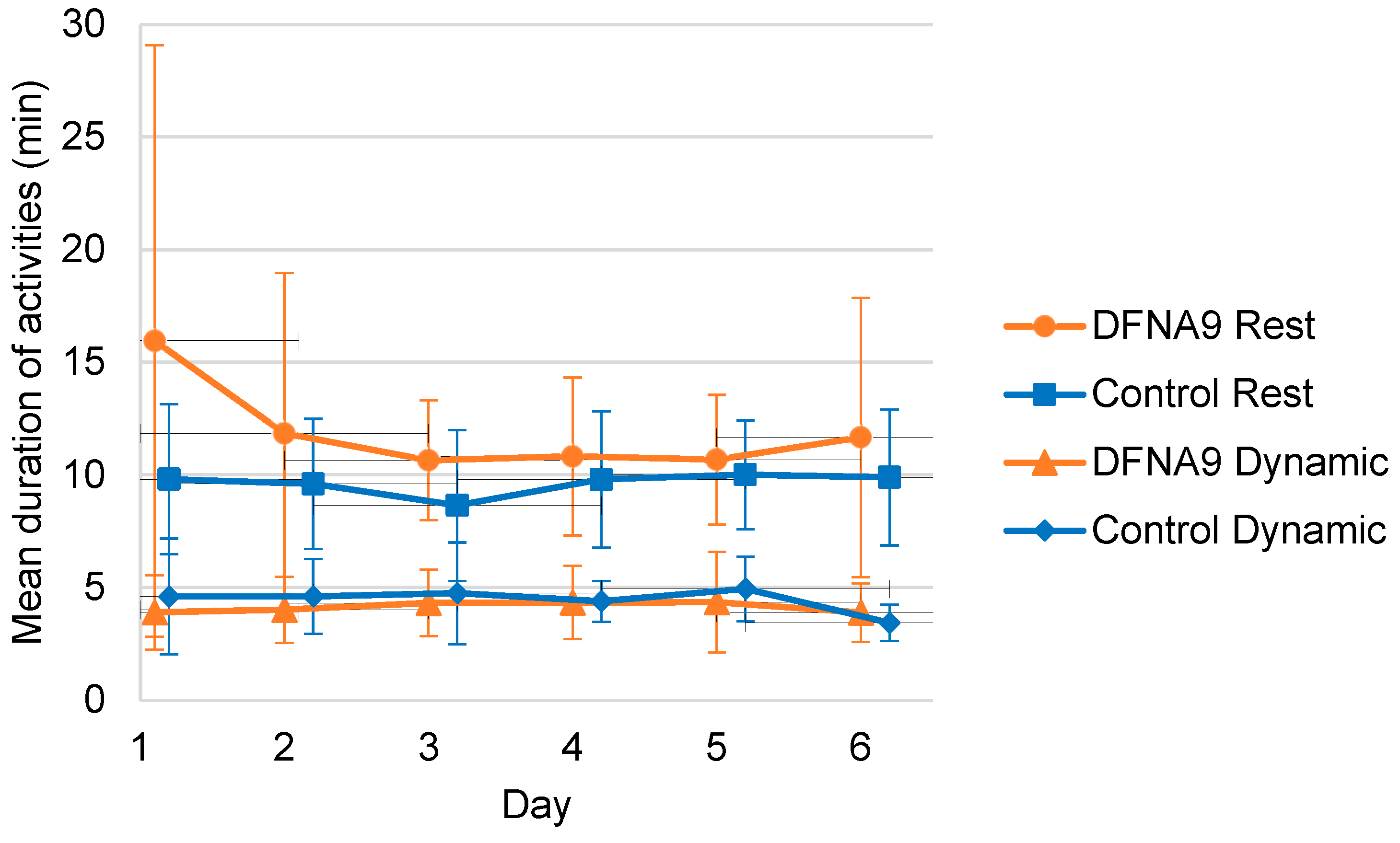
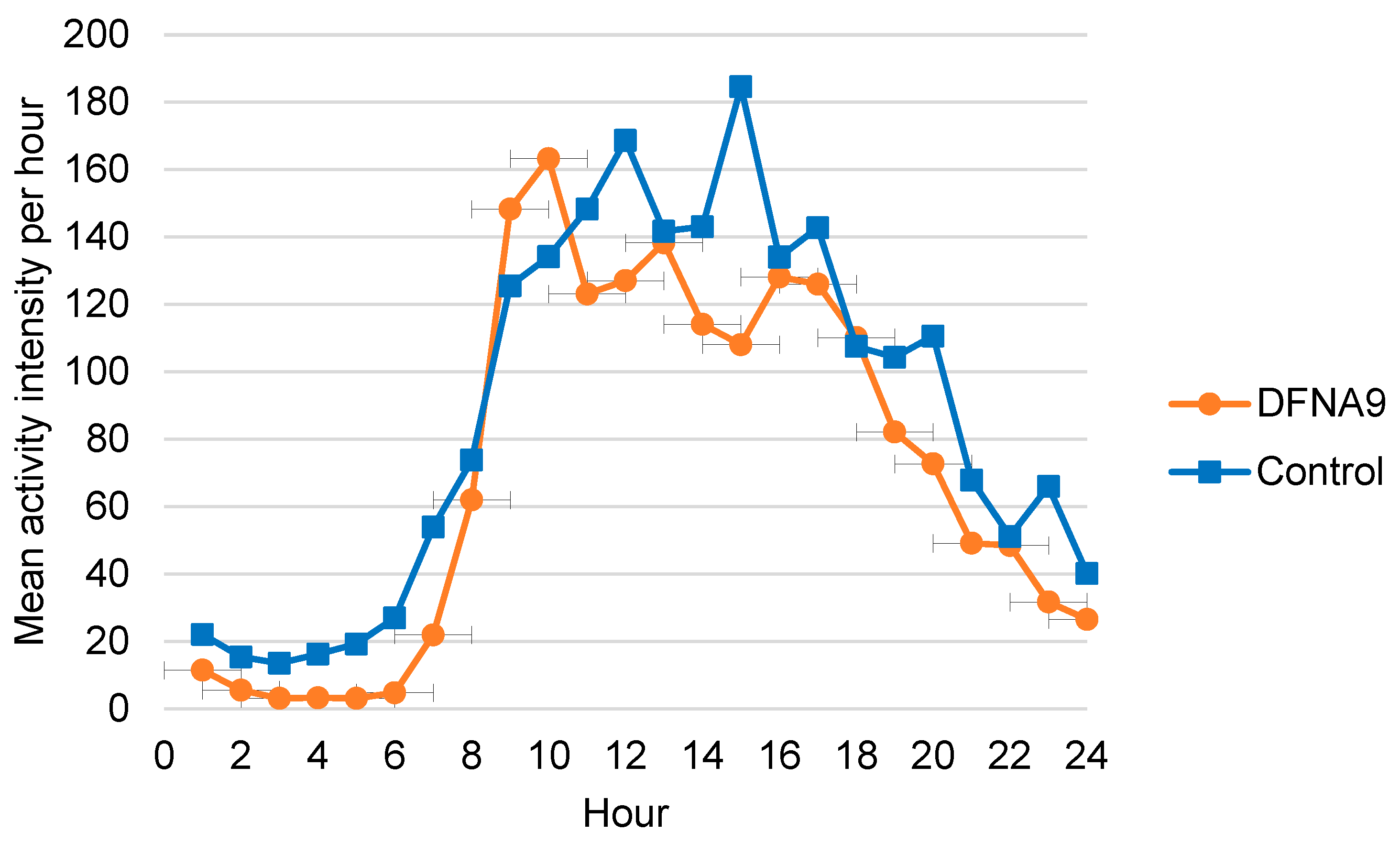
3.4. Objective Measurements of Physical Activity
3.5. Self-Reported Functional Limitations and Tiredness Related to Mean Physical Activity Intensities
3.6. Comparison of Activity Patterns
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kingma, H.; van de Berg, R. Anatomy, physiology, and physics of the peripheral vestibular system. Handb. Clin. Neurol. 2016, 137, 1–16. [Google Scholar]
- Angelaki, D.E.; Cullen, K.E. Vestibular system: The many facets of a multimodal sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef]
- Lucieer, F.; Vonk, P.; Guinand, N.; Stokroos, R.; Kingma, H.; van de Berg, R. Bilateral Vestibular Hypofunction: Insights in Etiologies, Clinical Subtypes, and Diagnostics. Front. Neurol. 2016, 7, 26. [Google Scholar] [CrossRef]
- Van Dooren, T.S.; Lucieer, F.M.P.; Duijn, S.; Janssen, A.M.L.; Guinand, N.; Perez Fornos, A.; Van Rompaey, V.; Kingma, H.; Ramat, S.; van de Berg, R. The Functional Head Impulse Test to Assess Oscillopsia in Bilateral Vestibulopathy. Front. Neurol. 2019, 10, 365. [Google Scholar] [CrossRef]
- Bisdorff, A.; Von Brevern, M.; Lempert, T.; Newman-Toker, D.E. Classification of vestibular symptoms: Towards an international classification of vestibular disorders. J. Vestib. Res. Equilib. Orientat. 2009, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.G.; Furman, J.M. Psychiatric consequences of vestibular dysfunction. Curr. Opin. Neurol. 2001, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zingler, V.C.; Cnyrim, C.; Jahn, K.; Weintz, E.; Fernbacher, J.; Frenzel, C.; Brandt, T.; Strupp, M. Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann. Neurol. 2007, 61, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Gazquez, I.; Lopez-Escamez, J.A. Genetics of recurrent vertigo and vestibular disorders. Curr Genom. 2011, 2, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.G.; Lu, L.; Heller, S.; Merchant, S.N.; Eavey, R.D.; McKenna, M.; Nadol, J.B.; Miyamoto, R.T.; Linthicum, F.H.; Neto, J.F.L.; et al. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat. Genet. 1998, 20, 299–303. [Google Scholar] [CrossRef] [PubMed]
- JanssensdeVarebeke, S.P.; Moyaert, J.; Fransen, E.; Bulen, B.; Neesen, C.; Devroye, K.; van de Berg, R.; Pennings, R.J.; Topsakal, V.; Vanderveken, O.; et al. Genotype-phenotype Correlation Study in a Large Series of Patients Carrying the p.Pro51Ser (p.P51S) Variant in COCH (DFNA9): Part I-A Cross-sectional Study of Hearing Function in 111 Carriers. Ear Hear 2021, 42, 1508–1524. [Google Scholar] [CrossRef] [PubMed]
- JanssensdeVarebeke, S.P.F.; Moyaert, J.; Fransen, E.; Bulen, B.; Neesen, C.; Devroye, K.; van de Berg, R.; Pennings, R.J.E.; Topsakal, V.; Vanderveken, O.; et al. Genotype-Phenotype Correlation Study in a Large Series of Patients Carrying the p.Pro51Ser (p.P51S) Variant in COCH (DFNA9) Part II: A Prospective Cross-Sectional Study of the Vestibular Phenotype in 111 Carriers. Ear Hear 2021, 42, 1525–1543. [Google Scholar] [CrossRef]
- Vermeire, K.; Brokx, J.P.L.; Wuyts, F.L.; Cochet, E.; Hofkens, A.; De Bodt, M.; Van de Heyning, P.H. Good speech recognition and quality-of-life scores after cochlear implantation in patients with DFNA9. Otol. Neurotol. 2006, 27, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Perez Fornos, A.; Cavuscens, S.; Ranieri, M.; van de Berg, R.; Stokroos, R.; Kingma, H.; Guyot, J.P.; Guinand, N. The vestibular implant: A probe in orbit around the human balance system. J. Vestib. Res. 2017, 27, 51–61. [Google Scholar] [CrossRef]
- Guinand, N.; van de Berg, R.; Ranieri, M.; Cavuscens, S.; DiGiovanna, J.; Nguyen, T.A.K.; Micera, S.; Stokroos, R.; Kingma, H.; Guyot, J.P.; et al. Vestibular implants: Hope for improving the quality of life of patients with bilateral vestibular loss. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, Milan, Italy, 25–29 August 2015; Volume 2015, pp. 7192–7195. [Google Scholar]
- Lucieer, F.; Duijn, S.; Van Rompaey, V.; Pérez Fornos, A.; Guinand, N.; Guyot, J.P.; Kingma, H.; Van De Berg, R. Full Spectrum of Reported Symptoms of Bilateral Vestibulopathy Needs Further Investigation—A Systematic Review. Front. Neurol. 2018, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Lucieer, F.M.; Van Hecke, R.; van Stiphout, L.; Duijn, S.; Perez-Fornos, A.; Guinand, N.; Van Rompaey, V.; Kingma, H.; Joore, M.; van de Berg, R. Bilateral vestibulopathy: Beyond imbalance and oscillopsia. J. Neurol. 2020, 267 (Suppl. S1), 241–255. [Google Scholar] [CrossRef]
- Danneels, M.; Van Hecke, R.; Keppler, H.; Degeest, S.; Cambier, D.; van de Berg, R.; Van Rompaey, V.; Maes, L. Psychometric Properties of Cognitive-Motor Dual-Task Studies With the Aim of Developing a Test Protocol for Persons With Vestibular Disorders: A Systematic Review. Ear Hear. 2020, 41, 3–16. [Google Scholar] [CrossRef]
- Sun, D.Q.; Ward, B.K.; Semenov, Y.R.; Carey, J.P.; Della Santina, C.C. Bilateral Vestibular Deficiency: Quality of Life and Economic Implications. JAMA Otolaryngol.—Head Neck Surg. 2014, 140, 527–534. [Google Scholar] [CrossRef]
- Ward, B.K.; Agrawal, Y.; Hoffman, H.J.; Carey, J.P.; Della Santina, C.C. Prevalence and impact of bilateral vestibular hypofunction: Results from the 2008 US National Health Interview Survey. JAMA Otolaryngol.—Head Neck Surg. 2013, 139, 803–810. [Google Scholar] [CrossRef] [PubMed]
- van Stiphout, L.; Lucieer, F.; Guinand, N.; Pérez Fornos, A.; van de Berg, M.; Van Rompaey, V.; Widdershoven, J.; Kingma, H.; Joore, M.; van de Berg, R. Bilateral vestibulopathy patients’ perspectives on vestibular implant treatment: A qualitative study. J. Neurol. 2021, 1–9. [Google Scholar] [CrossRef]
- Guinand, N.; Boselie, F.; Guyot, J.P.; Kingma, H. Quality of life of patients with bilateral vestibulopathy. Ann. Otol. Rhinol. Laryngol. 2012, 121, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Schniepp, R.; Mohwald, K.; Wuehr, M. Clinical and automated gait analysis in patients with vestibular, cerebellar, and functional gait disorders: Perspectives and limitations. J. Neurol. 2019, 266 (Suppl. S1), 118–122. [Google Scholar] [CrossRef]
- Aparicio Ugarriza, R.; Mielgo Ayuso, J.; Benito Peinado, P.J.; Pedrero Chamizo, R.; Ara, I.; Gonzalez Gross, M.M. Physical activity assessment in the general population; instrumental methods and new technologies. Nutr. Hosp. 2015, 31, 219–226. [Google Scholar]
- Silfee, V.J.; Haughton, C.F.; Jake-Schoffman, D.; Lopez-Cepero, A.; May, C.N.; Sreedhara, M.; Rosal, M.C.; Lemon, S.C. Objective measurement of physical activity outcomes in lifestyle interventions among adults: A systematic review. Prev. Med. Rep. 2018, 11, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Decramer, M.; Gosselink, R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2005, 86, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Bijnens, W.; Aarts, J.; Stevens, A.; Ummels, D.; Meijer, K. Optimization and Validation of an Adjustable Activity Classification Algorithm for Assessment of Physical Behavior in Elderly. Sensors 2019, 19, 5344. [Google Scholar] [CrossRef]
- van der Weegen, S.; Essers, H.; Spreeuwenberg, M.; Verwey, R.; Tange, H.; de Witte, L.; Meijer, K. Concurrent validity of the MOX activity monitor compared to the ActiGraph GT3X. Telemed. J. e-Health 2015, 21, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Instruments, M. MOX Maastricht, The Netherlands. Available online: www.accelerometry.eu (accessed on 1 July 2019).
- Zwergal, A.; Brandt, T.; Magnusson, M.; Kennard, C. DIZZYNET—A European network initiative for vertigo and balance research: Visions and aims. J. Neurol. 2016, 263 Suppl. 1, 2–9. [Google Scholar] [CrossRef]
- Myin-Germeys, I.; Oorschot, M.; Collip, D.; Lataster, J.; Delespaul, P.; van Os, J. Experience sampling research in psychopathology: Opening the black box of daily life. Psychol. Med. 2009, 39, 1533–1547. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.C.; Leue, C.; Delespaul, P.; Peeters, F.; Janssen, A.M.L.; Lousberg, R.; Erdkamp, A.; van de Weijer, S.; Widdershoven, J.; Blom, H.; et al. Introducing the DizzyQuest: An app-based diary for vestibular disorders. J. Neurol. 2020, 267 (Suppl. S1), 3–14. [Google Scholar] [CrossRef]
- Hartmann, J.A.; Wichers, M.; Menne-Lothmann, C.; Kramer, I.; Viechtbauer, W.; Peeters, F.; Schruers, K.R.J.; Van Bemmel, A.L.; Myin-Germeys, I.; Delespaul, P.; et al. Experience Sampling-Based Personalized Feedback and Positive Affect: A Randomized Controlled Trial in Depressed Patients. PLoS ONE 2015, 10, e0128095. [Google Scholar] [CrossRef]
- Broen, M.P.; Marsman, V.A.; Kuijf, M.L.; Van Oostenbrugge, R.J.; van Os, J.; Leentjens, A.F. Unraveling the Relationship between Motor Symptoms, Affective States and Contextual Factors in Parkinson’s Disease: A Feasibility Study of the Experience Sampling Method. PLoS ONE 2016, 11, e0151195. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Kim, J.-S.; Murofushi, T.; Straumann, D.; Jen, J.C.; Rosengren, S.M.; Della Santina, C.C.; Kingma, H. Bilateral vestibulopathy: Diagnostic criteria Consensus document of the Classification Committee of the Barany Society. J. Vestib. Res. 2017, 27, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Dobbels, B.; Mertens, G.; Gilles, A.; Claes, A.; Moyaert, J.; Van De Berg, R.; Van De Heyning, P.; Vanderveken, O.; Van Rompaey, V. Cognitive Function in Acquired Bilateral Vestibulopathy: A Cross-Sectional Study on Cognition, Hearing, and Vestibular Loss. Front. Neurosci. 2019, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Bersch, S.D.; Azzi, D.; Khusainov, R.; Achumba, I.E.; Ries, J. Sensor Data Acquisition and Processing Parameters for Human Activity Classification. Sensors 2014, 14, 4239–4270. [Google Scholar] [CrossRef] [PubMed]
- Mathie, M.J.; Coster, A.C.F.; Lovell, N.H.; Celler, B.G. Detection of daily physical activities using a triaxial accelerometer. Med. Biol. Eng. Comput. 2003, 41, 296–301. [Google Scholar] [CrossRef]
- Snijders, T.A.B.; Bosker, R.J. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling, 2nd ed.; Angeles, L., Ed.; SAGE: London, UK, 2012. [Google Scholar]
- Paraschiv-Ionescu, A.; Buchser, E.; Aminian, K. Unraveling dynamics of human physical activity patterns in chronic pain conditions. Sci. Rep. 2013, 3, 2019. [Google Scholar] [CrossRef]
- Wuehr, M.; Huppert, A.; Schenkel, F.; Decker, J.; Jahn, K.; Schniepp, R. Independent domains of daily mobility in patients with neurological gait disorders. J. Neurol. 2020, 267 (Suppl. S1), 292–300. [Google Scholar] [CrossRef]
- Biesecker, B.B.; Erby, L. Adaptation to living with a genetic condition or risk: A mini-review. Clin. Genet. 2008, 74, 401–407. [Google Scholar] [CrossRef]
- Adams, A.; Cox, A. Questionnaires, in-depth interviews and focus groups. In Research Methods for Human-Computer Interaction; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Cohen, H.S.; Kimball, K.T.; Adams, A.S. Application of the Vestibular Disorders Activities of Daily Living Scale. Laryngoscope 2000, 110, 1204–1209. [Google Scholar] [CrossRef]
- Najafi, B.; Aminian, K.; Paraschiv-Ionescu, A.; Loew, F.; Büla, C.; Prince, R. Ambulatory System for Human Motion Analysis Using a Kinematic Sensor: Monitoring of Daily Physical Activity in the Elderly. IEEE Trans. Bio-Med. Eng. 2003, 50, 711–723. [Google Scholar] [CrossRef]
- Petrella, J.K.; Cress, M.E. Daily Ambulation Activity and Task Performance in Community-Dwelling Older Adults Aged 63–71 Years With Preclinical Disability. J. Gerontol. Ser. A 2004, 59, M264–M267. [Google Scholar] [CrossRef]
- Schniepp, R.; Wühr, M.; Huth, S.; Pradhan, C.; Brandt, T.; Jahn, K. Gait characteristics of patients with phobic postural vertigo: Effects of fear of falling, attention, and visual input. J. Neurol. 2014, 261, 738–746. [Google Scholar] [CrossRef]
- Morimoto, H.; Asai, Y.; Johnson, E.G.; Koide, Y.; Niki, J.; Sakai, S.; Nakayama, M.; Kabaya, K.; Fukui, A.; Mizutani, Y.; et al. Objective measures of physical activity in patients with chronic unilateral vestibular hypofunction, and its relationship to handicap, anxiety and postural stability. Auris Nasus Larynx 2019, 46, 70–77. [Google Scholar] [CrossRef]
- Dietrich, H.; Heidger, F.; Schniepp, R.; MacNeilage, P.R.; Glasauer, S.; Wuehr, M. Head motion predictability explains activity-dependent suppression of vestibular balance control. Sci. Rep. 2020, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P.; Hallam, R.; Beyts, J.; Hinchlife, R. Dizziness: Behavioural, subjective and organic aspects. J. Psychosom. Res. 1988, 32, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.C.; Verkaik, R.; Stultiens, J.J.A.; van de Berg, M.R.; Janssen, A.M.L.; Leue, C.; Delespaul, P.; Peeters, F.; Widdershoven, J.; Erdkamp, A.; et al. The DizzyQuest: Relation between self-reported hearing loss, tinnitus and objective hearing thresholds in patients with Meniere’s disease. J. Neurol. 2021, 1–10. [Google Scholar] [CrossRef]
- Kim, S.; Oh, Y.M.; Koo, J.W.; Kim, J.S. Bilateral vestibulopathy: Clinical characteristics and diagnostic criteria. Otol. Neurotol. 2011, 32, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Harrison Bush, A.L.; Lister, J.J.; Lin, F.R.; Betz, J.; Edwards, J.D. Peripheral Hearing and Cognition: Evidence From the Staying Keen in Later Life (SKILL) Study. Ear Hear. 2015, 36, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol.—Head Neck Surg. 2018, 144, 115–126. [Google Scholar] [CrossRef]
- Dobbels, B.; Peetermans, O.; Boon, B.; Mertens, G.; Van de Heyning, P.; Van Rompaey, V. Impact of Bilateral Vestibulopathy on Spatial and Nonspatial Cognition: A Systematic Review. Ear Hear. 2019, 40, 757–765. [Google Scholar] [CrossRef] [PubMed]
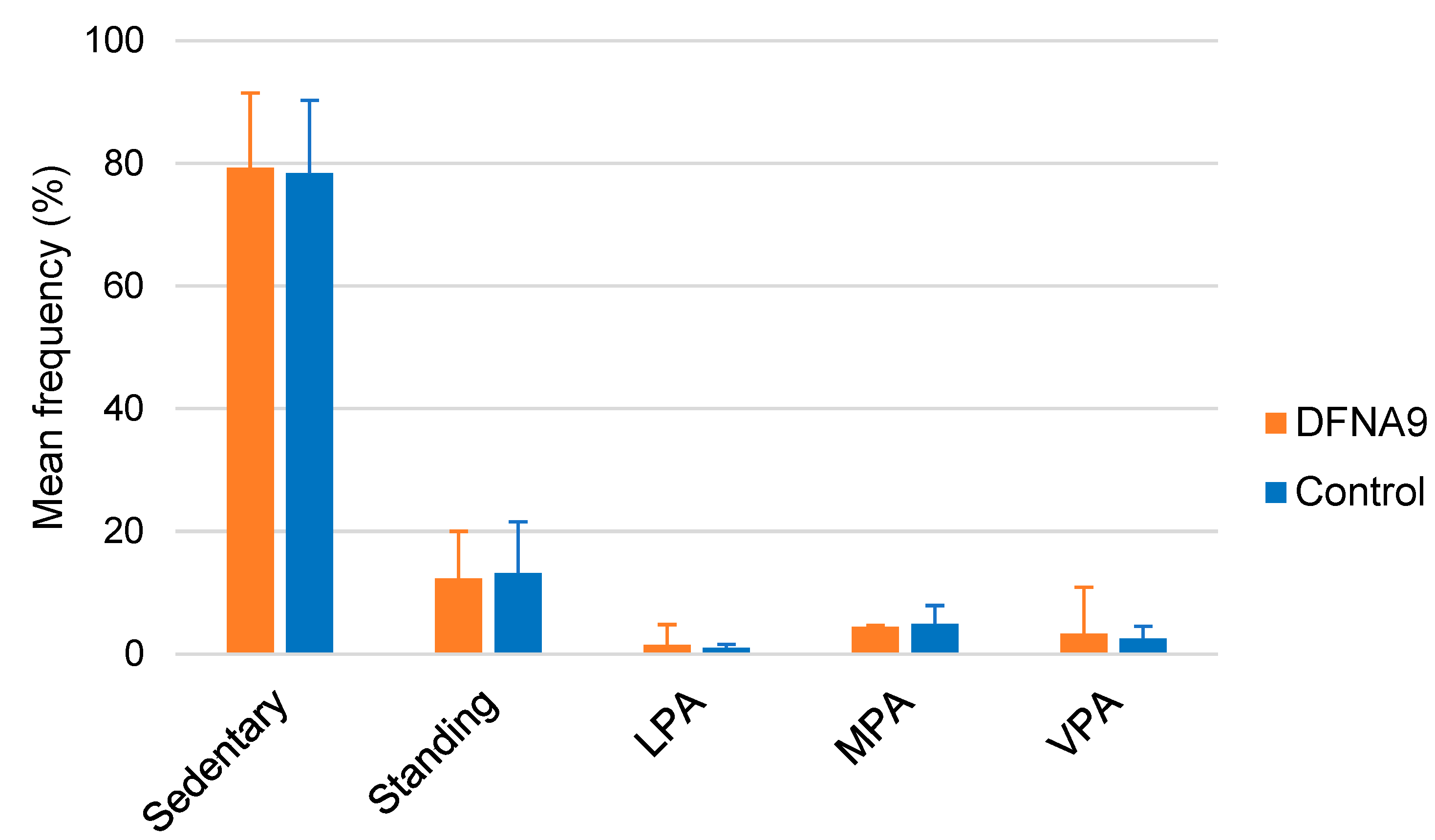
| Patient Number | Gender (M/F) | Age (Years) | vHIT (Gain) | Caloric Test (Sum of Bithermal Max. Peak SPV) | Audiometry (PTA dB HL) | |||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |||
| 1. | M | 54 | 0.56 | 0.08 | - | - | 30 | 40 |
| 2. | F | 52 | 0.38 | 0.38 | - | - | 70 | 70 |
| 3. | F | 50 | 0.39 | 0.63 | - | - | 50 | 20 |
| 4. | M | 63 | 0.03 | 0.15 | - | - | 70 | 60 |
| 5. | F | 71 | 0.21 | 0.47 | - | - | - | - |
| 6. | F | 61 | 0.58 | 0.26 | 3 | 3 | 10 | 10 |
| 7. | F | 54 | 0.28 | 0.32 | 3 | 1 | 10 | 10 |
| 8. | M | 61 | - | - | 0 | 0 | 70 | 70 |
| 9. | F | 70 | - | - | 0 | 0 | - | - |
| 10. | M | 75 | - | - | 0 | 0 | 70 | 70 |
| 11. | F | 59 | 0 | 0 | - | - | 60 | 30 |
| 12. | F | 72 | - | - | 0 | 0 | - | - |
| 13. | F | 66 | 0.32 | 0.04 | - | - | - | - |
| 14. | F | 54 | - | - | 1 | 2 | 30 | 80 |
| 15. | F | 64 | 0.03 | 0.06 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, E.; de Hoon, S.; Stultiens, J.; Janssen, M.; Essers, H.; Meijer, K.; Bijnens, W.; van de Berg, M.; Herssens, N.; Janssens de Varebeke, S.; et al. The DizzyQuest Combined with Accelerometry: Daily Physical Activities and Limitations among Patients with Bilateral Vestibulopathy Due to DFNA9. J. Clin. Med. 2024, 13, 1131. https://doi.org/10.3390/jcm13041131
Martin E, de Hoon S, Stultiens J, Janssen M, Essers H, Meijer K, Bijnens W, van de Berg M, Herssens N, Janssens de Varebeke S, et al. The DizzyQuest Combined with Accelerometry: Daily Physical Activities and Limitations among Patients with Bilateral Vestibulopathy Due to DFNA9. Journal of Clinical Medicine. 2024; 13(4):1131. https://doi.org/10.3390/jcm13041131
Chicago/Turabian StyleMartin, Erik, Sofie de Hoon, Joost Stultiens, Miranda Janssen, Hans Essers, Kenneth Meijer, Wouter Bijnens, Maurice van de Berg, Nolan Herssens, Sebastien Janssens de Varebeke, and et al. 2024. "The DizzyQuest Combined with Accelerometry: Daily Physical Activities and Limitations among Patients with Bilateral Vestibulopathy Due to DFNA9" Journal of Clinical Medicine 13, no. 4: 1131. https://doi.org/10.3390/jcm13041131
APA StyleMartin, E., de Hoon, S., Stultiens, J., Janssen, M., Essers, H., Meijer, K., Bijnens, W., van de Berg, M., Herssens, N., Janssens de Varebeke, S., Hallemans, A., Van Rompaey, V., Guinand, N., Perez-Fornos, A., Widdershoven, J., & van de Berg, R. (2024). The DizzyQuest Combined with Accelerometry: Daily Physical Activities and Limitations among Patients with Bilateral Vestibulopathy Due to DFNA9. Journal of Clinical Medicine, 13(4), 1131. https://doi.org/10.3390/jcm13041131









