Statistical Associations between Vestibular Pathologies and Hypothyroidism: A Retrospective Study
Abstract
1. Introduction
1.1. Hypothyroidism and BPPV
1.2. Hypothyroidism and Meniere Disease
1.3. Impact of Hypothyroidism on Vestibular Disorders Symptoms
2. Materials and Methods
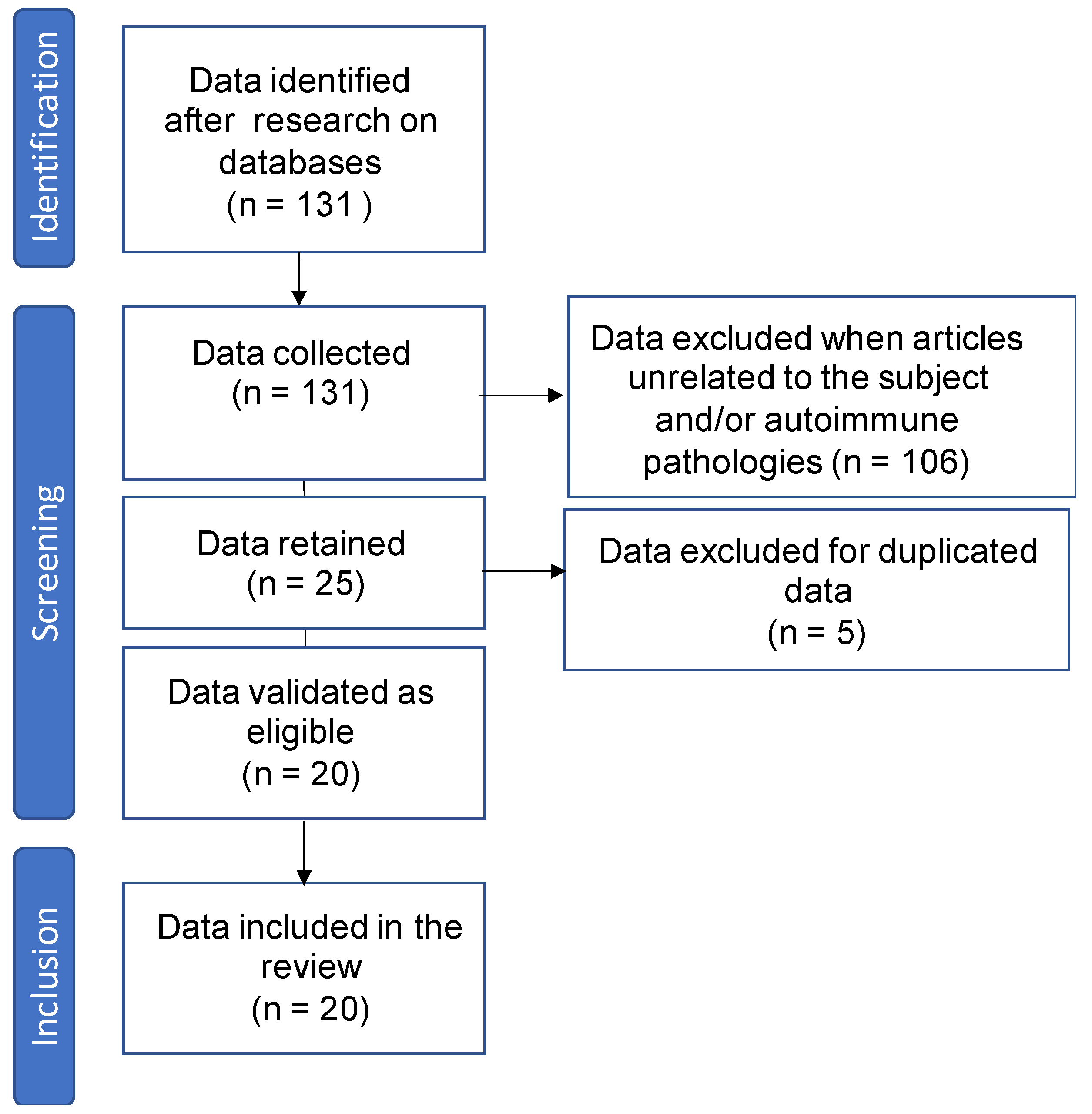
2.1. Collection of Bibliographical Data
2.2. Participants
2.3. Data Analysis
3. Results
3.1. Demographics and Clinical Characteristics of the Study Group
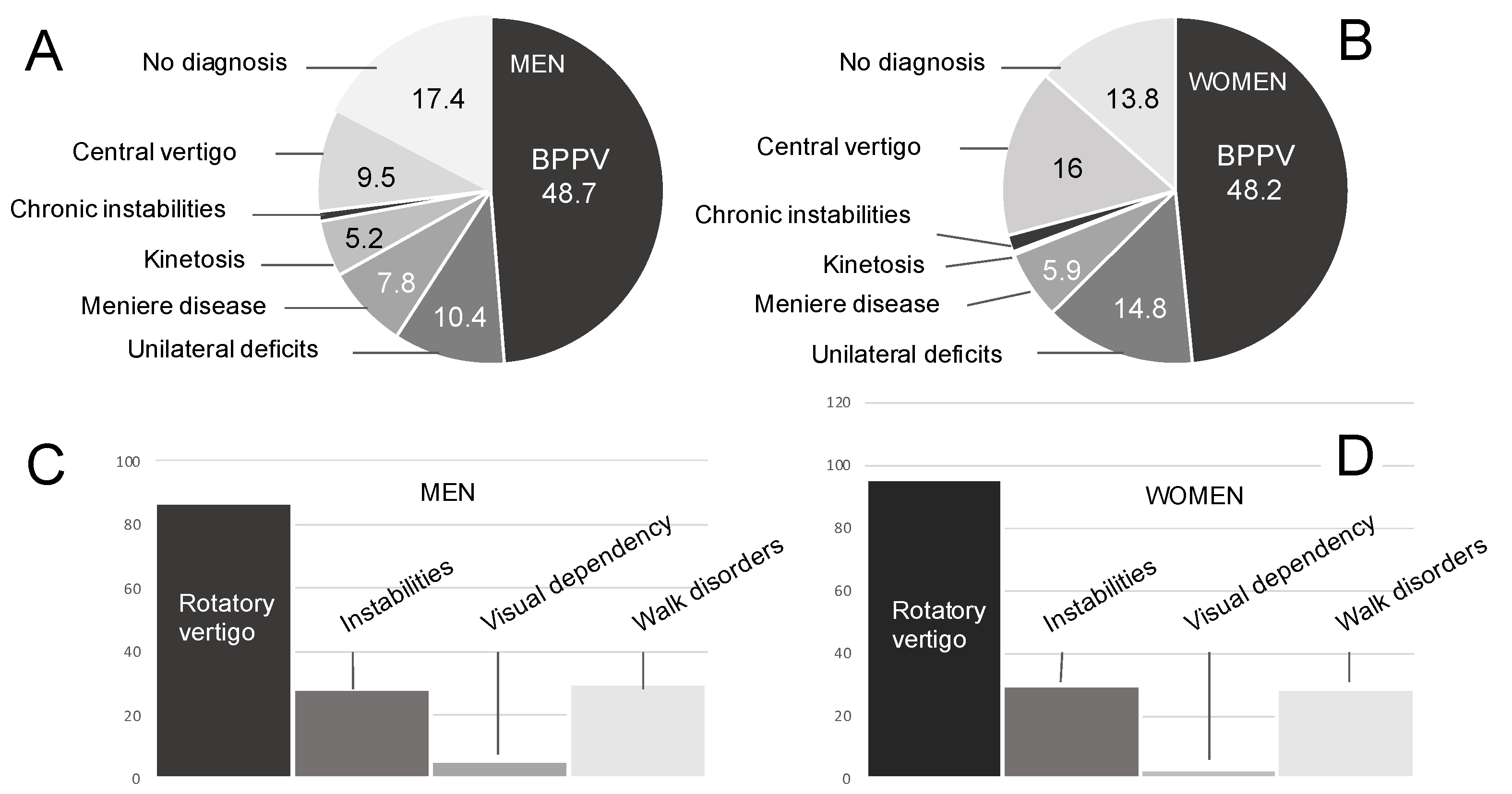
3.1.1. Pathologies
3.1.2. Expressed Symptoms
3.1.3. Thyroid Status
3.2. Studies of Statistical Associations between Vestibular Pathologies and Expressed Symptoms
3.3. Studies of Statistical Associations between Clinical Characteristics and Thyroid Conditions
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rybak, L.P. Metabolic disorders of the vestibular system. Otolaryngol. Head Neck Surg. 1995, 112, 128–132. [Google Scholar] [CrossRef] [PubMed]
- El Khiati, R.; Tighilet, B.; Besnard, S.; Chabbert, C. Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives. Cells 2023, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Shambaugh, G.E., Jr. Endocrine aspects of Meniere’s disease. Laryngoscope 1959, 69, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Power, W.N. Metabolic aspects of Meniere’s disease. Laryngoscope 1978, 88, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Ritter, F.N. The effects of hypothyroidism upon the ear, nose and throat. A clinical and experimental study. Laryngoscope 1967, 77, 1427–1479. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Muskett, J.; King, K.A.; Zalewski, C.K.; Shawker, T.; Reynolds, J.C.; Butman, J.A.; Brewer, C.C.; Stewart, A.K.; Alper, S.L.; et al. Hereditary hearing loss with thyroid abnormalities. Adv. Otorhinolaryngol. 2011, 70, 43–49. [Google Scholar] [PubMed]
- Chiarella, G.; Russo, D.; Monzani, F.; Petrolo, C.; Fattori, B.; Pasqualetti, G.; Cassandro, E.; Costante, G. Hashimoto thyroiditis and vestibular dysfunction. Endocr. Pract. 2017, 23, 863–868. [Google Scholar] [CrossRef]
- Modugno, G.C.; Pirodda, A.; Ferri, G.G.; Montana, R.; Rasciti, L.; Ceroni, A.R. A relationship between autoimmune thyroiditis and benign paroxysmal positional vertigo? Med. Hypotheses 2000, 54, 614–615. [Google Scholar] [CrossRef]
- Papi, G.; Corsello, S.M.; Milite, M.T.; Zanni, M.; Ciardullo, A.V.; Donato, C.D.; Pontecorvi, A. Association between benign paroxysmal positional vertigo and auto-immune chronic thyroiditis. Clin. Endocrinol. 2009, 70, 169–170. [Google Scholar] [CrossRef]
- Papi, G.; Guidetti, G.; Corsello, S.M.; Di Donato, C.; Pontecorvi, A. The association between benign paroxysmal positional vertigo and autoimmune chronic thyroiditis is not related to thyroid status. Thyroid 2010, 20, 237–238. [Google Scholar] [CrossRef]
- Sari, K.; Yildirim, T.; Borekci, H.; Akin, I.; Aydin, R.; Ozkiris, M. The relationship between benign paroxysmal positional vertigo and thyroid autoimmunity. Acta Otolaryngol. 2015, 135, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Teggi, R.; Guidetti, R.; Gatti, O.; Guidetti, G. Recurrence of benign paroxysmal positional vertigo: Experience in 3042 patients. Acta Otorhinolaryngol. Ital. 2021, 41, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Song, Y.S.; Wee, J.H.; Min, C.; Yoo, D.M.; Kim, S.Y. Analyses of the Relation between BPPV and Thyroid Diseases: A Nested Case-Control Study. Diagnostics 2021, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Fattori, B.; Nacci, A.; Dardano, A.; Dallan, I.; Grosso, M.; Traino, C.; Mancini, V.; Ursino, F.; Monzani, F. Possible association between thyroid autoimmunity and Menière’s disease. Clin. Exp. Immunol. 2008, 152, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.D.; Bittar, R.S. Vertigo and metabolic disorders. Int. Tinnitus J. 2012, 17, 16–20. [Google Scholar] [PubMed]
- Chiarella, G.; Tognini, S.; Nacci, A.; Sieli, R.; Costante, G.; Petrolo, C.; Mancini, V.; Guzzi, P.H.; Pasqualetti, G.; Cassandro, E.; et al. Vestibular disorders in euthyroid patients with Hashimoto’s thyroiditis: Role of thyroid autoimmunity. Clin. Endocrinol. 2014, 81, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Santosh, U.P.; Rao, M.S. Incidence of Hypothyroidismin Meniere’s Disease. J. Clin. Diagn. Res. 2016, 10, MC01–MC03. [Google Scholar] [CrossRef]
- Lin, W.L.; Chen, C.Y.; Hsu, T.Y.; Chen, W.K.; Lin, C.L.; Chen, H.C. Hypothyroidism is an independent risk factor for Meniere’s disease: A population-based cohort study. Medicine 2019, 98, e15166. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, Y.S.; Wee, J.H.; Min, C.; Yoo, D.M.; Choi, H.G. Association between Meniere’s disease and thyroid diseases: A nested case-control study. Sci. Rep. 2020, 10, 18224. [Google Scholar] [CrossRef]
- Bakdounes, A.; Akashe, N.; Alchallah, M.O.; Alolabi, H.; Bakdounes, D.; Mohsen, F.; Darjazini, N. Prevalence of Ménière’s Disease in Syrian Patients with hypothyroidism: Cross-sectional study. Ann. Med. Surg. 2022, 81, 104405. [Google Scholar] [CrossRef]
- Miśkiewicz-Orczyk, K.; Vlaykov, A.; Lisowska, G.; Strzelczyk, J.; Kos-Kudła, B. Does Thyroid Hormone Metabolism Correlate with the Objective Assessment of the Vestibular Organ in Patients with Vertigo? J. Clin. Med. 2022, 11, 6771. [Google Scholar] [CrossRef] [PubMed]
- Mammarella, F.; Loperfido, A.; Keeling, E.G.; Bellocchi, G.; Marsili, L. Ménière’s Disease: Insights from an Italian Nationwide Survey. Audiol. Res. 2023, 13, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, W.; Kong, W.; Fan, J.; He, G.; Zheng, Y.; Ren, J.; Dong, C. Risk factors for Meniere disease: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2022, 279, 5485–5496. [Google Scholar] [CrossRef] [PubMed]
- Bringuier, C.M.; Hatat, B.; Boularand, R.; Chabbert, C.; Tighilet, B. Characterization of Thyroid Hormones Antivertigo Effects in a Rat Model of Excitotoxically-Induced Vestibulopathy. Front. Neurol. 2022, 13, 877319. [Google Scholar] [CrossRef] [PubMed]
- Polaczkiewicz, L.; Olszewski, O. Analyze causes and results of VNG examinations in patients with vertigo and balance disorders in the private ENT practice. J. Otolaryngol. Pol. 2019, 74, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Saadi, R.; Patel, V.A.; Liaw, J.; Isildak, H. Thyroid Dysfunction in Ménière’s Disease: A Comprehensive Review. ORL J. Otorhinolaryngol. Relat. Spec. 2021, 83, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, L.; Di Cesare, T.; Galli, J.; Fetoni, A.R.; Paludetti, G.; Picciotti, P.M. Benign paroxysmal positional vertigo: Is hypothyroidism a risk factor for recurrence? Acta Otorhinolaryngol. Ital. 2022, 42, 465–470. [Google Scholar] [CrossRef]
- Rastoldo, G.; Marouane, E.; El-Mahmoudi, N.; Péricat, D.; Watabe, I.; Lapotre, A.; Tonetto, A.; López-Juárez, A.; El-Ahmadi, A.; Caron, P.; et al. L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells 2022, 11, 684. [Google Scholar] [CrossRef]
- Guerra, J.; Devesa, J. Causes and treatment of idiopathic benign paroxysmal positional vertigo based on endocrinological and other metabolic factors. J. Otol. 2020, 15, 155–160. [Google Scholar] [CrossRef]
- Rastoldo, G.; Tighilet, B. Thyroid Axis and Vestibular Physiopathology: From Animal Model to Pathology. Int. J. Mol. Sci. 2023, 24, 9826. [Google Scholar] [CrossRef]
- Sreenivas, V.; Sima, N.H.; Philip, S. The Role of Comorbidities in Benign Paroxysmal Positional Vertigo. Ear Nose Throat J. 2021, 100, NP225–NP230. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol. Head Neck Surg. 2017, 156, S1–S47. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Bisdorff, A.; Furman, J.; Hornibrook, J.; Jahn, K.; Maire, R.; Newman-Toker, D.; Magnusson, M. Acute unilateral vestibulopathy/vestibular neuritis: Diagnostic criteria. J. Vestib. Res. 2022, 32, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Basura, G.J.; Adams, M.E.; Monfared, A.; Schwartz, S.R.; Antonelli, P.J.; Burkard, R.; Bush, M.L.; Bykowski, J.; Colandrea, M.; Derebery, J.; et al. Clinical Practice Guideline: Ménière’s Disease Executive Summary. Otolaryngol. Head Neck Surg. 2020, 162, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.-H.; Golding, J.F.; Keshavarz, B.; Furman, J.; Kim, J.-S.; Lopez-Escamez, J.A.; Magnusson, M.; Yates, B.J.; Lawson, B.D.; Staab, J.; et al. Motion sickness diagnostic criteria: Consensus Document of the Classification Committee of the Bárány Society. J. Vestib. Res. 2021, 31, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.P.; Eckhardt-Henn, A.; Horii, A.; Jacob, R.; Strupp, M.; Brandt, T.; Bronstein, A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2017, 27, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Lui, F.; Foris, L.A.; Willner, K.; Tadi, P. Central Vertigo. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hsu, A.; Tsou, Y.A.; Wang, T.C.; Chang, W.D.; Lin, C.L.; Tyler, R.S. Hypothyroidism and related comorbidities on the risks of developing tinnitus. Sci. Rep. 2022, 12, 3401. [Google Scholar] [CrossRef]
| Variable | Mean ± SD | Range |
|---|---|---|
| Gender n (%) | ||
| Women | 306 (72.5) | |
| Men | 116 (27.5) | |
| Age (years) | 61.5 ± 14.7 | |
| Women | 61.9 ± 14.5 | 24–91 |
| Men | 60.4 ± 15.4 | 19–88 |
| Symptoms/ Pathologies | Rotatory Vertigo | Instabilities | Visual Dependency | Walk Disorders |
|---|---|---|---|---|
| BPPV | OR = 10.549 p < 0.001 | OR = 0.133 p < 0.001 | OR = 0.029 p < 0.001 | OR = 0.222 p < 0.001 |
| Unilateral Deficit | OR = 0.881 p = 0.787 | OR = 3.064 p < 0.001 | OR = 0.931 p = 0.926 | OR = 3.387 p < 0.001 |
| Menière | OR = 0.928 p = 0.906 | OR = 5.211 p < 0.001 | OR = 3.373 p = 0.070 | OR = 3.290 p = 0.002 |
| Kinetosis | OR = 0.015 p < 0.001 | OR = 3.278 p = 0.124 | OR = 91.818 p < 0.001 | OR = 0.400 p = 0.399 |
| Central Vertigo | OR = 0.180 p < 0.001 | OR = 3.354 p < 0.001 | OR = 1.413 p = 0.598 | OR = 2.267 p = 0.004 |
| Chronic Instabilities | OR = 0.101 p = 0.006 | OR = 4.933 p = 0.067 | OR = 5.347 p = 0.096 | OR = 12.627 p = 0.021 |
| Symptoms/Pathologies Euthyroidism (0)/Hypothyroidism (1) | Rotatory Vertigo | Instabilities | Visual Dependency | Walk Disorders | |
|---|---|---|---|---|---|
| BPPV | 0 | OR = 10.642 | OR = 0.144 | OR = 0.032 | OR = 0.232 |
| (n = 367) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| 1 | OR = 3.983 | OR = 0.078 | OR = 0.433 | OR = 0.160 | |
| (n = 54) | p = 0.214 | p < 0.001 | p = 0.385 | p = 0.006 | |
| Unilateral Deficit | 0 | OR = 1.054 | OR = 3.151 | OR = 1.085 | OR = 3.420 |
| (n = 367) | p = 0.918 | p < 0.001 | p = 0.917 | p < 0.001 | |
| 1 | OR = 0.130 | OR = 2.844 | OR = 2.067 | OR = 3.143 | |
| (n = 54) | p = 0.112 | p = 0.192 | p = 0.697 | p = 0.152 | |
| Menière Disease | 0 | OR = 0.821 | OR = 4.757 | OR = 4.312 | OR = 2.688 |
| (n = 367) | p = 0.758 | p < 0.001 | p = 0.022 | p = 0.016 | |
| 1 | OR = 0.579 | OR = 9.067 | OR = 2.939 | OR = 10.000 | |
| (n = 54) | p = 0.645 | p = 0.028 | p = 0.747 | p = 0.020 | |
| Kinetosis | 0 | OR = 0.017 | OR = 3.467 | OR = 97.50 | OR = 0.410 |
| (n = 367) | p < 0.001 | p = 0.988 | p < 0.001 | p = 0.397 | |
| 1 | OR = NaN | OR = NaN | OR = NaN | OR = NaN | |
| (n = 54) | p = NaN | p = NaN | p= NaN | p = NaN | |
| Central Vertigo | 0 | OR = 0.166 | OR = 2.737 | OR = 0.477 | OR = 2.009 |
| (n = 367) | p < 0.001 | p < 0.001 | p = 0.471 | p = 0.024 | |
| 1 | OR = 0.182 | OR = 9.625 | OR = 16.059 | OR = 5.500 | |
| (n = 54) | p = 0.197 | p = 0.003 | p = 0.024 | p = 0.020 | |
| Chronic Instabilities | 0 | OR = 0.074 | OR = 10.480 | OR = 6.712 | OR = 28.731 |
| (n = 367) | p < 0.001 | p = 0.010 | p = 0.057 | p < 0.001 | |
| 1 | OR = 0.146 | OR = 0.590 | OR = 11.667 | OR = 0.640 | |
| (n = 54) | p = 0.843 | p = 0.457 | p = 0.890 | p = 0.475 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougerolle, V.; El Khiati, R.; El Ahmadi, A.; Tighilet, B.; Besnard, S.; Chabbert, C. Statistical Associations between Vestibular Pathologies and Hypothyroidism: A Retrospective Study. J. Clin. Med. 2024, 13, 1099. https://doi.org/10.3390/jcm13041099
Bougerolle V, El Khiati R, El Ahmadi A, Tighilet B, Besnard S, Chabbert C. Statistical Associations between Vestibular Pathologies and Hypothyroidism: A Retrospective Study. Journal of Clinical Medicine. 2024; 13(4):1099. https://doi.org/10.3390/jcm13041099
Chicago/Turabian StyleBougerolle, Virginie, Rhizlane El Khiati, Abdessadek El Ahmadi, Brahim Tighilet, Stéphane Besnard, and Christian Chabbert. 2024. "Statistical Associations between Vestibular Pathologies and Hypothyroidism: A Retrospective Study" Journal of Clinical Medicine 13, no. 4: 1099. https://doi.org/10.3390/jcm13041099
APA StyleBougerolle, V., El Khiati, R., El Ahmadi, A., Tighilet, B., Besnard, S., & Chabbert, C. (2024). Statistical Associations between Vestibular Pathologies and Hypothyroidism: A Retrospective Study. Journal of Clinical Medicine, 13(4), 1099. https://doi.org/10.3390/jcm13041099






