Learning Curve for Starting a Successful Single-Centre TAVR Programme with Multiple Devices: Early and Mid-Term Follow-Up
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Population
2.3. Device Description and Procedure
2.4. Access Site
2.5. Study Endpoints and Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Procedural Outcomes
Device Success and Re-Intervention
3.3. VARC-2 Outcomes at Follow-Ups
3.3.1. VARC-2 Outcomes at 30 Days
3.3.2. VARC-2 Outcomes at 1-Year
3.3.3. VARC-2 Outcomes at 3 Years
3.4. Echocardiographic Outcomes and Valve Durability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2012, 42, S1–S44. [Google Scholar]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; DeFrain, M.; Muppala, M.; et al. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 81, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. Engl. Ed. 2022, 75, 524. [Google Scholar] [CrossRef]
- Alli, O.; Rihal, C.S.; Suri, R.M.; Greason, K.L.; Waksman, R.; Minha, S.; Torguson, R.; Pichard, A.D.; Mack, M.; Svensson, L.G.; et al. Learning curves for transfemoral transcatheter aortic valve replacement in the PARTNER-I trial: Technical performance. Catheter. Cardiovasc. Interv. 2016, 87, 154–162. [Google Scholar] [CrossRef]
- Gurvitch, R.; Tay, E.L.; Wijesinghe, N.; Ye, J.; Nietlispach, F.; Wood, D.A.; Lichtenstein, S.; Cheung, A.; Webb, J.G. Transcatheter aortic valve implantation: Lessons from the learning curve of the first 270 high-risk patients. Catheter. Cardiovasc. Interv. 2011, 78, 977–984. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Thorac. Cardiovasc. Surg. 2013, 145, 6–23. [Google Scholar] [CrossRef]
- Sinning, J.-M.; Hammerstingl, C.; Vasa-Nicotera, M.; Adenauer, V.; Lema Cachiguango, S.J.; Scheer, A.-C.; Hausen, S.; Sedaghat, A.; Ghanem, A.; Müller, C.; et al. Aortic Regurgitation Index Defines Severity of Peri-Prosthetic Regurgitation and Predicts Outcome in Patients after Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. 2012, 59, 1134–1141. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Levy, F.; Laurent, M.; Monin, J.L.; Maillet, J.M.; Pasquet, A.; Le Tourneau, T.; Petit-Eisenmann, H.; Gori, M.; Jobic, Y.; Bauer, F.; et al. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: A European multicenter study. J. Am. Coll. Cardiol. 2008, 51, 1466–1472. [Google Scholar] [CrossRef]
- Monin, J.L.; Quéré, J.P.; Monchi, M.; Petit, H.; Baleynaud, S.; Chauvel, C.; Pop, C.; Ohlmann, P.; Lelguen, C.; Dehant, P.; et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation 2003, 108, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Cueff, C.; Serfaty, J.M.; Cimadevilla, C.; Laissy, J.P.; Himbert, D.; Tubach, F.; Duval, X.; Iung, B.; Enriquez-Sarano, M.; Vahanian, A.; et al. Measurement of aortic valve calcification using multislice computed tomography: Correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2011, 97, 721–726. [Google Scholar] [CrossRef]
- Clavel, M.A.; Messika-Zeitoun, D.; Pibarot, P.; Aggarwal, S.R.; Malouf, J.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; Capoulade, R.; et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler echocardiographic and computed tomographic study. J. Am. Coll. Cardiol. 2013, 62, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Pibarot, P.; Messika-Zeitoun, D.; Capoulade, R.; Malouf, J.; Aggarval, S.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J. Am. Coll. Cardiol. 2014, 64, 1202–1213. [Google Scholar] [CrossRef]
- Webb, J.; Rodés-Cabau, J.; Fremes, S.; Pibarot, P.; Ruel, M.; Ibrahim, R.; Welsh, R.; Feindel, C.; Lichtenstein, S. Transcatheter aortic valve implantation: A Canadian Cardiovascular Society position statement. Can. J. Cardiol. 2012, 28, 520–528. [Google Scholar] [CrossRef]
- Cremer, J.; Heinemann, M.K.; Mohr, F.W.; Diegeler, A.; Beyersdorf, F.; Niehaus, H.; Ensminger, S.; Schlensak, C.; Reichenspurner, H.; Rastan, A.; et al. Commentary by the German Society for Thoracic and Cardiovascular Surgery on the positions statement by the German Cardiology Society on quality criteria for transcatheter aortic valve implantation (TAVI). Thorac. Cardiovasc. Surg. 2014, 62, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Minha, S.; Waksman, R.; Satler, L.P.; Torguson, R.; Alli, O.; Rihal, C.S.; Mack, M.; Svensson, L.G.; Rajeswaran, J.; Blackstone, E.H.; et al. Learning curves for transfemoral transcatheter aortic valve replacement in the PARTNER-I trial: Success and safety. Catheter. Cardiovasc. Interv. 2016, 87, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wassef, A.W.A.; Rodes-Cabau, J.; Liu, Y.; Webb, J.G.; Barbanti, M.; Muñoz-García, A.J.; Tamburino, C.; Dager, A.E.; Serra, V.; Amat-Santos, I.J.; et al. The Learning Curve and Annual Procedure Volume Standards for Optimum Outcomes of Transcatheter Aortic Valve Replacement: Findings From an International Registry. JACC Cardiovasc. Interv. 2018, 11, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Kaier, K.; Reinecke, H.; Schmoor, C.; Frankenstein, L.; Vach, W.; Hehn, P.; Zirlik, A.; Bode, C.; Zehender, M.; Reinöhl, J. Learning Curves Among All Patients Undergoing Transcatheter Aortic Valve Implantation in Germany: A Retrospective Observational Study. Int. J. Cardiol. 2017, 235, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kumamaru, H.; Torikai, K.; Kohsaka, S.; Takayama, M.; Kobayashi, J.; Ogawa, H.; Shirato, H.; Ishii, K.; Koike, K.; et al. Learning Curve for Transcatheter Aortic Valve Implantation Under a Controlled Introduction System—Initial Analysis of a Japanese Nationwide Registry. Circ. J. 2018, 82, 1951–1958. [Google Scholar] [CrossRef]
- Storz, C.; Geisler, T.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Role of Imaging in Transcatheter Aortic Valve Replacement. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Kaier, K.; Oettinger, V.; Reinecke, H.; Schmoor, C.; Frankenstein, L.; Vach, W.; Hehn, P.; von Zur Mühlen, C.; Bode, C.; Zehender, M.; et al. Volume-outcome relationship in transcatheter aortic valve implantations in Germany 2008–2014: A secondary data analysis of electronic health records. BMJ Open 2018, 8, e020204. [Google Scholar] [CrossRef]
- Das, R.; Puri, R. Transcatheter Treatment of Bicuspid Aortic Valve Disease: Imaging and Interventional Considerations. Front. Cardiovasc. Med. 2018, 5, 91. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Zhao, Z.; Du, R.; Staniloae, C.; Saric, M.; Neuburger, P.J.; Querijero, M.; Vainrib, A.; Hisamoto, K.; Ibrahim, H.; et al. Minimizing Permanent Pacemaker Following Repositionable Self-Expanding Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1796–1807. [Google Scholar] [CrossRef]

| Baseline Characteristic of Study Population | ||||
|---|---|---|---|---|
| Overall (n = 122) | Cohort A (First 63 Patients) | Cohort B (Last 1 Year, n = 59) | p Value | |
| Age (yrs) | 77.9 ± 6.1 | 78.7 ± 5.6 | 77.6 ± 6.6 | 0.325 |
| Men | 48 | 24 | 24 | 0.77 |
| Body mass index (kg/m2) | 29.3 ± 4.8 | 28.7 ± 4.5 | 29.9 ± 5.1 | 0.175 |
| Body surface area (m2) | 1.87 ± 0.20 | 1.86 ± 0.2 | 1.87 ± 0.21 | 0.834 |
| Hypertension | 113 | 60 | 56 | 0.934 |
| Diabetes mellitus | 32 | 19 | 13 | 0.308 |
| Hyperlipidemia | 87 | 43 | 43 | 0.575 |
| NYHA class III or IV | 60 | 28 | 32 | 0.28 |
| Ischaemic Heart Disease | 56 | 29 | 26 | 0.828 |
| Prior Myocardial Infarction | 17 | 7 | 10 | 0.352 |
| Prior PCI | 40 | 21 | 19 | 0.894 |
| Prior CABG | 15 | 9 | 6 | 0.489 |
| Peripheral artery disease | 21 | 4 | 5 | 0.654 |
| Cerebrovascular disease | 10 | 9 | 2 | 0.036 |
| Pulmonary disease | 15 | 6 | 9 | 0.335 |
| Previous BAV | 21 | 14 | 6 | 0.066 |
| Permanent PM | 9 | 6 | 3 | 0.349 |
| Atrial fibrillation | 25 | 15 | 10 | 0.348 |
| Logistic EuroSCORE | 15.9 ± 14.6 | 16.6 ± 12.8 | 15.2 ± 13.6 | 0.554 |
| Logistic EuroSCORE II | 4.2 ± 4.5 | 4.7 ± 4.2 | 4.3 ± 4.8 | 0.667 |
| STS score (%) | 6.9 ± 4.68 | 7.2 ± 3.6 | 6.7 ± 5 | 0.497 |
| Serum creatinine (μmol/L) | 100.3 ± 46.4 | 99.9 ± 42.2 | 100.7 ± 50.8 | 0.936 |
| Estimated GFR (mL/min) | 60.5 ± 24.2 | 59.6 ± 26.6 | 61.4 ± 21.6 | 0.678 |
| Estimated GFR < 60 mL/min | 70 | 40 | 28 | 0.075 |
| Bicuspid valve | 7 | 3 | 4 | 0.632 |
| Prior MVR | 2 | 0 | 2 | 0.141 |
| Prior AVR | 1 | 0 | 1 | 0.299 |
| Dialysis | 0 | 0 | 0 | NA |
| Procedure status | ||||
| elective | 114 | 61 | 53 | 0.119 |
| urgent | 8 | 2 | 6 | 0.119 |
| acute | 0 | 0 | 0 | NA |
| Echocardiographic Parameters of the Study Population (n = 122) | |
|---|---|
| Mean LVEF | 55.9 ± 10.6 |
| Mean AoVmax (m/s) | 4.62 ± 0.62 |
| Aortic peak gradient (Hgmm) | 87.6 ± 22.7 |
| Aortic mean gradient (Hgmm) | 52.7 ± 15.9 |
| Mitral insufficiency III or IV | 21 |
| Tricuspid insufficiency III or IV | 19 |
| sPAP ≥ 60 Hgmm | 9 |
| High-gradient AS | n = 106 (86.9%) |
| Mean LVEF | 57.6 ± 6.3 |
| Mean AoVmax (m/s) | 4.7 ± 0.6 |
| Aortic peak gradient (Hgmm) | 90.2 ± 22.6 |
| Aortic mean gradient (Hgmm) | 53.8 ± 16 |
| Low-flow, low-gradient AS | n = 14 (11.5%) |
| Mean LVEF | 31.3 ± 5.5 |
| Mean AoVmax (m/s) | 3.91 ± 0.61 |
| Aortic peak gradient (Hgmm) | 67.8 ± 19 |
| Aortic mean gradient (Hgmm) | 37.5 ± 9.5 |
| Paradox low-flow, low-gradient AS | n = 2 (1.6%) |
| Mean LVEF | 62.5 ± 9.2 |
| Mean AoVmax (m/s) | 3.84 ± 0.34 |
| Aortic peak gradient (Hgmm) | 59 ± 11.3 |
| Aortic mean gradient (Hgmm) | 34.5 ± 4.9 |
| Variable | Overall (n = 122) | Medtronic Corevalve (n = 82, 67.2%) | Portico (n = 13, 10.6%) | Myval (n = 11, 9%) | Acurate-Neo (n = 6, 8.2%) | Lotus (n = 10, 8.2%) |
|---|---|---|---|---|---|---|
| Type of anesthesia | ||||||
| general | 118 | 81 | 10 | 11 | 6 | 10 |
| local | 4 | 1 | 3 | 0 | 0 | 0 |
| Access site | ||||||
| femoral | 117 | 78 | 13 | 11 | 6 | 10 |
| subclavia | 2 | 2 | 0 | 0 | 0 | 0 |
| axillaris | 2 | 2 | 0 | 0 | 0 | 0 |
| direct aortic | 1 | 1 | 0 | 0 | 0 | 0 |
| Contrast agent | 154 ± 119 | 144.5 ± 125.6 | 193.1 ± 98.3 | 244 ± 98 | 158.6 ± 76.7 | 91.7 ± 72.6 |
| Operation duration (min) | 89.6 ± 38.6 | 91.1 ± 40.9 | 85.1 ± 36.1 | 91.4 ± 41.1 | 69 ± 14.4 | 100.1 ± 32.4 |
| Predilatation | 39 | 10 | 13 | 10 | 6 | 0 |
| Postdilatation | 17 | 9 | 3 | 1 | 2 | 2 |
| Preimpl. peak AV gradient | 91.8 ± 24.7 | 86.5 ± 21.3 | 103.9 ± 36.4 | 96.5 ± 28.5 | 95.5 ± 16.2 | 99.3 ± 21.7 |
| Preimpl. mean AV gradient | 53.01 ± 14.9 | 52.36 ± 12.1 | 56.9 ± 21.1 | 56.7 ± 16.8 | 53.5 ± 12.5 | 58.4 ± 10.3 |
| Postimpl. peak AV gradient | 34.2 ± 9.6 | 33.6 ± 9.4 | 38.5 ± 8.1 | 34.3 ± 11.1 | 42.3 ± 4.1 | 28.6 ± 11.5 |
| Postimpl. mean AV gradient | 11.9 ± 6.4 | 12.5 ± 6.1 | 12.2 ± 4.4 | 8.4 ± 9.1 | 9.2 ± 7.5 | 13.9 ± 6.5 |
| ARI | 26.2 ± 9.8 | 26.3 ± 9.8 | 23.6 ± 9.6 | 27.2 ± 11.1 | 23 ± 4.7 | 30.7 ± 11.3 |
| Permanent PM impl. | 14 (12.4%) | 10 (12.1%) | 2 (15.3%) | 1 (9.1%) | 0 | 1 (10%) |
| Postprocedural Outcomes <72 h after the Index Procedure | ||||
|---|---|---|---|---|
| Outcome | Overall (n = 122) | Cohort A (First 63 Patients) | Cohort B (Last 1 Year, n = 59) | p Value |
| No. (%) of events | ||||
| In-hospital mortality | 5 (4.1%) | 4 (6.3%) | 1 (1.7%) | 0.195 |
| Device success | 119 (97.5%) | 62 (98.4%) | 58 (98.3%) | 0.963 |
| Myocardial infarction | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Coronary obstruction | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Stroke or TIA | 3 (2.4%) | 2 (3.2%) | 1 (1.7%) | 0.598 |
| Acute kidney injure, stage 2 or 3 | 3 (2.4%) | 3 (4.7%) | 0 (0%) | 0.09 |
| Major vascular complications | 4 (3.3%) | 0 (0%) | 4 (6.8%) | 0.036 |
| Cardiac tamponade | 0 (0%) | 1 (0%) | 1 (0%) | 0.963 |
| Annulus rupture | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Valve malpositioning | 2 (1.6%) | 1 (1.6%) | 1 (1.7%) | 0.963 |
| Need for a second valve | 2 (1.6%) | 1 (1.6%) | 1 (1.7%) | 0.963 |
| Posptocedural AR grade III or IV | 1 (0.9%) | 1 (0.9%) | 0 (0%) | 0.331 |
| Non Pm (n = 108, 88.5%) | PM (n = 14, 12.5%) | p Value | |
|---|---|---|---|
| Age | 78.6 ± 5.8 | 76.3 ± 7.6 | 0.232 |
| Euroscore | 15.8 ± 13.3 | 16.2 ± 12.9 | 0.933 |
| Euroscore II | 4.4 ± 4.5 | 5.02 ± 4.5 | 0.650 |
| STS score | 6.8 ± 3.8 | 8.1 ± 7.3 | 0.523 |
| Ca score | 2888 ± 1866 | 2767 ± 1108 | 0.822 |
| Ca in LVOT | 20 | 5 | 0.149 |
| THV implantation depth | |||
| Left coronary side (mm) | 7.67 ± 2.9 | 7.99 ± 3.01 | 0.695 |
| Non coronary side (mm) | 7.6 ± 2.6 | 8.0 ± 3.6 | 0.584 |
| Right coronary side (mm) | 7.67 ± 2.6 | 8.23 ± 3.2 | 0.472 |
| Average implantation depth (mm) | 7.7 ± 2.6 | 8.1 ± 3.2 | 0.568 |
| VARC-2 Outcomes at 30-Day, 1-Year, and 3-Year Follow-Ups | ||||
|---|---|---|---|---|
| Outcome | Overall (n = 122) | Cohort A (First 63 Patients) | Cohort B (Last 1 Year, n = 59) | p Value |
| 30 days cumulative clinical outcomes (n = 122) | ||||
| All-cause mortality | 5 (4.1%) | 4 (6.3%) | 1 (1.7%) | 0.195 |
| Cardiac mortality | 4 (3.3%) | 3 (4.7%) | 1 (1.7%) | 0.341 |
| All stroke | 2 (1.6%) | 1 (1.6%) | 1 (1.7%) | 0.962 |
| Life-threatening bleeding | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Acute kidney injury, stage 2 or 3 | 1 (0.8%) | 0 (0%) | 1 (1.7%) | 0.299 |
| Coronary artery obstruction | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Major vascular complication | 4 (3.3%) | 0 (0%) | 4 (6.8%) | 0.035 |
| New pacemaker implantation | 14 (12.4%) | 2 (3.2%) | 12 (20.3%) | 0.002 |
| Valve-related dysfunction requiring repeat procedure (BAV, TAVI, or SAVR) | 2 (1.6%) | 1 (1.6%) | 1 (1.7%) | 0.962 |
| One-year cumulative clinical outcomes (n = 113, 92.6%) | ||||
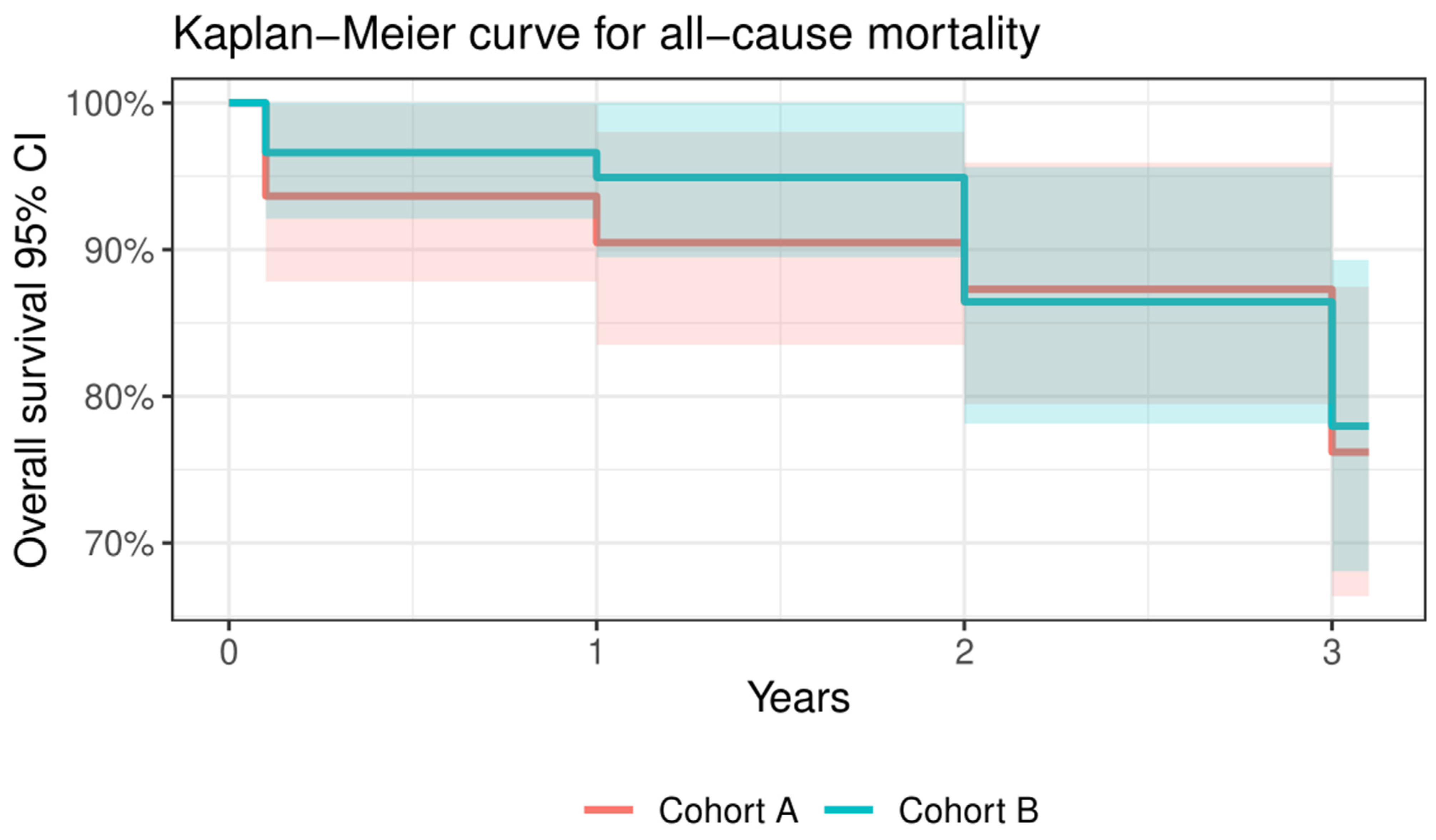
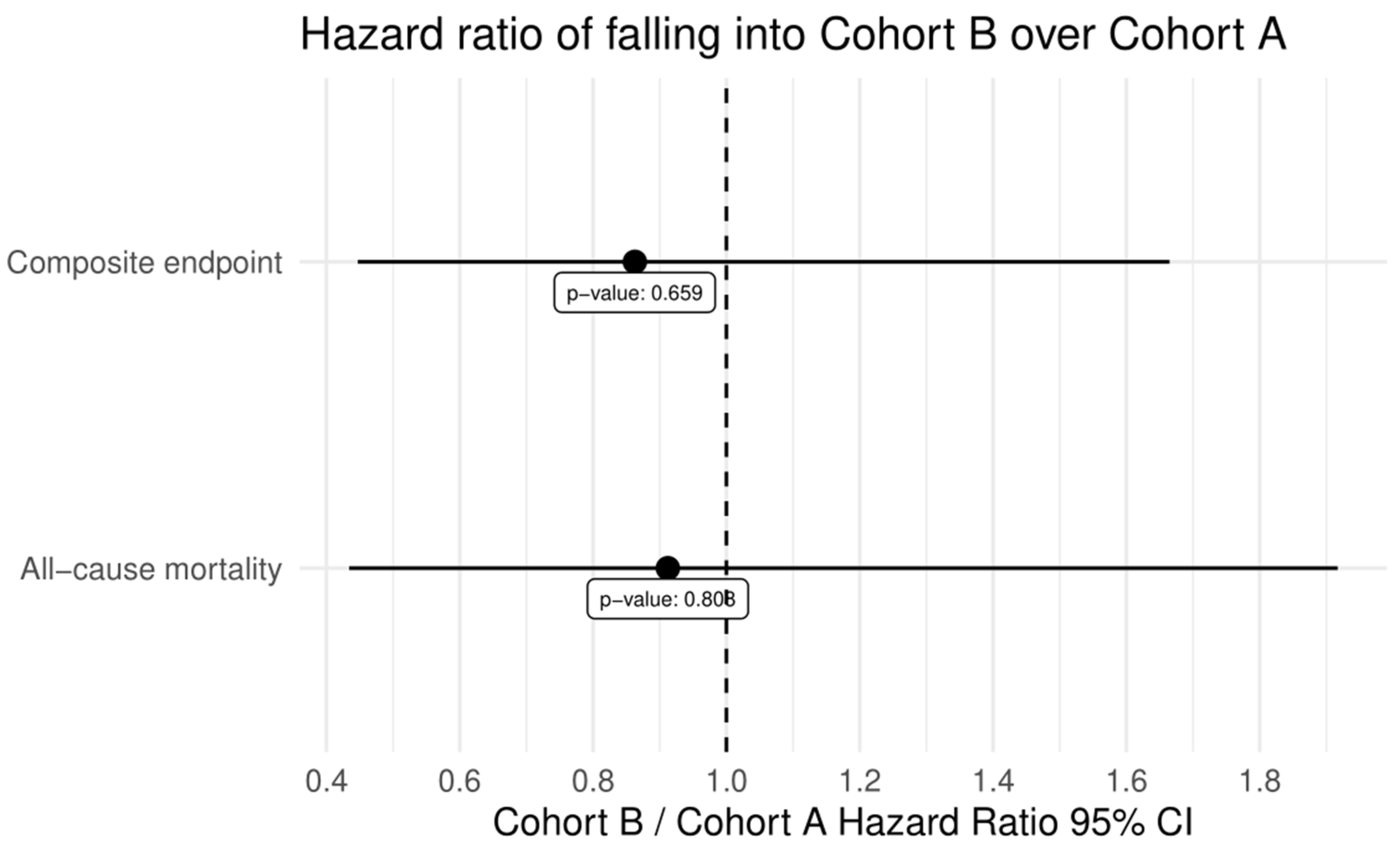
| All-cause mortality | 9 | 6 | 3 | 0.348 |
| Cardiac mortality | 6 | 4 | 2 | 0.450 |
| All stroke | 3 | 1 | 2 | 0.520 |
| Requiring hospitalizations for worsening heart failure | 0 | 0 (0%) | 0 (0%) | - |
| NYHA class III or IV | 1 | 0 | 1 | 0.299 |
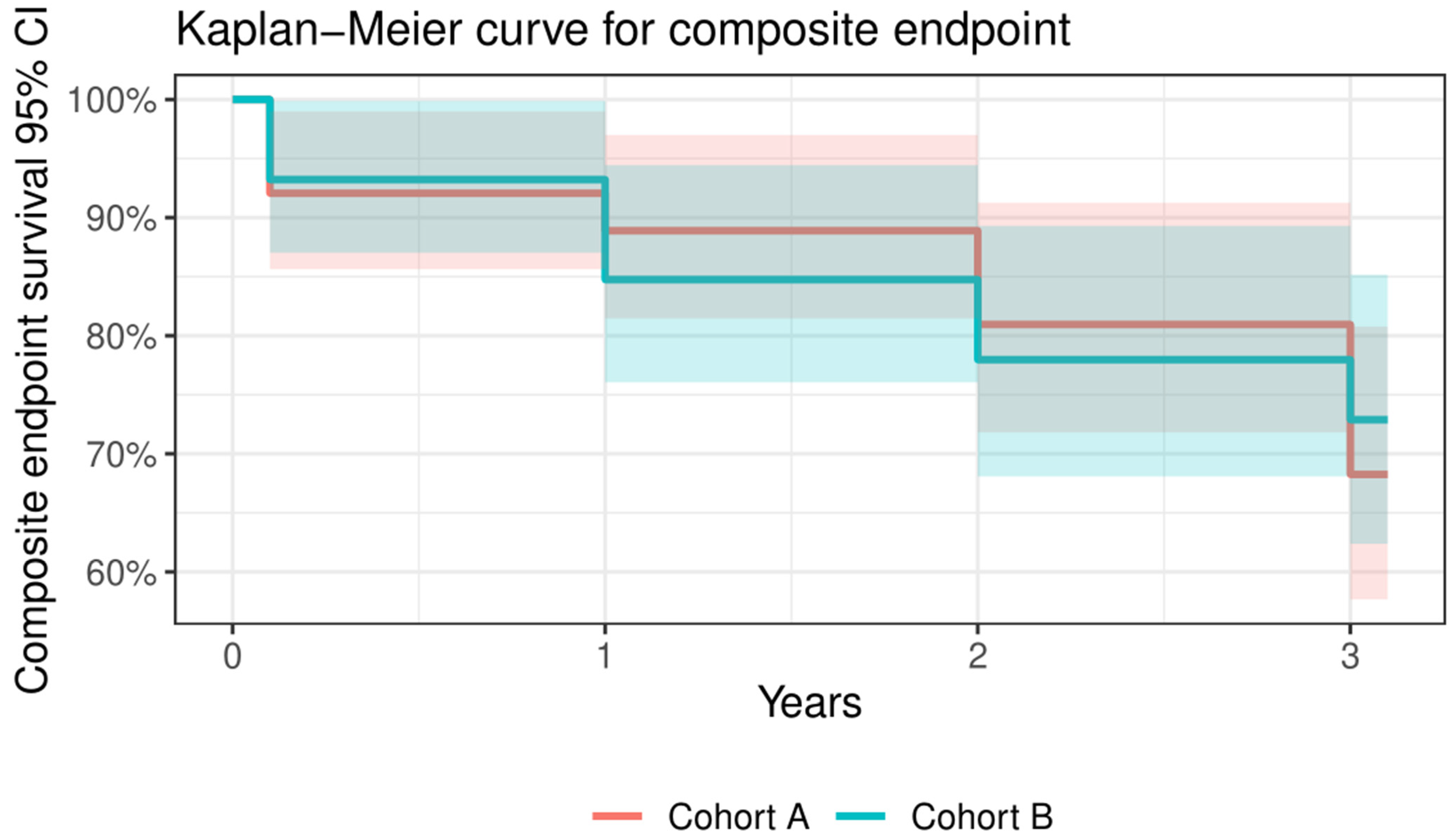
| Valve-related dysfunction | 5 | 1 | 4 | 0.148 |
| Three-year cumulative clinical outcomes (n = 94, 77.0%) | ||||
| All-cause mortality | 28 | 15 | 13 | 0.815 |
| Cardiac mortality | 8 | 5 | 3 | 0.524 |
| All stroke | 5 | 3 | 2 | 0.702 |
| Requiring hospitalizations for worsening heart failure | 4 | 1 | 3 | 0.278 |
| NYHA class III or IV | 0 | 0 | 0 | - |
| Valve-related dysfunction | 3 | 2 | 1 | 0.597 |
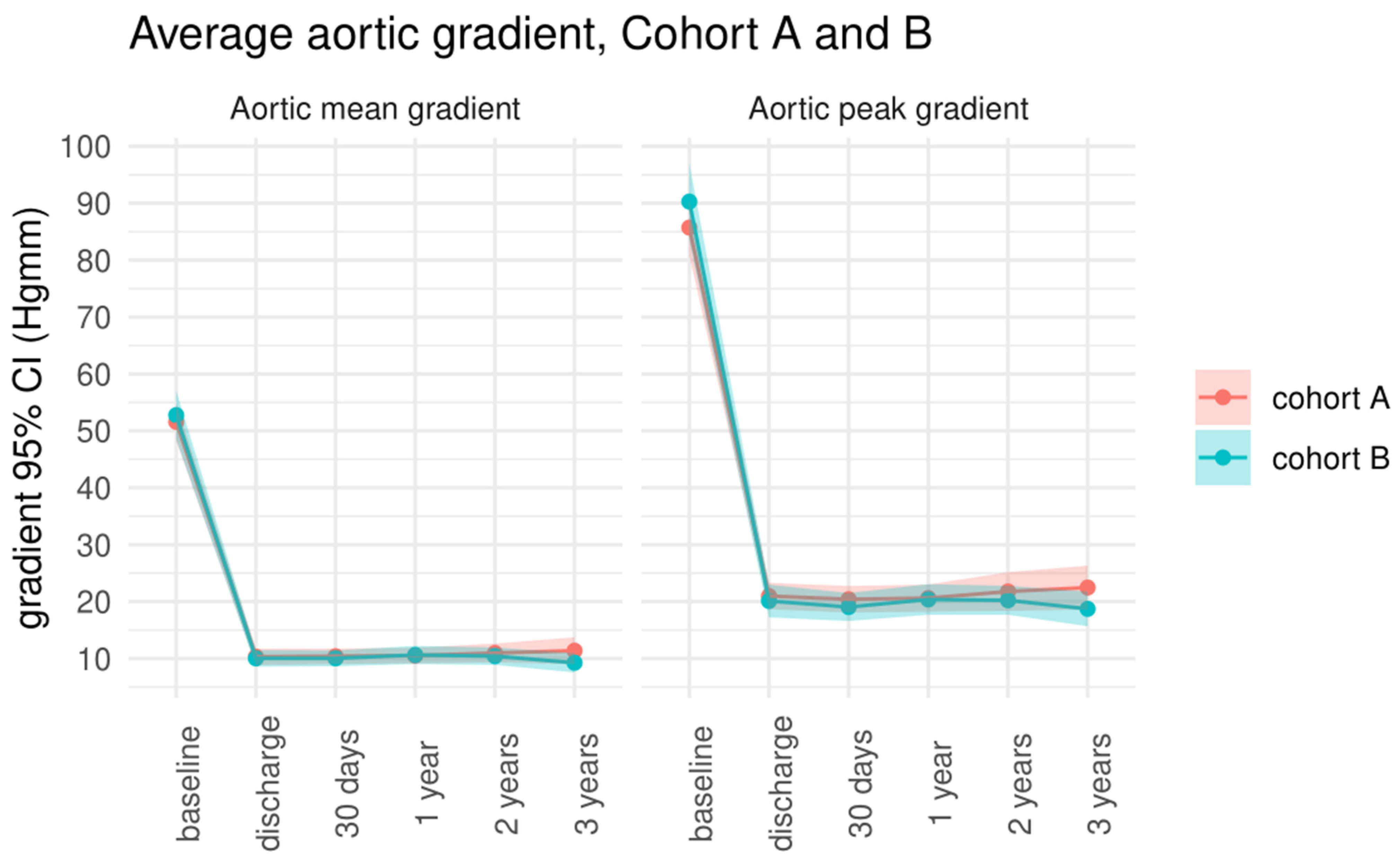
| Cohort A | Cohort B | p Value | |
|---|---|---|---|
| Peak aortic gradient (mmHg) | |||
| baseline | 85.7 ± 19.1 | 90.3 ± 25.7 | 0.272 |
| discharge | 20.9 ± 8.9 | 21.1 ± 10.8 | 0.635 |
| 30-day follow-up | 20.4 ± 8.8 | 19 ± 9.2 | 0.429 |
| 1-year follow-up | 20.6 ± 8.8 | 20.4 ± 9.8 | 0.893 |
| 2-year follow-up | 21.7 ± 11.6 | 20.2 ± 8.8 | 0.478 |
| 3-year follow-up | 22.4 ± 12.7 | 18.7 ± 9.8 | 0.130 |
| Mean aortic gradient (mmHg) | |||
| baseline | 51.6 ± 12.6 | 52.7 ± 16.5 | 0.662 |
| discharge | 10.3 ± 5.5 | 10 ± 5.4 | 0.778 |
| 30-day follow-up | 10.3 ± 5.1 | 10 ± 5.2 | 0.745 |
| 1-year follow-up | 10.6 ± 5.3 | 10.6 ± 5.7 | 0.960 |
| 2-year follow-up | 10.9 ± 5.7 | 10.4 ± 5.2 | 0.641 |
| 3-year follow-up | 11.4 ± 7.7 | 9.3 ± 5.5 | 0.147 |
| Global ejection fraction (%) | |||
| baseline | 55.2 ± 10.7 | 59.1 ± 10.8 | 0.051 |
| discharge | 58.9 ± 9.7 | 58.7 ± 8.7 | 0.901 |
| 30-day follow-up | 58.3 ± 9 | 60 ± 9.1 | 0.306 |
| 1-year follow-up | 57.2 ± 8.7 | 61 ± 7 | 0.015 * |
| 2-year follow-up | 57.6 ± 8.6 | 59.8 ± 8.7 | 0.229 |
| 3-year follow-up | 58.8 ± 10.9 | 60.4 ± 10.6 | 0.499 |
| Transthoracic Echocardiography Follow-Up Data | ||||||
|---|---|---|---|---|---|---|
| Variable | Baseline (n = 122) | Discharge (n = 117, 95.9%) | 30 Days (n = 117, 95.9%) | 1 Year (n = 113, 92.6%) | 2 Year (n = 104, 85.2%) | 3 Year (n = 90, 73.8%) |
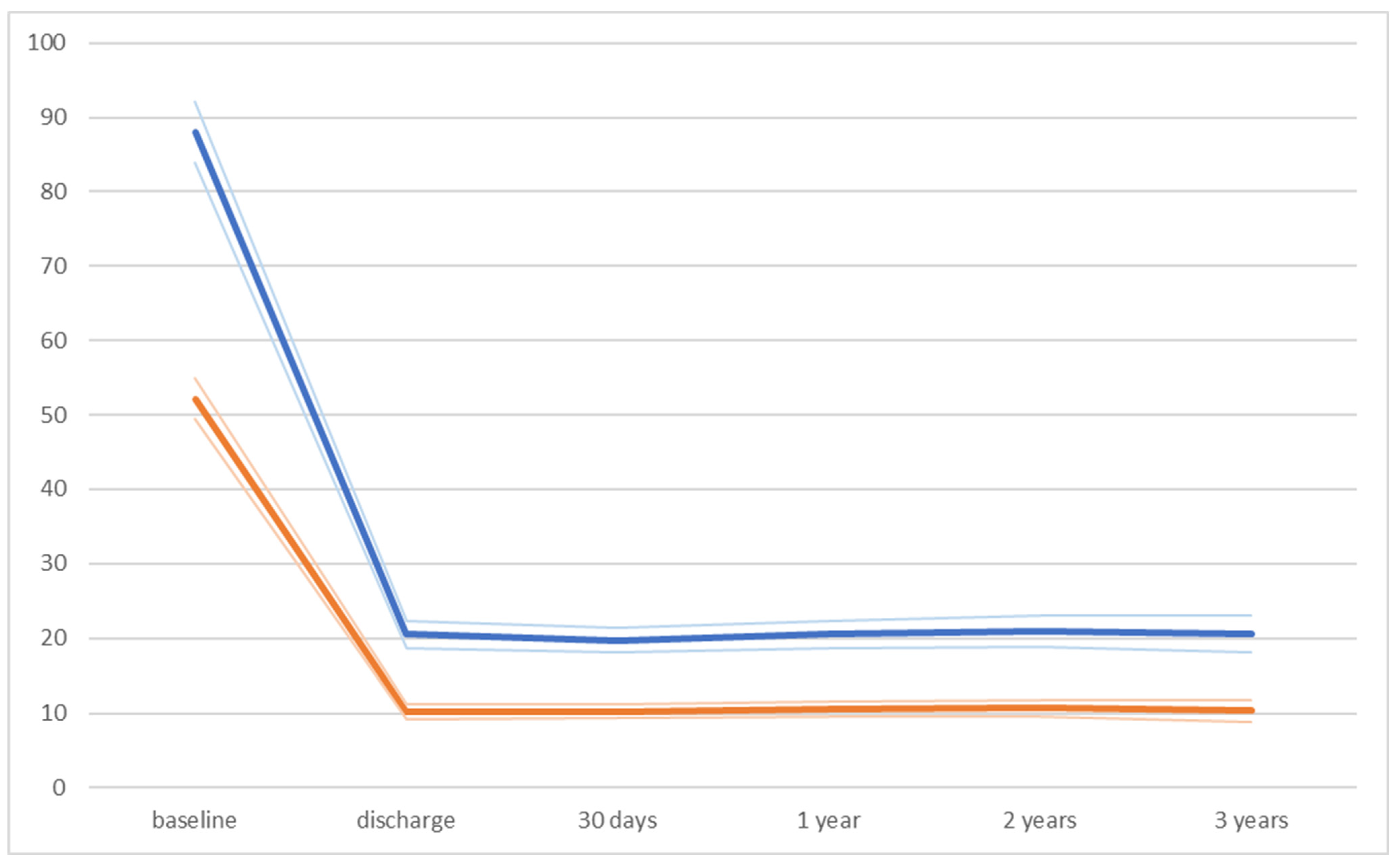
| Peak aortic gradient, mmHg | 88.3 ± 22.9 | 20.5 ± 9.9 | 19.7 ± 8.9 | 20.5 ± 9.3 | 21 ± 10.3 | 20.6 ± 11.5 |
| Mean aortic gradient, mmHg | 52.4 ± 14.7 | 10.2 ± 5.5 | 10.2 ± 5.1 | 10.6 ± 5.5 | 10.6 ± 5.4 | 10.3 ± 6.8 |
| LVEF, % | 57 ± 11.1 | 58.8 ± 9.2 | 59.2 ± 9.1 | 59.1 ± 8.1 | 59.1 ± 8.4 | 59.8 ± 10.6 |
| Aortic regurgitation grade 2 or above | 8 | 10 | 9 | 10 | 19 | 11 |
| Paravalvular leak grading mild/mild-to-moderate | NA | 0/0 | 0/0 | 0/0 | 1/0 | 3/2 |
| Mitral regurgitation grade 3 or 4 | 18 | 6 | 3 | 8 | 12 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magyari, B.; Kittka, B.; Goják, I.; Schönfeld, K.; Szapáry, L.B.; Simon, M.; Kiss, R.; Bertalan, A.; Várady, E.; Gyimesi, A.; et al. Learning Curve for Starting a Successful Single-Centre TAVR Programme with Multiple Devices: Early and Mid-Term Follow-Up. J. Clin. Med. 2024, 13, 1088. https://doi.org/10.3390/jcm13041088
Magyari B, Kittka B, Goják I, Schönfeld K, Szapáry LB, Simon M, Kiss R, Bertalan A, Várady E, Gyimesi A, et al. Learning Curve for Starting a Successful Single-Centre TAVR Programme with Multiple Devices: Early and Mid-Term Follow-Up. Journal of Clinical Medicine. 2024; 13(4):1088. https://doi.org/10.3390/jcm13041088
Chicago/Turabian StyleMagyari, Balázs, Bálint Kittka, Ilona Goják, Kristóf Schönfeld, László Botond Szapáry, Mihály Simon, Rudolf Kiss, Andrea Bertalan, Edit Várady, András Gyimesi, and et al. 2024. "Learning Curve for Starting a Successful Single-Centre TAVR Programme with Multiple Devices: Early and Mid-Term Follow-Up" Journal of Clinical Medicine 13, no. 4: 1088. https://doi.org/10.3390/jcm13041088
APA StyleMagyari, B., Kittka, B., Goják, I., Schönfeld, K., Szapáry, L. B., Simon, M., Kiss, R., Bertalan, A., Várady, E., Gyimesi, A., Szokodi, I., & Horváth, I. G. (2024). Learning Curve for Starting a Successful Single-Centre TAVR Programme with Multiple Devices: Early and Mid-Term Follow-Up. Journal of Clinical Medicine, 13(4), 1088. https://doi.org/10.3390/jcm13041088





