Characteristics of Functional Hyperthermia Detected in an Outpatient Clinic for Fever of Unknown Origin
Abstract
1. Introduction
2. Patients and Methods
2.1. Inclusion of Patients
2.2. Definition of FH
2.3. Laboratory Examination
2.4. Statistical Analysis
3. Results
3.1. Clinical Backgrounds of the Patients with FUO
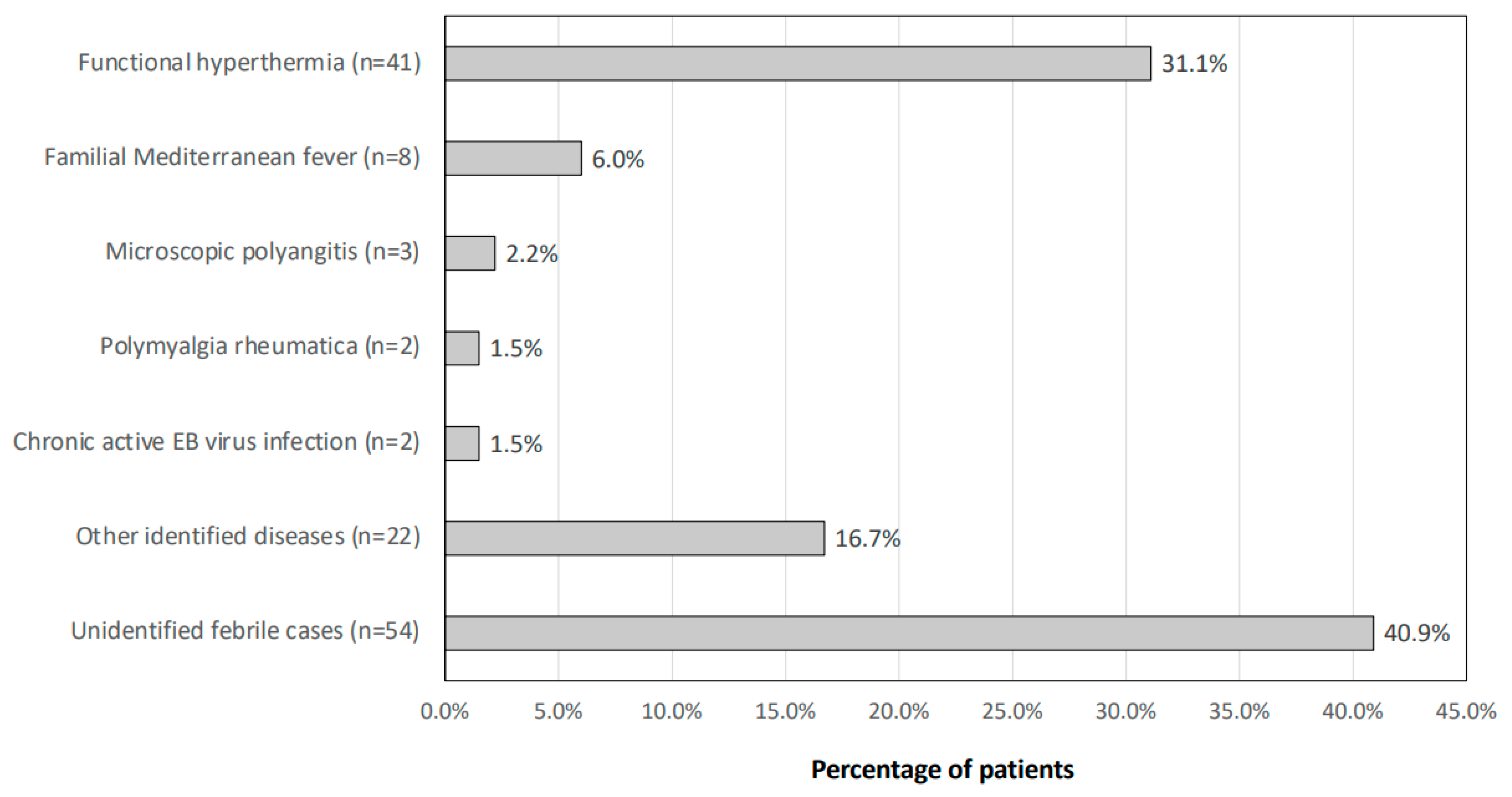
3.2. Breakdown of the Final Diagnosis of FUO
3.3. Distributions of Ages and Genders of Patients with FH
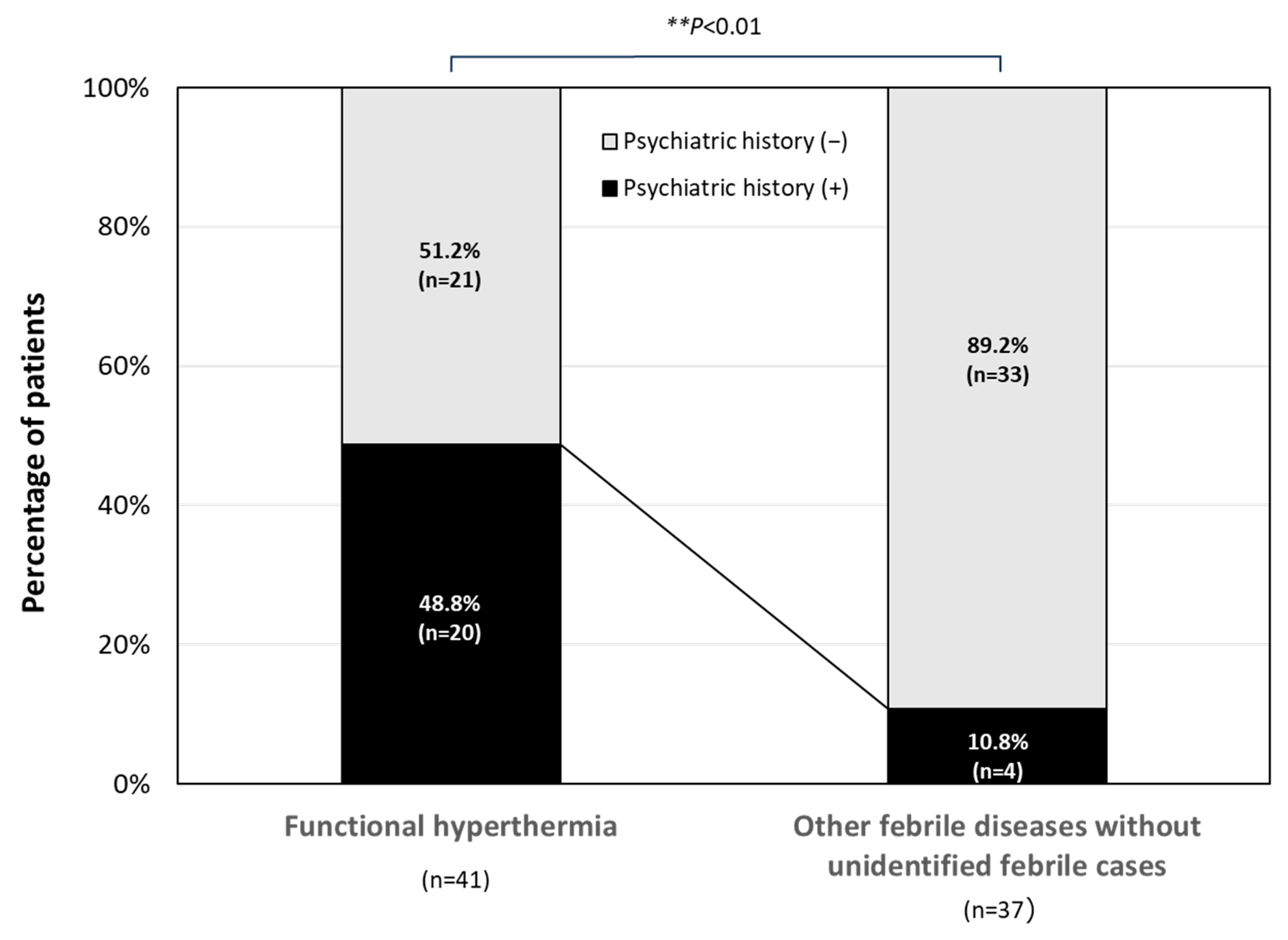
3.4. Past Psychiatric Disorders in the Patients with FH
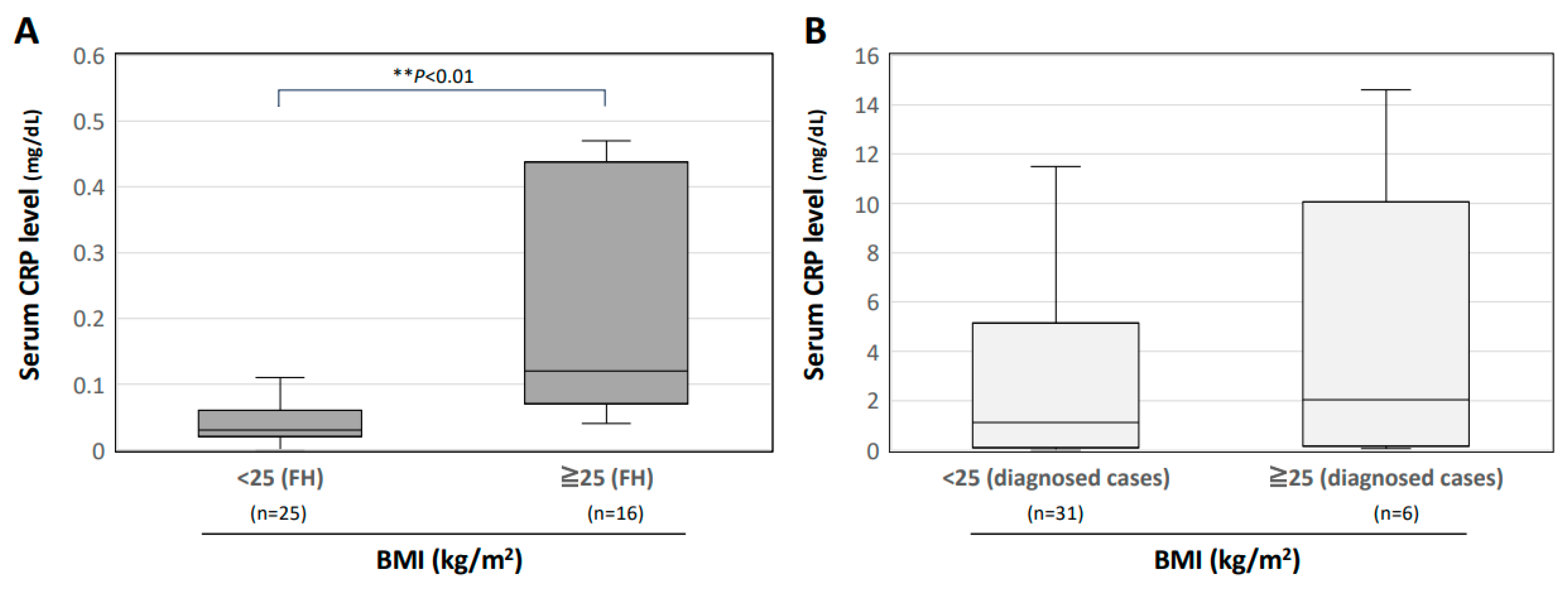
3.5. Interrelationship between Serum CRP Level and BMI in Patients with FH
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Oka, T. Stress-induced hyperthermia and hypothermia. Handb. Clin. Neurol. 2018, 157, 599–621. [Google Scholar]
- Oka, T. Psychogenic fever: How psychological stress affects body temperature in the clinical population. Temperature 2015, 2, 368–378. [Google Scholar] [CrossRef]
- Singer, R.; Harker, C.T.; Vander, A.J.; Kluger, M.J. Hyperthermia induced by open-field stress is blocked by salicylate. Physiol. Behav. 1986, 36, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Soszynski, D.; Kozak, W.; Kluger, M.J. Endotoxin tolerance does not alter open field-induced fever in rats. Physiol. Behav. 1998, 63, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Prinz, S.; Schwaninger, M. The role of interleukin-6 in stress-induced hyperthermia and emotional behaviour in mice. Behav. Brain Res. 2003, 144, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Long, N.C.; Vander, A.J.; Kunkel, S.L.; Kluger, M.J. Antiserum against tumor necrosis factor increases stress hyperthermia in rats. Am. J. Physiol. 1990, 258 Pt 2, R591–R595. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Nakamori, T.; Morimoto, K.; Tan, N.; Murakami, N. The central role of corticotrophin-releasing factor (CRF-41) in psychological stress in rats. J. Physiol. 1993, 460, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Oka, K.; Kobayashi, T.; Sugimoto, Y.; Ichikawa, A.; Ushikubi, F.; Narumiya, S.; Saper, C.B. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J. Physiol. 2003, 551 Pt 3, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Nagasaka, T. Contribution of nonshivering thermogenesis to stress-induced hyperthermia in rats. Jpn. J. Physiol. 1982, 32, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Nagasaka, T. Role of sympathetic nervous system in immobilization- and cold-induced brown adipose tissue thermogenesis in rats. Jpn. J. Physiol. 1984, 34, 103–111. [Google Scholar] [CrossRef]
- Gao, B.; Kikuchi-Utsumi, K.; Ohinata, H.; Hashimoto, M.; Kuroshima, A. Repeated immobilization stress increases uncoupling protein 1 expression and activity in Wistar rats. Jpn. J. Physiol. 2003, 53, 205–213. [Google Scholar] [CrossRef][Green Version]
- Ootsuka, Y.; Blessing, W.W.; Nalivaiko, E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress 2008, 11, 125–133. [Google Scholar] [CrossRef]
- Borsini, F.; Lecci, A.; Volterra, G.; Meli, A. A model to measure anticipatory anxiety in mice? Psychopharmacology 1989, 98, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zethof, T.J.J.; Van Der Heyden, J.A.M.; Tolboom, J.T.B.M.; Olivier, B. Stress-induced hyperthermia as a putative anxiety model. Eur. J. Pharmacol. 1995, 294, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Vinkers, C.H.; van Bogaert, M.J.V.; Klanker, M.; Korte, S.M.; Oosting, R.; Hanania, T.; Hopkins, S.C.; Olivier, B.; Groenink, L. Translational aspects of pharmacological research into anxiety disorders: The stress-induced hyperthermia (SIH) paradigm. Eur. J. Pharmacol. 2008, 585, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Beig, M.I.; Baumert, M.; Walker, F.R.; Day, T.A.; Nalivaiko, E. Blockade of 5-HT2A receptors suppresses hyperthermic but not cardiovascular responses to psychosocial stress in rats. Neuroscience 2009, 159, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, S.; Oka, T.; Mera, T.; Tsuji, S. Repeated social defeat stress induces chronic hyperthermia in rats. Physiol. Behav. 2010, 101, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lkhagvasuren, B.; Nakamura, Y.; Oka, T.; Sudo, N.; Nakamura, K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur. J. Neurosci. 2011, 34, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Lkhagvasuren, B.; Oka, T.; Nakamura, Y.; Hayashi, H.; Sudo, N.; Nakamura, K. Distribution of Fos-immunoreactive cells in rat forebrain and midbrain following social defeat stress and diazepam treatment. Neuroscience 2014, 272, 34–57. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Ootsuka, Y.; Blessing, W. Brown adipose tissue thermogenesis contributes to emotional hyperthermia in a resident rat suddenly confronted with an intruder rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R394–R400. [Google Scholar] [CrossRef]
- Yokoi, Y. Effect of ambient temperature upon emotional hyperthermia and hypothermia in rabbits. J. Appl. Physiol. 1966, 21, 1795–1798. [Google Scholar] [CrossRef] [PubMed]
- Kohlhause, S.; Hoffmann, K.; Schlumbohm, C.; Fuchs, E.; Flügge, G. Nocturnal hyperthermia induced by social stress in male tree shrews: Relation to low testosterone and effects of age. Physiol. Behav. 2011, 104, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Schmelting, B.; Corbach-Söhle, S.; Kohlhause, S.; Schlumbohm, C.; Flügge, G.; Fuchs, E. Agomelatine in the tree shrew model of depression: Effects on stress-induced nocturnal hyperthermia and hormonal status. Eur. Neuropsychopharmacol. 2014, 24, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Pedernera-Romano, C.; Ruiz de la Torre, J.L.; Badiella, L.; Manteca, X. Effect of perphenazine enanthate on open-field test behaviour and stress-induced hyperthermia in domestic sheep. Pharmacol. Biochem. Behav. 2010, 94, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Muchlinski, A.E.; Baldwin, B.C.; Padick, D.A.; Lee, B.Y.; Salguero, H.S.; Gramajo, R. California ground squirrel body temperature regulation patterns measured in the laboratory and in the natural environment. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 120, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Padick, D.A.; Muchlinski, A.E. Stress fever magnitude in laboratory-maintained California ground squirrels varies with season. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 325–330. [Google Scholar] [CrossRef]
- Parr, L.A.; Hopkins, W.D. Brain temperature asymmetries and emotional perception in chimpanzees, Pan troglodytes. Physiol. Behav. 2000, 71, 363–371. [Google Scholar] [CrossRef]
- Meyer, L.C.R.; Fick, L.; Matthee, A.; Mitchell, D.; Fuller, A. Hyperthermia in captured impala (Aepyceros melampus): A fright not flight response. J. Wildl. Dis. 2008, 44, 404–416. [Google Scholar] [CrossRef]
- Gray, D.A.; Maloney, S.K.; Kamerman, P.R. Restraint increases afebrile body temperature but attenuates fever in Pekin ducks (Anas platyrhynchos). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1666–R1671. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Bittencourt, M.; Melleu, F.F.; Marino-Neto, J. Stress-induced core temperature changes in pigeons (Columba livia). Physiol. Behav. 2015, 139, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Rudy, T.A.; Yaksh, T.L.; Viswanathan, C.T. An extensive exploration of the rat brain for sites mediating prostaglandin-induced hyperthermia. Brain Res. 1977, 120, 251–262. [Google Scholar] [CrossRef]
- Nakamura, K.; Matsumura, K.; Hübschle, T.; Nakamura, Y.; Hioki, H.; Fujiyama, F.; Boldogköi, Z.; König, M.; Thiel, H.-J.; Gerstberger, R.; et al. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 2004, 24, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Rathner, J.A.; Madden, C.J.; Morrison, S.F. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R343–R354. [Google Scholar] [CrossRef]
- Lazarus, M.; Yoshida, K.; Coppari, R.; Bass, C.E.; Mochizuki, T.; Lowell, B.B.; Saper, C.B. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007, 10, 1131–1133. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74–104. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Matsumura, K.; Kaneko, T.; Kobayashi, S.; Katoh, H.; Negishi, M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J. Neurosci. 2002, 22, 4600–4610. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, N.; Hioki, H.; Kaneko, T.; Nakamura, K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014, 20, 346–358. [Google Scholar] [CrossRef]
- Kataoka, N.; Shima, Y.; Nakajima, K.; Nakamura, K. A central master driver of psychosocial stress responses in the rat. Science 2020, 367, 1105–1112. [Google Scholar] [CrossRef]
- Kuroshima, A.; Habara, Y.; Uehara, A.; Murazumi, K.; Yahata, T.; Ohno, T. Cross adaption between stress and cold in rats. Pflug. Arch. 1984, 402, 402–408. [Google Scholar] [CrossRef]
- Kuroshima, A.; Yahata, T. Changes in the colonic temperature and metabolism during immobilization stress in repetitively immobilized or cold-acclimated rats. Jpn. J. Physiol. 1985, 35, 591–597. [Google Scholar] [CrossRef]
- Nozu, T.; Okano, S.; Kikuchi, K.; Yahata, T.; Kuroshima, A. Effect of immobilization stress on in vitro and in vivo thermogenesis of brown adipose tissue. Jpn. J. Physiol. 1992, 42, 299–308. [Google Scholar] [CrossRef][Green Version]
- Kaneda, Y.; Tsuji, S.; Oka, T. Age distribution and gender differences in psychogenic fever patients. Biopsychosoc. Med. 2009, 3, 6. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K. Age and gender differences of psychogenic fever: A review of the Japanese literature. Biopsychosoc. Med. 2007, 1, 11. [Google Scholar] [CrossRef]
- Hiramoto, T.; Oka, T.; Yoshihara, K.; Kubo, C. Pyrogenic cytokines did not mediate a stress interview-induced hyperthermic response in a patient with psychogenic fever: A case report. Psychosom. Med. 2009, 71, 932–936. [Google Scholar] [CrossRef] [PubMed]
- McNeil, G.N.; Leighton, L.H.; Elkins, A.M. Possible psychogenic fever of 103.5 degrees F in a patient with borderline personality disorder. Am. J. Psychiatry 1984, 141, 896–897. [Google Scholar] [PubMed]
- Oka, T.; Hayashida, S.; Kaneda, Y.; Kodama, N.; Hashimoto, T.; Tsuji, S. Why do chronic stress-induced hyperthermia patients worry about slightly elevated body temperature? Jpn. J. Psychosom. Intern. Med. 2006, 10, 243–246. [Google Scholar]
- Petersdorf, R.G.; Beeson, P.B. Fever of unexplained origin: Report on 100 cases. Medicine 1961, 40, 1–30. [Google Scholar] [CrossRef]
- Naito, T.; Mizooka, M.; Mitsumoto, F.; Kanazawa, K.; Torikai, K.; Ohno, S.; Morita, H.; Ukimura, A.; Mishima, N.; Otsuka, F.; et al. Diagnostic workup for fever of unknown origin: A multicenter collaborative retrospective study. BMJ Open 2013, 3, e003971. [Google Scholar] [CrossRef]
- Naito, T.; Tanei, M.; Ikeda, N.; Ishii, T.; Suzuki, T.; Morita, H.; Yamasaki, S.; Tamura, J.; Akazawa, K.; Yamamoto, K.; et al. Key diagnostic characteristics of fever of unknown origin in Japanese patients: A prospective multicentre study. BMJ Open. 2019, 9, e032059. [Google Scholar] [CrossRef]
- Goto, M.; Koyama, H.; Takahashi, O.; Fukui, T. A retrospective review of 226 hospitalized patients with fever. Intern. Med. 2007, 46, 17–22. [Google Scholar] [CrossRef]
- Ryuko, H.; Otsuka, F. A comprehensive analysis of 174 febrile patients admitted to Okayama University Hospital. Acta Med. Okayama 2013, 67, 227–237. [Google Scholar]
- Araki, J.; Oka, K.; Yamamoto, K.; Hanayama, Y.; Tokumasu, K.; Hagiya, H.; Ogawa, H.; Itoshima, K.; Otsuka, F. Interrelationships Between Serum Levels of Procalcitonin and Inflammatory Markers in Patients Who Visited a General Medicine Department. Acta Med. Okayama 2021, 75, 299–306. [Google Scholar]
- Oka, K.; Hanayama, Y.; Sato, A.; Omura, D.; Yasuda, M.; Hasegawa, K.; Obika, M.; Otsuka, F. Clinical Characteristics of Febrile Outpatients: Possible Involvement of Thyroid Dysfunction in Febrile Tachycardia. Acta Med. Okayama 2018, 72, 447–456. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Knockaert, D.C.; Dujardin, K.S.; Bobbaers, H.J. Long-term follow-up of patients with undiagnosed fever of unknown origin—PubMed. Arch. Intern. Med. 1996, 156, 618–620. [Google Scholar] [CrossRef]
- Oka, T. Functional hyperthermia and comorbid psychiatric disorders. Biopsychosoc. Med. 2023, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Salit, I.E. Precipitating factors for the chronic fatigue syndrome. J. Psychiatr. Res. 1997, 31, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Kanemitsu, Y.; Sudo, N.; Hayashi, H.; Oka, K. Psychological stress contributed to the development of low-grade fever in a patient with chronic fatigue syndrome: A case report. Biopsychosoc. Med. 2013, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Ginier-Gillet, M. “Functional hyperthermia”: A historical overview. Biopsychosoc. Med. 2023, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.C.; Mukamal, K.J.; Huang, A.; Davis, R.B.; McCarthy, E.P.; Mittleman, M.A. Obesity and C-reactive protein levels among white, black, and hispanic US adults. Obesity 2008, 16, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, J. Gairai de Miru Humeinetsu, 1st ed.; Nakayama-Shoten: Tokyo, Japan, 2017; pp. 19–30. ISBN 978-4-521-74539-8. [Google Scholar]

| Patients’ Background | ||
|---|---|---|
| Age | Case Number (Total 132 Patients) | Male/Female |
| Median [IQR], years | 44 [28–61.5] | 47/85 |
| 20–29 years | 42 (31.8%) | 16/26 |
| 30–39 years | 16 (12.1%) | 4/12 |
| 40–49 years | 20 (15.2%) | 5/15 |
| 50–59 years | 18 (13.6%) | 9/9 |
| 60–69 years | 11 (8.3%) | 3/8 |
| 70–79 years | 16 (12.1%) | 7/9 |
| 80–89 years | 9 (6.8%) | 3/6 |
| Body temperature | ||
| Median [IQR] (°C) | 36.9 [36.6–37.2] | |
| Fever duration | ||
| Median [IQR] (days) | 63 [30–240] | |
| BMI | ||
| Median [IQR] (kg/m2) | 21.1 [18.5–26.1] | |
| Final Diagnosis |
|---|
| Adult-onset Still’s disease |
| Aseptic meningitis |
| Aspiration pneumonia |
| Carcinomatous pleurisy |
| Clostridium difficile-associated diarrhea |
| Cytomegalovirus infection |
| Dermatomyositis |
| Drug allergy |
| Fitz–Hugh–Curtis syndrome |
| Graves’ disease |
| Human immunodeficiency virus infection |
| Inflammatory lymphadenitis |
| Lung abscess |
| Miliary tuberculosis |
| Myelodysplastic syndromes |
| Neurogenic fever |
| PFAPA syndrome |
| Pharyngeal carcinoma |
| Post-vaccination reaction to coronavirus vaccine |
| Pseudogout |
| Rheumatoid arthritis |
| Ureteral obstruction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oka, K.; Tokumasu, K.; Hagiya, H.; Otsuka, F. Characteristics of Functional Hyperthermia Detected in an Outpatient Clinic for Fever of Unknown Origin. J. Clin. Med. 2024, 13, 889. https://doi.org/10.3390/jcm13030889
Oka K, Tokumasu K, Hagiya H, Otsuka F. Characteristics of Functional Hyperthermia Detected in an Outpatient Clinic for Fever of Unknown Origin. Journal of Clinical Medicine. 2024; 13(3):889. https://doi.org/10.3390/jcm13030889
Chicago/Turabian StyleOka, Kosuke, Kazuki Tokumasu, Hideharu Hagiya, and Fumio Otsuka. 2024. "Characteristics of Functional Hyperthermia Detected in an Outpatient Clinic for Fever of Unknown Origin" Journal of Clinical Medicine 13, no. 3: 889. https://doi.org/10.3390/jcm13030889
APA StyleOka, K., Tokumasu, K., Hagiya, H., & Otsuka, F. (2024). Characteristics of Functional Hyperthermia Detected in an Outpatient Clinic for Fever of Unknown Origin. Journal of Clinical Medicine, 13(3), 889. https://doi.org/10.3390/jcm13030889






