The Prognostic Role of Volumetric MRI Evaluation in the Surgical Treatment of Glioblastoma
Abstract
1. Introduction
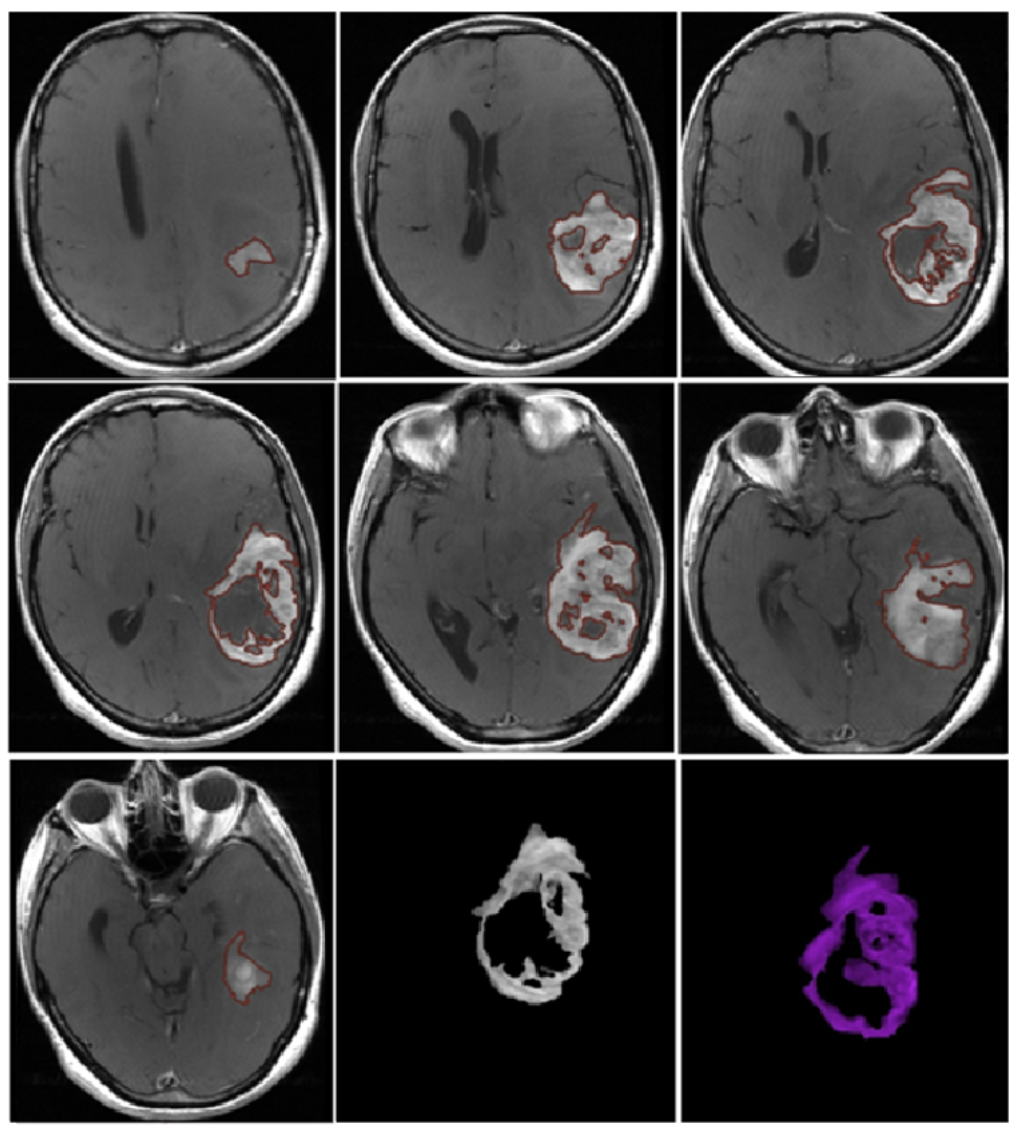
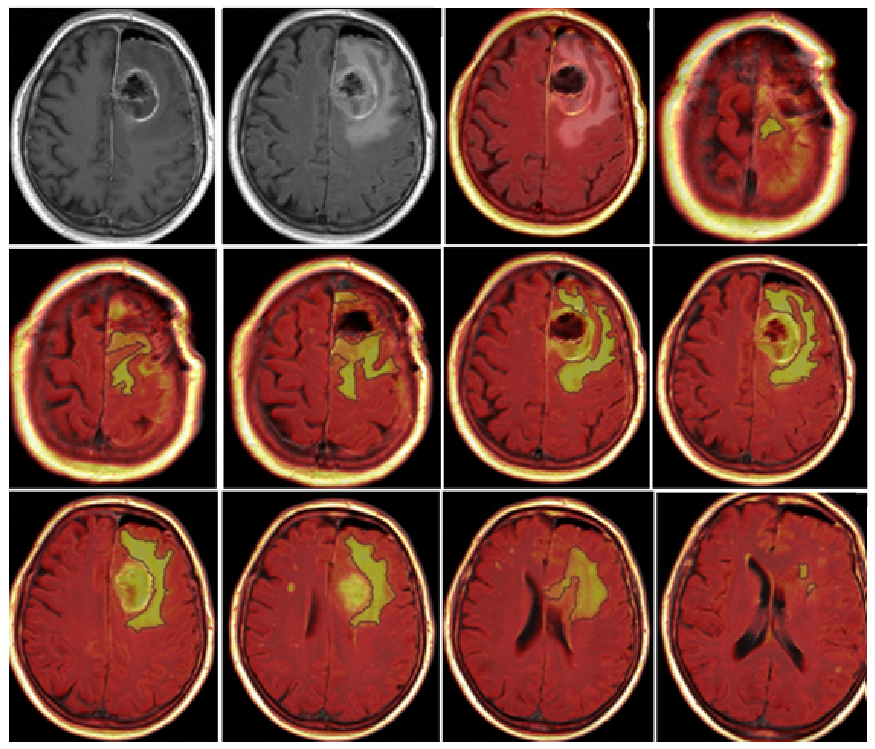
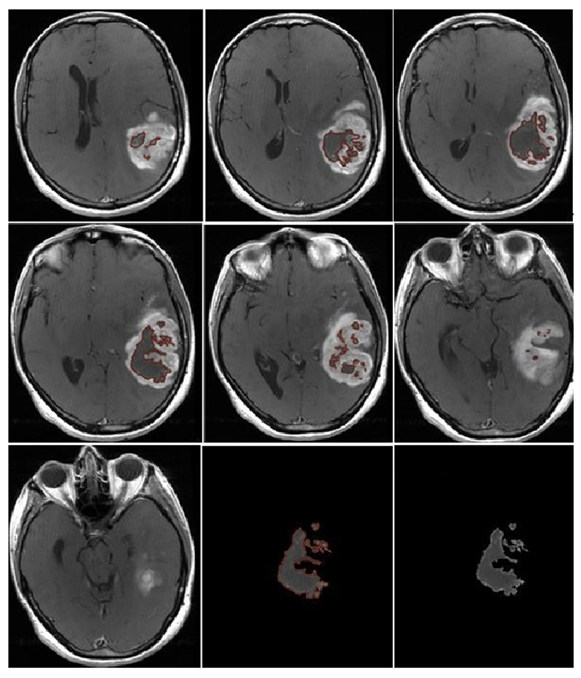
2. Materials and Methods
3. Results
3.1. Qualitative Analysis
- Localization: All tumors had a supratentorial localization; 31 (36%) were in the frontal lobe, 18 (21%) were in the parietal lobe, 36 (41%) in the temporal lobe, and 2 (2%) in the occipital lobe.
- Eloquent areas: 35 of the 87 lesions (40%) were in eloquent areas.
- Ependymal involvement: Ependymal involvement was observed in 52 (60%) patients; 35 (40%) lesions had no connection with the periventricular zone.
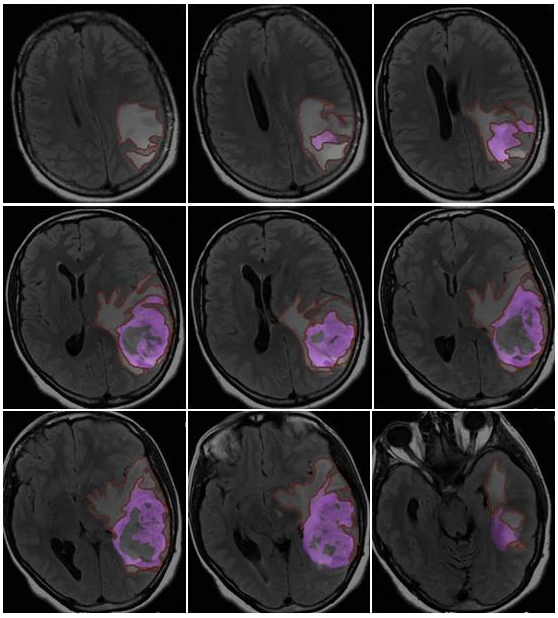
- Morphological appearance: We divided GB lesions into three categories based on the enhancing wall thickness: thin, <3 mm; thin-nodular, when the enhancing wall showed focal thickenings > 3 mm; and nodular, when solid appearance was predominant and intratumoral necrosis was absent or <1.5 cm3. A total of 11 (13%) masses showed a thin pattern, 51 (58%) showed a thin-nodular pattern, and 25 (29%) showed a nodular pattern.
- Multifocal disease: Multifocal disease was found in 20 (23.3%) patients (Table 1).
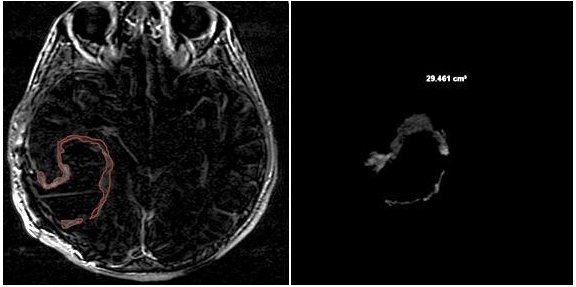
3.2. Quantitative Analysis
3.3. Overall Survival—Univariate Analysis
- Chemotherapy: The median OS values were 15 months for patients who received adjuvant chemotherapy (n = 61) and 3 months for patients who did not (n = 26); the difference between the two groups was statistically significant (p < 0.0001).
- Radiotherapy: The median OS values were 14 months for patients who received adjuvant radiotherapy (n = 64) and 3 months for patients who did not (n = 23); the difference between the two groups was statistically significant (p < 0.0001).
- Chemoradiotherapy: The median OS values were 16 months for patients who received chemoradiotherapy (n = 57), 6 months for patients who received radiotherapy alone (n = 7), and 5 months for patients who received chemotherapy alone (n = 4); the median OS value was 2 months for patients who did not receive any adjuvant treatment (n = 19). The difference was statistically significant (p < 0.0001).
- CE-RTV: The median OS value was 19 months for patients with CE-RTV < 5.8 cm3 and 9 months for patients with CE-RTV > 5.8 cm3. The difference was statistically significant (p < 0.004) (Table 2).
3.4. Overall Survival—Multivariate Analysis
3.5. Progression-Free Survival—Univariate Analysis
- Gender: The median PFS value was 4 months for men and 5 months for women. The difference was statistically significant (p = 0.02).
- Chemotherapy: The median PFS value was 6 months for patients who underwent chemotherapy and 1 month for patients who did not. The difference was statistically significant (p < 0.0001).
- Radiotherapy: The median PFS value was 5 months for patients who received radiotherapy and 1 month for patients who did not. The difference was statistically significant (p < 0.0001).
- Postoperative KPS: The median PFS value was 3 months in patients with postoperative KPS < 80 and 7 months in patients with postoperative KPS > 80. The difference was statistically significant (p < 0.0001).
- CE-RTV: The median PFS value was 5 months in patients with CE-RTV < 5.8 cm3 and 4 months in patients with CE-RTV > 5.8 cm3. The difference was statistically significant (p = 0.04).
- Surgical resection: The median PFS value was 6 months in patients with a surgical resection percentage > 95% and 4 months if the surgical resection percentage was < 95%. The difference was statistically significant (p = 0.02).
3.6. Progression-Free Survival—Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System tumours: A practical update on what neurosurgeons need to know—A minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Stoyanov, G.S.; Lyutfi, E.; Georgieva, R.; Georgiev, R.; Dzhenkov, D.L.; Petkova, L.; Ivanov, B.D.; Kaprelyan, A.; Ghenev, P. Reclassification of Glioblastoma Multiforme According to the 2021 World Health Organization Classification of Central Nervous System Tumors: A Single Institution Report and Practical Significance. Cureus 2022, 14, e21822. [Google Scholar] [CrossRef] [PubMed]
- Melhem, J.M.; Detsky, J.; Lim-Fat, M.J.; Perry, J.R. Updates in IDH-Wildtype Glioblastoma. Neurotherapeutics 2022, 19, 1705–1723. [Google Scholar] [CrossRef] [PubMed]
- Lakomy, R.; Kazda, T.; Selingerova, I.; Poprach, A.; Pospisil, P.; Belanova, R.; Fadrus, P.; Vybihal, V.; Smrcka, M.; Jancalek, R.; et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen after a Decade. Front. Oncol. 2020, 10, 840. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Polonara, G.; Aiudi, D.; Iacoangeli, A.; Raggi, A.; Ottaviani, M.M.; Antonini, R.; Iacoangeli, M.; Dobran, M. Glioblastoma: A Retrospective Analysis of the Role of the Maximal Surgical Resection on Overall Survival and Progression Free Survival. Biomedicines 2023, 11, 739. [Google Scholar] [CrossRef]
- Al-Holou, W.N.; Hodges, T.R.; Everson, R.G.; Freeman, J.; Zhou, S.; Suki, D.; Rao, G.; Ferguson, S.D.; Heimberger, A.B.; McCutcheon, I.E.; et al. Perilesional Resection of Glioblastoma Is Independently Associated with Improved Outcomes. Neurosurgery 2020, 86, 112–121. [Google Scholar] [CrossRef]
- Pessina, F.; Navarria, P.; Cozzi, L.; Ascolese, A.M.; Simonelli, M.; Santoro, A.; Clerici, E.; Rossi, M.; Scorsetti, M.; Bello, L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: Is it useful and safe? A single institution retrospective experience. J. Neurooncol. 2017, 135, 129–139. [Google Scholar] [CrossRef]
- Hooper, G.W.; Ansari, S.; Johnson, J.M.; Ginat, D.T. Advances in the Radiological Evaluation of and Theranostics for Glioblastoma. Cancers 2023, 15, 4162. [Google Scholar] [CrossRef]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Trevisan, E.; Bertero, L.; Cassoni, P.; Morra, I.; Fabrini, M.G.; Pasqualetti, F.; Lolli, I.; Castiglione, A.; Ciccone, G.; et al. Bevacizumab and fotemustine for recurrent glioblastoma: A phase II study of AINO (Italian Association of Neuro-Oncology). J. Neurooncol. 2014, 116, 533–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wangaryattawanich, P.; Hatami, M.; Wang, J.; Thomas, G.; Flanders, A.; Kirby, J.; Wintermark, M.; Huang, E.S.; Bakhtiari, A.S.; Luedi, M.M.; et al. Multicenter imaging outcomes study of the Cancer Genome Atlas glioblastoma patient cohort: Imaging predictors of overall and progression-free survival. Neuro Oncol. 2015, 17, 1525–1537. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Harris, R.J.; Woodworth, D.C.; Leu, K.; Zaw, O.; Mason, W.P.; Sahebjam, S.; Abrey, L.E.; Aftab, D.T.; Schwab, G.M.; et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: Evidence from single and multicenter trials. Neuro Oncol. 2017, 19, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013, 15 (Suppl. S2), ii1–ii56, Erratum in: Neuro Oncol. 2014, 16, 760. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Beck, T.; Pongratz, T.; Meinel, T.; Kreth, F.W.; Tonn, J.C.; Stummer, W. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- deSouza, R.M.; Shaweis, H.; Han, C.; Sivasubramaniam, V.; Brazil, L.; Beaney, R.; Sadler, G.; Al-Sarraj, S.; Hampton, T.; Logan, J.; et al. Has the survival of patients with glioblastoma changed over the years? Br. J. Cancer 2016, 114, 146–150, Erratum in: Br. J. Cancer 2016, 114, e20. [Google Scholar] [CrossRef]
- Stewart, L.A. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002, 359, 1011–1018. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Walid, M.S. Prognostic factors for long-term survival after glioblastoma. Perm. J. 2008, 12, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol. 2023, 25, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Abdal, K.; Darvish, M.; Ahmadi, M.H. Rapid Progression of Primary Glioblastoma to the Maxillofacial Area in a 29-year-old Woman. World J. Dent. 2016, 9, 122–125. [Google Scholar] [CrossRef]
- Grossman, R.; Shimony, N.; Shir, D.; Gonen, T.; Sitt, R.; Kimchi, T.J.; Harosh, C.B.; Ram, Z. Dynamics of FLAIR Volume Changes in Glioblastoma and Prediction of Survival. Ann. Surg. Oncol. 2017, 24, 794–800. [Google Scholar] [CrossRef]


| Localization | n (%) | Morphology | n (%) |
|---|---|---|---|
| Frontal lobe | 31 (36%) | Multifocal | 20 (23.3%) |
| Parietal lobe | 18 (21%) | Thin (d < 3 mm) | 11 (13%) |
| Temporal lobe | 36 (41%) | Thin-nodular (d > 3 mm) | 51 (58%) |
| Occipital lobe | 2 (2%) | Nodular | 25 (29%) |
| Eloquent areas | 35 (40%) | Pseudocapsulated | 62 (71%) |
| Ependymal involvment | 52 (60%) |
| Median OS Treated (n) | Median OS Not Treated (n) | p-Value | |
|---|---|---|---|
| AC | 15 (n = 61) | 3 (n = 26) | <0.0001 |
| ART | 14 (n = 64) | 3 (n = 23) | <0.0001 |
| CRT | 16 (n = 57) | 2 (n = 19) | <0.0001 |
| CT | 5 (n = 4) | ||
| RT | 6 (n = 7) | ||
| NT | 2 (n = 19) | ||
| CE-RTV | <5.8 cm3: 19 | >5.8 cm3: 9 | <0.004 |
| Median PFS (Months) | p-Value | ||
|---|---|---|---|
| Sex (M/F) | Male 4 | Female 5 | p = 0.02 |
| Chemotherapy | With: 6 / | Without: 1 | p < 0.0001 |
| Radiotherapy | With: 5 / | Without: 1 | p < 0.0001 |
| Postoperative KPS | <80: 3 | >80: 7 | p < 0.0001 |
| CE-RTV | <5.8 cm3: 5 | >5.8 cm3: 4 | p = 0.04 |
| Surgical resection (%) | >95%: 6 | >95%: 4 | p = 0.02 |
| Pseudocapsulated | with: 6 | Without: 3 | p < 0.0001 |
| Patient | Semiautomatic CE-PTV (cm3) | Manual CE-PTV (cm3) | CE-RTV (cm3) |
|---|---|---|---|
| 1 | 34.89 | 31.14 | |
| 2 | 30.00 | 33.51 | 16.37 |
| 3 | 27.32 | 33.33 | 0.00 |
| 4 | 17.53 | 15.46 | |
| 5 | 12.21 | 5.24 | |
| 6 | 5.21 | 4.70 | 0.00 |
| 7 | 21.99 | 24.04 | 10.71 |
| 8 | 59.10 | 38.78 | 5.82 |
| 9 | 20.15 | 21.31 | 6.98 |
| 10 | 64.52 | 72.11 | |
| 11 | 11.83 | 10.58 | 10.12 |
| 12 | 49.81 | 49.72 | |
| 13 | 11.01 | 10.17 | |
| 14 | 20.87 | 17.97 | |
| 15 | 32.55 | 39.27 | |
| 16 | 46.11 | 42.10 | |
| 17 | 35.88 | 36.29 | |
| 18 | 23.65 | 39.15 | |
| 19 | 5.87 | 5.21 | 4.65 |
| 20 | 25.75 | 24.16 | 10.49 |
| 21 | 17.78 | 14.68 | |
| 22 | 20.43 | 21.26 | |
| 23 | 1.18 | 0.39 | 0.00 |
| 24 | 10.93 | 11.26 | |
| 25 | 16.06 | 14.84 | 0.00 |
| 26 | 20.11 | 18.65 | 13.28 |
| 27 | 25.89 | 25.40 | 0.00 |
| 28 | 19.49 | 13.90 | 0.00 |
| 29 | 39.34 | 42.64 | 16.67 |
| 30 | 38.90 | 42.41 | |
| 31 | 20.24 | 22.89 | |
| 32 | 43.90 | 42.09 | 4.17 |
| 33 | 14.30 | 13.22 | 8.44 |
| 34 | 24.87 | 23.45 | 6.85 |
| 35 | 4.51 | 5.29 | 2.85 |
| 36 | 28.13 | 24.56 | |
| 37 | 68.11 | 61.67 | |
| 38 | 51.79 | 54.13 | |
| 39 | 59.65 | 64.19 | |
| 40 | 83.58 | 65.28 | |
| 41 | 18.34 | 13.45 | |
| 42 | 8.03 | 10.69 | |
| 43 | 49.64 | 28.78 | 5.27 |
| 44 | 62.41 | 51.74 | |
| 45 | 22.34 | 12.37 | |
| 46 | 5.69 | 9.09 | |
| 47 | 20.00 | 17.02 | 0.00 |
| 48 | 47.05 | 44.89 | |
| 49 | 2.12 | 1.60 | |
| 50 | 31.19 | 34.89 | |
| 51 | 6.73 | 5.93 | |
| 52 | 28.91 | 34.66 | |
| 53 | 8.54 | 8.50 | 3.30 |
| 54 | 50.48 | 60.56 | |
| 55 | 12.72 | 11.05 | 7.75 |
| 56 | 28.14 | 27.22 | 12.23 |
| 57 | 37.39 | 32.12 | |
| 58 | 37.98 | 31.05 | 0.00 |
| 59 | 36.26 | 51.17 | |
| 60 | 22.56 | 27.87 | 5.32 |
| 61 | 28.90 | 44.36 | |
| 62 | 16.00 | 15.98 | 14.46 |
| 63 | 5.97 | 17.10 | |
| 64 | 17.40 | 17.61 | 7.38 |
| 65 | 14.28 | 15.88 | 5.78 |
| 66 | 8.80 | 10.30 | |
| 67 | 46.00 | 49.34 | 28.97 |
| 68 | 0.79 | 0.97 | |
| 69 | 15.53 | 12.24 | 0.73 |
| 70 | 29.56 | 41.62 | |
| 71 | 10.79 | 11.25 | |
| 72 | 37.61 | 51.02 | |
| 73 | 21.61 | 29.19 | |
| 74 | 24.86 | 33.77 | |
| 75 | 18.46 | 20.99 | |
| 76 | 42.89 | 46.64 | 42.96 |
| 77 | 42.32 | 54.07 | |
| 78 | 24.09 | 24.08 | 0.58 |
| 79 | 83.44 | 84.80 | 29.46 |
| 80 | 12.51 | 11.59 | |
| 81 | 14.12 | 10.71 | 1.30 |
| 82 | 37.00 | 54.50 | |
| 83 | 47.58 | 37.95 | |
| 84 | 2.35 | 2.98 | 0.00 |
| 85 | 12.98 | 12.60 | 11.45 |
| 86 | 15.53 | 21.01 | |
| 87 | 39.18 | 42.70 | 8.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiudi, D.; Iacoangeli, A.; Dobran, M.; Polonara, G.; Chiapponi, M.; Mattioli, A.; Gladi, M.; Iacoangeli, M. The Prognostic Role of Volumetric MRI Evaluation in the Surgical Treatment of Glioblastoma. J. Clin. Med. 2024, 13, 849. https://doi.org/10.3390/jcm13030849
Aiudi D, Iacoangeli A, Dobran M, Polonara G, Chiapponi M, Mattioli A, Gladi M, Iacoangeli M. The Prognostic Role of Volumetric MRI Evaluation in the Surgical Treatment of Glioblastoma. Journal of Clinical Medicine. 2024; 13(3):849. https://doi.org/10.3390/jcm13030849
Chicago/Turabian StyleAiudi, Denis, Alessio Iacoangeli, Mauro Dobran, Gabriele Polonara, Mario Chiapponi, Andrea Mattioli, Maurizio Gladi, and Maurizio Iacoangeli. 2024. "The Prognostic Role of Volumetric MRI Evaluation in the Surgical Treatment of Glioblastoma" Journal of Clinical Medicine 13, no. 3: 849. https://doi.org/10.3390/jcm13030849
APA StyleAiudi, D., Iacoangeli, A., Dobran, M., Polonara, G., Chiapponi, M., Mattioli, A., Gladi, M., & Iacoangeli, M. (2024). The Prognostic Role of Volumetric MRI Evaluation in the Surgical Treatment of Glioblastoma. Journal of Clinical Medicine, 13(3), 849. https://doi.org/10.3390/jcm13030849







