Short-Term Effects of 3D-Printed Occlusal Splints and Conventional Splints on Sleep Bruxism Activity: EMG–ECG Night Recordings of a Sample of Young Adults
Abstract
1. Introduction
- -
- Do different OA fabrication techniques (i.e., 3D or traditional) influence SB?
- -
- Do different OA materials influence perceived comfort during usage?
- -
- Is there a difference in nanomechanical properties between the materials used to produce the two devices?
2. Materials and Methods
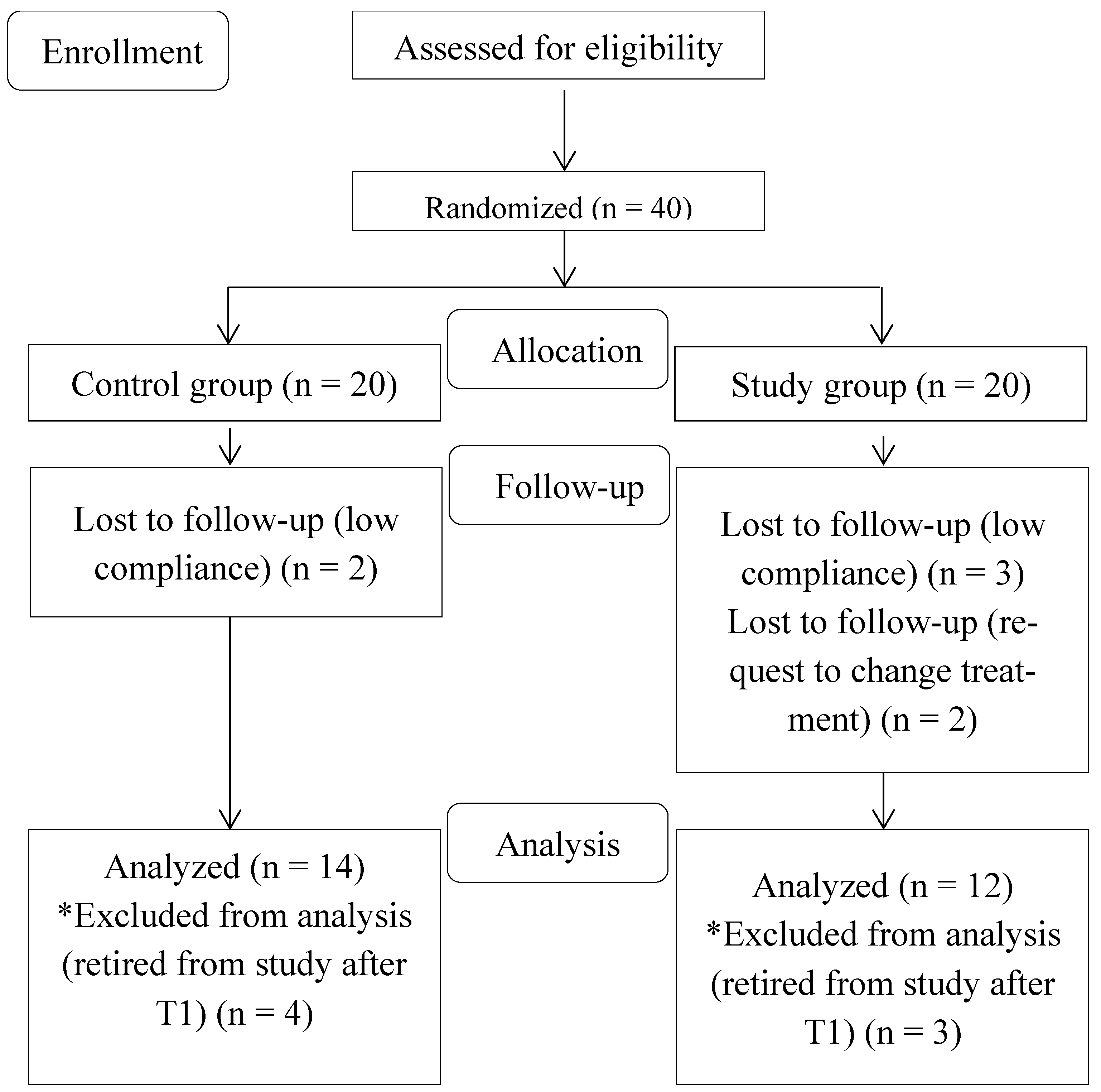
2.1. Study Design
2.2. Participants
2.3. Randomization and Blinding
2.4. Outcome Measures
2.5. Sample Size Calculation
2.6. Statistical Analysis
2.7. Sensitivity Analysis
2.8. Experimental Section
- (1)
- Study group: Splints were manufactured thanks to the 3D printer, a SolFlex 650 (VOCO® GmbH, Cuxhaven, Germany), with an additive technique. The light-cured V-Print Splint resin (VOCO® GmbH, Cuxhaven, Germany) was used for this purpose, classified as class IIa medical disposal [47] (medium risk, non-active disposal), biocompatible, tasteless, transparent, and with high resistance to abrasion (reported flexural strength: 75 Mpa). A 0.20 mm offset value was used for the additive technique, as suggested by Lo Giudice et al. [48]. After printing, all debris was removed with an ultrasonic bath of isopropyl alcohol, and the plates were dried with compressed air. Fifteen minutes after the final contact with isopropyl alcohol, the plates were post-cured on both sides with an OtoFlash-Polymerization Unit (VOCO® GmbH, Cuxhaven, Germany): 3.5 min per side with a frequency of 10 flashes per second for a total amount of 2000 flashes. At the end of the process, all the plates were finished and polished by an expert dental technician of the Dental School, University of Torino, Torino (Italy).
- (2)
- Control group: Splints were manufactured with a 1.25 mm thermoformed retentive base of polyethylenterephthalat–glycol copolyester (PET-G) (DURAN® Scheu-Dental, Am Burgberg, Germany) (reported flexural strength: 69 Mpa) rebased with self-curing resin (Forestacry®, Forestadent Bernhard Förster GmbH Westliche, Pforzheim, Germany), classified as class I medical disposal [47] (non-critical and non-active disposal), biocompatible, tasteless, transparent, and with high resistance to abrasion (reported flexural strength: ≥50 Mpa). The plates were fabricated, finished, and polished by an external dental laboratory (GLOI Laboratorio Odontotecnico, Biella, Italy).
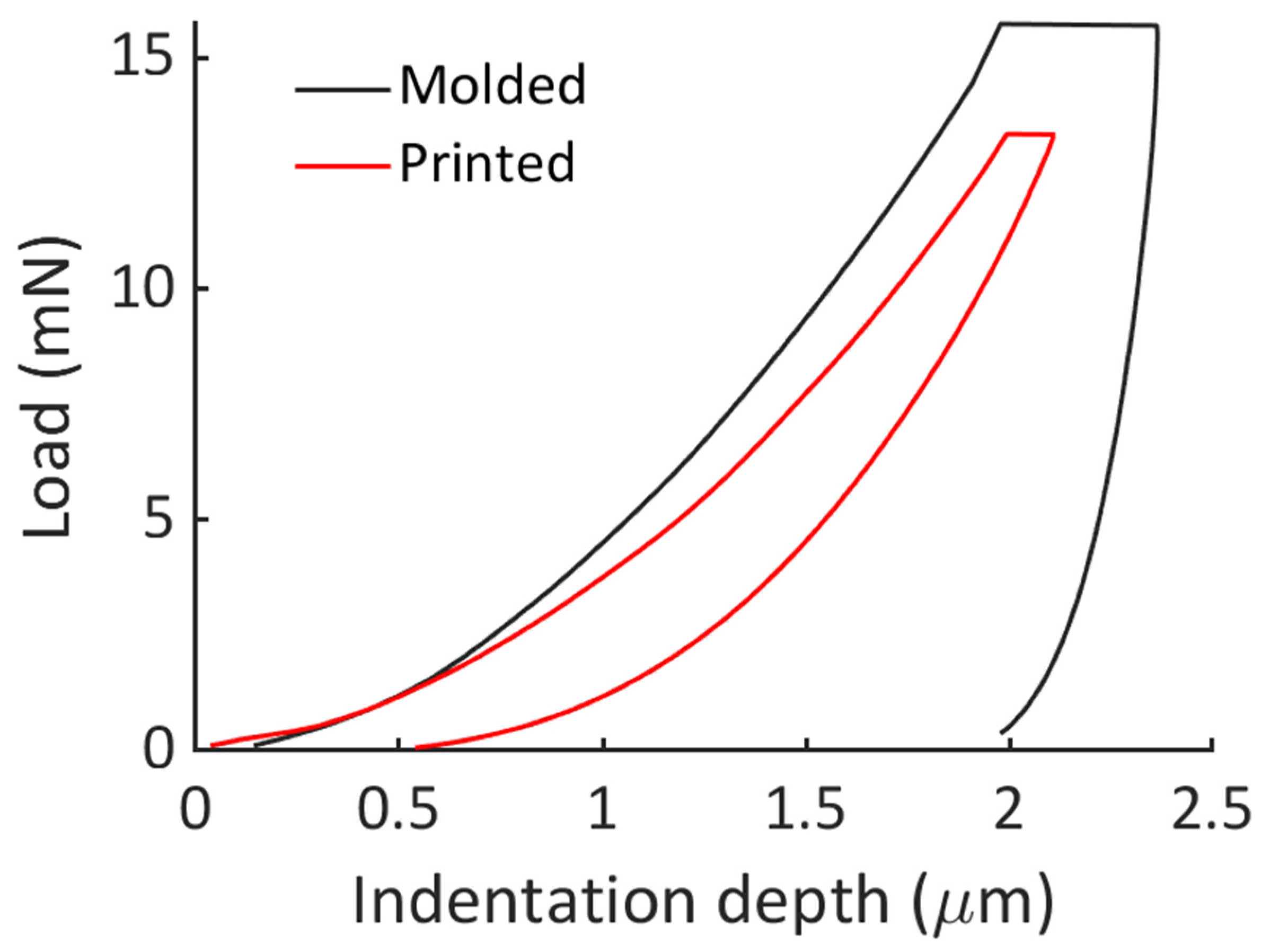
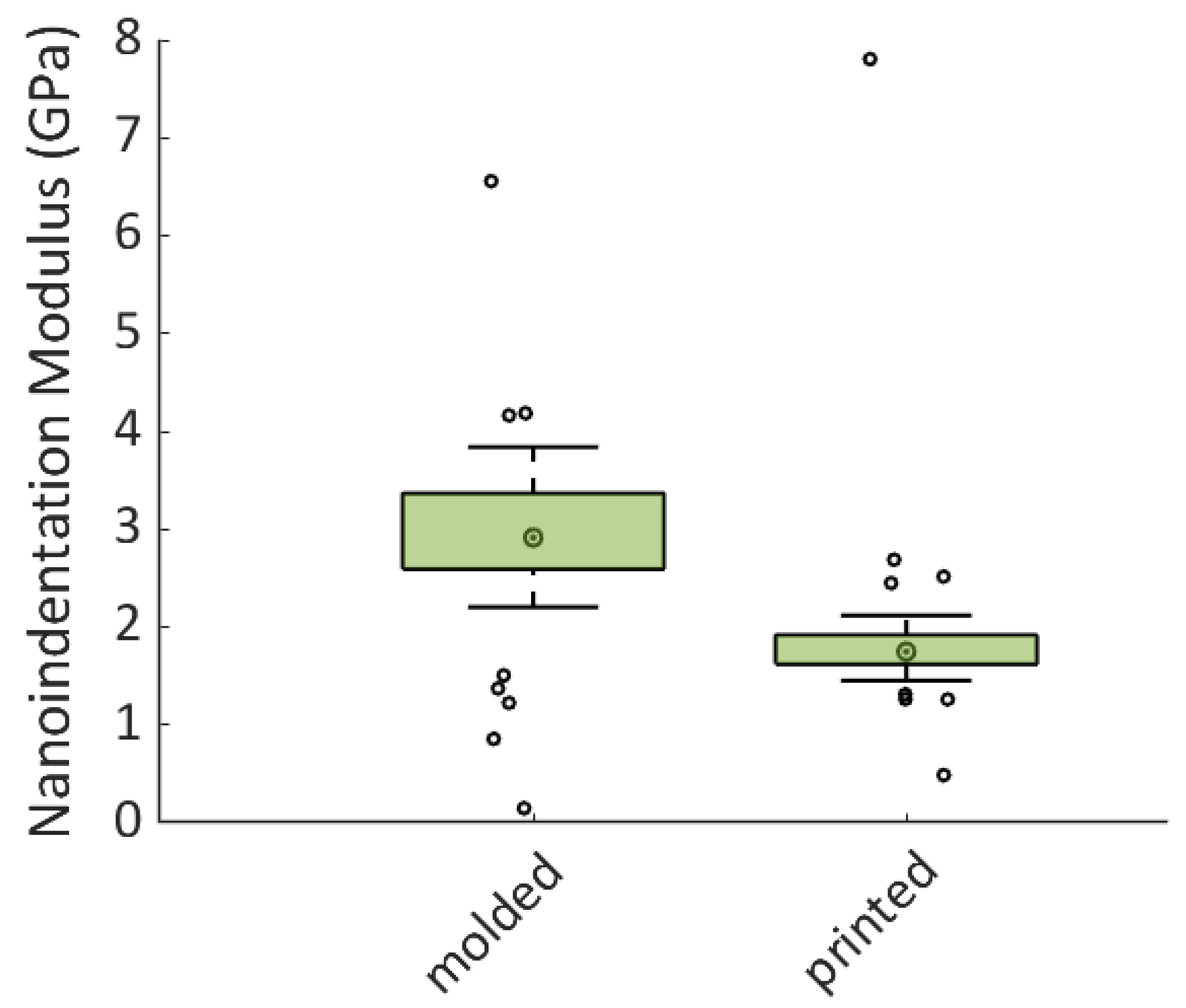
2.9. Nanoindentation Protocol
3. Results
Nanoindentation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sateia, M.J. International Classification of Sleep Disorders, 3rd ed.; Sleep Related Bruxism; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral. Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism physiology and pathology: An overview for clinicians. J. Oral. Rehabil. 2008, 35, 476–494. [Google Scholar] [CrossRef]
- Sforza, E.; Jouny, C.; Ibanez, V. Cardiac activation during arousal in humans: Further evidence for hierarchy in the arousal response. Clin. Neurophysiol. 2000, 111, 1611–1619. [Google Scholar] [CrossRef]
- Huynh, N.; Kato, T.; Rompré, P.H.; Okura, K.; Saber, M.; Lanfranchi, P.A.; Montplaisir, J.Y.; Lavigne, G.J. Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J. Sleep. Res. 2006, 15, 339–346. [Google Scholar] [CrossRef]
- Macaluso, G.M.; Guerra, P.; Di Giovanni, G.; Boselli, M.; Parrino, L.; Terzano, M.G. Sleep bruxism is a disorder related to periodic arousals during sleep. J. Dent. Res. 1998, 77, 565–573. [Google Scholar] [CrossRef]
- Kato, T.; Rompre, P.; Montplaisir, J.Y.; Sessle, B.J.; Lavigne, G.J. Sleep bruxism: An oromotor activity secondary to micro-arousal. J. Dent. Res. 2001, 80, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Khoury, S.; Rouleau, G.A.; Rompre, P.H.; Mayer, P.; Montplaisir, J.; Lavigne, G.J. A significant increase in breathing amplitude precedes sleep bruxism. Chest 2008, 134, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Nashed, A.; Lanfranchi, P.; Rompré, P.; Carra, M.C.; Mayer, P.; Colombo, R.; Huynh, N.; Lavigne, G. Sleep bruxism is associated with a rise in arterial blood pressure. Sleep 2012, 35, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Yazıcıoğlu, İ.; Çiftçi, V. Evaluation of signs and symptoms of temporomandibular disorders and incisal relationships among 7–10-year-old Turkish children with sleep bruxism: A cross-sectional study. Cranio 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Smardz, J.; Martynowicz, H.; Michalek-Zrabkowska, M.; Wojakowska, A.; Mazur, G.; Winocur, E.; Wieckiewicz, M. Sleep bruxism and occurrence of temporomandibular disorders-related pain: A polysomnographic study. Front. Neurol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Andrade de Alencar, N.; Nolasco Fernandes, A.B.; Gomes de Souza, M.M.; Luiz, R.R.; Fonseca-Gonçalves, A.; Maia, L.C. Lifestyle and oral facial disorders associated with sleep bruxism in children. Cranio 2017, 35, 168–174. [Google Scholar] [CrossRef]
- Marpaung, C.; van Selms, M.K.; Lobbezoo, F. Prevalence and risk indicators of pain-related temporomandibular disorders among Indonesian children and adolescents. Commun. Dent. Oral. Epidemiol. 2018, 46, 400–406. [Google Scholar] [CrossRef]
- Rubin, P.F.; Erez, A.; Peretz, B.; Birenboim-Wilensky, R.; Winocur, E. Prevalence of bruxism and temporomandibular disorders among orphans in southeast Uganda: A gender and age comparison. Cranio 2018, 36, 243–249. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Smardz, J.; Martynowicz, H.; Wojakowska, A.; Mazur, G.; Winocur, E. Distribution of temporomandibular disorders among sleep bruxers and non-bruxers—A polysomnographic study. J. Oral. Rehabil. 2020, 47, 820–826. [Google Scholar] [CrossRef]
- Lei, J.; Fu, J.; Yap, A.U.J.; Fu, K.Y. Temporomandibular disorders symptoms in Asian adolescents and their association with sleep quality and psychological distress. Cranio 2016, 34, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu-Ak, A.; Kurtulmus, H.; Basa, S.; Sabuncuoglu, O. Can sleeping habits be associated with sleep bruxism, temporomandibular disorders and dental caries among children? Dent. Med. Probl. 2022, 59, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Cheifetz, A.T.; Osganian, S.K.; Allred, E.M.; Needleman, H.L. Prevalence of bruxism and associated correlates in children as reported by parents. J. Dent. Child. 2005, 72, 67–73. [Google Scholar]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic basis of sleep bruxism and sleep apnea-response to a medical puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Gostner, J.M.; Fuchs, D. Mood, food, and cognition: Role of tryptophan and serotonin. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Fitzpatrick, P.F. Mechanisms of tryptophan and tyrosine hydroxylase. IUBMB Life 2013, 65, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and seroton in on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, H.; Fujisawa, M.; Abe, Y.; Iida, T.; Oki, K.; Okura, K.; Tanabe, N.; Nishiyama, A. Managements of sleep bruxism in adult: A systematic review. Jpn. Dent. Sci. Rev. 2022, 58, 124–136. [Google Scholar] [CrossRef]
- Cerón, L.; Pacheco, M.; Delgado Gaete, A.; Bravo Torres, W.; Astudillo Rubio, D. Therapies for sleep bruxism in dentistry: A critical evaluation of systematic reviews. Dent. Med. Probl. 2023, 60, 335–344. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Winocur, E.; Lobbezoo, F. Management of sleep bruxism in adults: A qualitative systematic literature review. J. Oral. Rehabil. 2015, 42, 862–874. [Google Scholar] [CrossRef]
- Melo, G.; Duarte, J.; Pauletto, P.; Porporatti, A.L.; Stuginski-Barbosa, J.; Winocur, E.; Flores-Mir, C.; De Luca Canto, G. Bruxism: An umbrella review of systematic reviews. J. Oral. Rehabil. 2019, 46, 666–690. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.; Chua, A.P. Sleep bruxism: Current knowledge and contemporary management. J. Conserv. Dent. 2016, 19, 383–389. [Google Scholar] [CrossRef]
- Mörmann, W.H.; Brandestini, M. State of the Art of CADS/CAM Restorations: 20 Years of CEREC; Mörmann, W.H., Ed.; Quintessence: London, UK, 2006; pp. 1–8. [Google Scholar]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Duret, F.; Preston, J.D. CAD/CAM imaging in dentistry. Curr. Opin. Dent. 1991, 1, 150–154. [Google Scholar]
- Blatz, M.B.; Conejo, J. The Current State of Chairside Digital Dentistry and Materials. Dent. Clin. N. Am. 2019, 63, 175–197. [Google Scholar] [CrossRef]
- Cunha, T.M.A.D.; Barbosa, I.D.S.; Palma, K.K. Orthodontic digital workflow: Devices and clinical applications. Dental Press. J. Orthod. 2021, 26, e21spe6. [Google Scholar] [CrossRef]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral. Health 2017, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Marcel, R.; Reinhard, H.; Andreas, K. Accuracy of CAD/CAM-fabricated bite splints: Milling vs. 3D printing. Clin. Oral. Investig. 2020, 24, 4607–4615. [Google Scholar] [CrossRef]
- Leib, A.M. Patient preference for light-cured composite bite splint compared to heat-cured acrylic bite splint. J. Periodontol. 2001, 72, 1108–1112. [Google Scholar] [CrossRef]
- Patzelt, S.B.M.; Krügel, M.; Wesemann, C.; Pieralli, S.; Nold, J.; Spies, B.C.; Vach, K.; Kohal, R.J. In Vitro Time Efficiency, Fit, and Wear of Conventionally- versus Digitally-Fabricated Occlusal Splints. Materials 2022, 15, 1085. [Google Scholar] [CrossRef] [PubMed]
- Zwarenstein, M.; Treweek, S.; Gagnier, J.J.; Altman, D.G.; Tunis, S.; Haynes, B.; Oxman, A.D.; Moher, D. Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ 2008, 337, a2390. [Google Scholar] [CrossRef] [PubMed]
- Casett, E.; Réus, J.C.; Stuginski-Barbosa, J.; Porporatti, A.L.; Carra, M.C.; Peres, M.A.; de Luca Canto, G.; Manfredini, D. Validity of different tools to assess sleep bruxism: A meta-analysis. J. Oral. Rehabil. 2017, 44, 722–734. [Google Scholar] [CrossRef]
- Castroflorio, T.; Mesin, L.; Tartaglia, G.M.; Sforza, C.; Farina, D. Use of electromyographic and electrocardiographic signals to detect sleep bruxism episodes in a natural environment. IEEE J. Biomed. Health Inform. 2013, 17, 994–1001. [Google Scholar] [CrossRef]
- Castroflorio, T.; Deregibus, A.; Bargellini, A.; Debernardi, C.; Manfredini, D. Detection of sleep bruxism: Comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J. Oral. Rehabil. 2014, 41, 163–169. [Google Scholar] [CrossRef]
- Deregibus, A.; Castroflorio, T.; Bargellini, A.; Debernardi, C. Reliability of a portable device for the detection of sleep bruxism. Clin. Oral. Investig. 2014, 18, 2037–2043. [Google Scholar] [CrossRef]
- Greene, J.C.; Vermillion, J.R. The simplified oral hygiene index. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef]
- Mayer, P.; Heinzer, R.; Lavigne, G. Sleep Bruxism in Respiratory Medicine Practice. Chest 2016, 149, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Vetter, T.R. Fundamentals of Research Data and Variables: The Devil Is in the Details. Anesth. Analg. 2017, 125, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Breusch, T.S.; Pagan, A.R. A Simple Test for Heteroscedasticity and Random Coefficient Variation. Econometrica 1979, 47, 1287. [Google Scholar] [CrossRef]
- Durbin, J.; Watson, G.S. Testing for Serial Correlation in Least Squares Regression. III. Biometrika 1971, 58, 1–19. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. [Google Scholar]
- Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No. 726/2004. 2019. Available online: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol1/reg2007139/reg20071394en.pdf (accessed on 8 April 2022).
- Lo Giudice, A.; Ronsivalle, V.; Pedullà, E.; Rugeri, M.; Leonardi, R. Digitally programmed (CAD) offset values for prototyped occlusal splints (CAM): Assessment of appliance-fitting using surface-based superimposition and deviation analysis. Int. J. Comput. Dent. 2021, 24, 53–63. [Google Scholar] [PubMed]
- Robinson, P.G.; Gibson, B.; Khan, F.A.; Birnbaum, W. A comparison of OHIP 14 and OIDP as interviews and questionnaires. Commun. Dent. Health. 2001, 18, 144–149. [Google Scholar]
- ISO 14577-1:2015; Metallic materials. Instrumented indentation test for hardness and materials parameters. Part 1: Test Method. ISO: Geneva, Switzerland, 2015.
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Bargellini, A.; Graziano, V.; Cugliari, G.; Deregibus, A.; Castroflorio, T. Effects on Sleep Bruxism Activity of Three Different Oral Appliances: One Year Longitudinal Cohort Study. Curr. Drug Deliv. 2022. epub ahead of printing. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tsukiyama, Y.; Kuwatsuru, R.; Koyano, K. The effect of intermittent use of occlusal splint devices on sleep bruxism: A 4-week observation with a portable electromyographic recording device. J. Oral. Rehabil. 2015, 42, 251–258. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Glaros, A.G.; Kato, T.; Koyano, K.; Lavigne, G.J.; de Leeuw, R.; Manfredini, D.; Svensson, P.; Winocur, E. Bruxism defined and graded: An international consensus. J. Oral. Rehabil. 2013, 40, 2–4. [Google Scholar] [CrossRef]
- Taira, M.; Maeda, Y.; Sawada, T.; Komatsu, M.; Kondo, H. Mechanical properties of 3D-printed thermoplastic materials for orthodontic retainers. Dent. Mat. J. 2020, 39, 607–613. [Google Scholar]
- Monzavi, A.; Li, W.; Li, Q.; Swain, M.V. Mechanical properties of 3D printed polymeric and ceramic orthodontic brackets. J. Mech. Behav. Biomed. Mat. 2018, 87, 277–285. [Google Scholar]
- Matinlinna, J.P.; Alomari, S.; Sadasivan, M.; Salehi, H.; Alagl, A.S.; Hamedani, S. Effect of material properties and manufacturing process of occlusal splints on wear resistance: A literature review. J. Prosth. Dent. 2020, 123, 46–52. [Google Scholar]
- Cheah, C.M.; Chua, C.K.; Tan, K.H.; Abu Bakar, M.S. Evaluation of mechanical properties and dimensional accuracy of 3D-printed occlusal splints. Int. J. Prosth. 2019, 32, 288–290. [Google Scholar]
- Hwang, Y.H.; Song, J.H.; Hong STKim, H.Y. Evaluation of the mechanical properties of 3D-printed occlusal splints made of biocompatible resin. Materials 2021, 14, 3867. [Google Scholar]
- Li, W.; Bai, S.; Zhou, J.; Xu, X.; Wang, Y. Comparison of mechanical properties between 3D-printed and traditionally polymerized occlusal splint materials. J. Prosth. Dent. 2020, 124, 98–105. [Google Scholar]
- Nayyer, M.; Savabi, O. Wear comparison of CAD/CAM milled and 3D printed occlusal splints. J. Prosth. Dent. 2020, 124, 639–645. [Google Scholar]
- Paradowska-Stolarz, A.; Wezgowiec, J.; Malysa, A.; Wieckiewicz, M. Effects of Polishing and Artificial Aging on Mechanical Properties of Dental LT Clear® Resin. J. Funct. Biomater. 2023, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.G.; Janal, M.N.; Sirois, D.A.; Dubrovsky, B.; Wigren, P.E.; Klausner, J.J.; Krieger, A.C.; Lavigne, G.J. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J. Oral. Rehabil. 2013, 40, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Manfredini, D.; Ohrbach, R. Creating patients: How technology and measurement approaches are misused in diagnosis and convert healthy individuals into TMD patients. Front. Dent. Med. 2023, 4, 1183327. [Google Scholar] [CrossRef]

|
|
|
|
|
|
|
|
|
|
|
| Outcome Variable | Group | Recording Nights (T0–T2) | Average Values (SD) with Splint | Average Values (SD) without Splint |
|---|---|---|---|---|
| SB index | 3D splint | T0 | 4.8 (1.6) | 5.1 (1.3) |
| T1 | 6.1 (3) | 7.3 (3.8) | ||
| T2 | 3 (2.1) | 5.4 (5.4) | ||
| Control splint | T0 | 6 (2) | 5.7 (2) | |
| T1 | 4.3 (2.2) | 6.2 (2.4) | ||
| T2 | 5.3 (3.5) | 5.5 (3.5) | ||
| Total sMMA contractions | 3D splint | T0 | 76.3 (50) | 69.7 (43.7) |
| T1 | 221.4 (134.4) | 217 (147.6) | ||
| T2 | 255.6 (117.2) | 265.3 (156.6) | ||
| Control splint | T0 | 84 (41.5) | 87 (45.6) | |
| T1 | 150 (92) | 212.8 (213.6) | ||
| T2 | 129.1 (83) | 155 (71) | ||
| Phasic sMMA contractions | 3D splint | T0 | 20.8 (18.3) | 26.7 (13.4) |
| T1 | 60.4 (42.9) | 61.3 (45.2) | ||
| T2 | 60.2 (20.6) | 60.4 (36) | ||
| Control splint | T0 | 26.9 (14) | 20.3 (11.5) | |
| T1 | 46.5 (37.5) | 54.1 (62) | ||
| T2 | 85.8 (144.4) | 44.7 (26) | ||
| Phasic sMMA contractions, SB-related | 3D splint | T0 | 6.9 (4.2) | 6.1 (3.7) |
| T1 | 10.2 (8.2) | 11.8 (9.1) | ||
| T2 | 4.3 (4.8) | 6.7 (4.9) | ||
| Control splint | T0 | 12.8 (6.1) | 11.3 (4.7) | |
| T1 | 8.2 (7.6) | 8 (5.9) | ||
| T2 | 16 (32.9) | 7 (5.6) | ||
| Tonic sMMA contractions | 3D splint | T0 | 22.4 (20.8) | 23.5 (19.6) |
| T1 | 69.2 (51.6) | 67.3 (37.3) | ||
| T2 | 91.6 (51.3) | 79 (50.6) | ||
| Control splint | T0 | 20.2 (15.1) | 22.6 (16.1) | |
| T1 | 42.7 (29.4) | 64.7 (70.8) | ||
| T2 | 31 (31.8) | 38.2 (19.9) | ||
| Tonic sMMA contractions, SB-related | 3D splint | T0 | 8.1 (5.1) | 8.5 (4.3) |
| T1 | 12 (7) | 17.5 (11.5) | ||
| T2 | 7.7 (5.5) | 9.6 (8.4) | ||
| Control splint | T0 | 10.4 (8.2) | 9.8 (10.4) | |
| T1 | 9.6 (9) | 8.7 (7.8) | ||
| T2 | 19.3 (48.5) | 8.5 (4.6) |
| Outcome Variable | Estimate | F Value | Pr (>F) |
|---|---|---|---|
| SB index | Group | 0.918 | 0.340 |
| Time | 1.659 | 0.196 | |
| Total sMMA contractions | Group | 3.139 | 0.0797 |
| Time | 0.613 | 0.5438 | |
| Phasic sMMA contractions | Group | 0.276 | 0.600464 |
| Time | 6.335 | 0.002646 ** | |
| Phasic sMMA contractions, SB-related | Group | 2.965 | 0.0885 |
| Time | 0.093 | 0.9114 | |
| Tonic sMMA contractions | Group | 11.659 | 0.000952 *** |
| Time | 12.928 | 0.00001 *** | |
| Tonic sMMA contractions, SB-related | Group | 0.710 | 0.4015 |
| Time | 0.465 | 0.6296 |
| Outcome Variable | Estimate | F Value | Pr (>F) |
|---|---|---|---|
| SB index | Group | 0.113 | 0.737 |
| Time | 1.885 | 0.158 | |
| Total sMMA contractions | Group | 0.738 | 0.39247 |
| Time | 4.501 | 0.01365 * | |
| Phasic sMMA contractions | Group | 0.145 | 0.70456 |
| Time | 9.551 | 0.00017 *** | |
| Phasic sMMA contractions, SB-related | Group | 0.863 | 0.35535 |
| Time | 2.158 | 0.12136 | |
| Tonic sMMA contractions | Group | 1.802 | 0.18281 |
| Time | 13.176 | 0.000009 *** | |
| Tonic sMMA contractions, SB-related | Group | 1.929 | 0.16823 |
| Time | 2.350 | 0.10104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bargellini, A.; Mannari, E.; Cugliari, G.; Deregibus, A.; Castroflorio, T.; Es Sebar, L.; Serino, G.; Roggia, A.; Scotti, N. Short-Term Effects of 3D-Printed Occlusal Splints and Conventional Splints on Sleep Bruxism Activity: EMG–ECG Night Recordings of a Sample of Young Adults. J. Clin. Med. 2024, 13, 776. https://doi.org/10.3390/jcm13030776
Bargellini A, Mannari E, Cugliari G, Deregibus A, Castroflorio T, Es Sebar L, Serino G, Roggia A, Scotti N. Short-Term Effects of 3D-Printed Occlusal Splints and Conventional Splints on Sleep Bruxism Activity: EMG–ECG Night Recordings of a Sample of Young Adults. Journal of Clinical Medicine. 2024; 13(3):776. https://doi.org/10.3390/jcm13030776
Chicago/Turabian StyleBargellini, Andrea, Elena Mannari, Giovanni Cugliari, Andrea Deregibus, Tommaso Castroflorio, Leila Es Sebar, Gianpaolo Serino, Andrea Roggia, and Nicola Scotti. 2024. "Short-Term Effects of 3D-Printed Occlusal Splints and Conventional Splints on Sleep Bruxism Activity: EMG–ECG Night Recordings of a Sample of Young Adults" Journal of Clinical Medicine 13, no. 3: 776. https://doi.org/10.3390/jcm13030776
APA StyleBargellini, A., Mannari, E., Cugliari, G., Deregibus, A., Castroflorio, T., Es Sebar, L., Serino, G., Roggia, A., & Scotti, N. (2024). Short-Term Effects of 3D-Printed Occlusal Splints and Conventional Splints on Sleep Bruxism Activity: EMG–ECG Night Recordings of a Sample of Young Adults. Journal of Clinical Medicine, 13(3), 776. https://doi.org/10.3390/jcm13030776










