Long-Term Kinetics of SARS-CoV-2 Neutralizing and Anti-Receptor Binding Domain Antibodies among Laboratory-Confirmed COVID-19 Cases in Delhi National Capital Region, India: A Prospective, One-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- da Rosa Mesquita, R.; Francelino Silva Junior, L.C.; Santos Santana, F.M.; Farias de Oliveira, T.; Campos Alcântara, R.; Monteiro Arnozo, G.; Rodrigues da Silva Filho, E.; Galdino dos Santos, A.G.; Oliveira da Cunha, E.J.; Salgueiro de Aquino, S.H.; et al. Clinical Manifestations of COVID-19 in the General Population: Systematic Review. Wien. Klin. Wochenschr. 2020, 133, 377–382. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 Detection: A Comprehensive Review of the FDA-EUA COVID-19 Testing Landscape. Biosens. Bioelectron. 2020, 165, 112454. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Zhao, R.; Gao, L.-J.; Gao, X.-F.; Wang, D.-P.; Cao, J.-M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Hotez, P.J.; Bottazzi, M.E. Potential for Developing a SARS-CoV Receptor-Binding Domain (RBD) Recombinant Protein as a Heterologous Human Vaccine against Coronavirus Infectious Disease (COVID)-19. Hum. Vaccin. Immunother. 2020, 16, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human Neutralizing Antibodies Elicited by SARS-CoV-2 Infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.A.P.M.; et al. Neutralizing Antibody Titres in SARS-CoV-2 Infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Kant, S.; Guleria, R.; Rai, S.; Jaiswal, A.; Mandal, S.; Medigeshi, G.; Ahmad, M.; Rahman, A.; Sangral, M.; et al. Antibody Response to SARS-CoV-2 among COVID-19 Confirmed Cases and Correlates with Neutralizing Assay in a Subgroup of Patients in Delhi National Capital Region, India. Vaccines 2022, 10, 1312. [Google Scholar] [CrossRef]
- India WHO. Coronavirus Disease (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int (accessed on 3 August 2023).
- India COVID-Coronavirus Statistics-Worldometer. Available online: https://www.worldometers.info/coronavirus/country/india/ (accessed on 3 August 2023).
- Lau, E.H.; Hui, D.S.; Tsang, O.T.; Chan, W.-H.; Kwan, M.Y.; Chiu, S.S.; Cheng, S.M.; Ko, R.L.; Li, J.K.; Chaothai, S.; et al. Long-Term Persistence of SARS-CoV-2 Neutralizing Antibody Responses after Infection and Estimates of the Duration of Protection. eClinicalMedicine 2021, 41, 101174. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 Infection Induces Long-Lived Bone Marrow Plasma Cells in Humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Haveri, A.; Ekström, N.; Solastie, A.; Virta, C.; Österlund, P.; Isosaari, E.; Nohynek, H.; Palmu, A.A.; Melin, M. Persistence of Neutralizing Antibodies a Year after SARS-CoV-2 Infection in Humans. Eur. J. Immunol. 2021, 51, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Pradenas, E.; Trinité, B.; Urrea, V.; Marfil, S.; Ávila-Nieto, C.; Rodríguez de la Concepción, M.L.; Tarrés-Freixas, F.; Pérez-Yanes, S.; Rovirosa, C.; Ainsua-Enrich, E.; et al. Stable Neutralizing Antibody Levels 6 Months after Mild and Severe COVID-19 Episodes. Med 2021, 2, 313–320.e4. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Choe, P.G.; Kim, K.H.; Kang, C.K.; Suh, H.J.; Kang, E.; Lee, S.Y.; Kim, N.J.; Yi, J.; Park, W.B.; Oh, M.D. Antibody Responses One Year after Mild SARS-CoV-2 Infection. J. Korean Med. Sci. 2021, 36, e157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.-H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally Enhanced Neutralizing Breadth against SARS-CoV-2 One Year after Infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Bruel, T.; Grzelak, L.; Guivel-Benhassine, F.; Staropoli, I.; Porrot, F.; Planchais, C.; Buchrieser, J.; Rajah, M.M.; Bishop, E.; et al. Sensitivity of Infectious SARS-CoV-2 B.1.1.7 and B.1.351 Variants to Neutralizing Antibodies. Nat. Med. 2021, 27, 917–924. [Google Scholar] [CrossRef]
- Breton, G.; Mendoza, P.; Hägglöf, T.; Oliveira, T.Y.; Schaefer-Babajew, D.; Gaebler, C.; Turroja, M.; Hurley, A.; Caskey, M.; Nussenzweig, M.C. Persistent Cellular Immunity to SARS-CoV-2 Infection. J. Exp. Med. 2021, 218, e20202515. [Google Scholar] [CrossRef]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis.. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 Infection Rates of Antibody-Positive Compared with Antibody-Negative Health-Care Workers in England: A Large, Multicentre, Prospective Cohort Study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2-Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.G.; Mann, P.; Kroidl, A.; Leroux-Roels, I.; Schindler, C.; Gabor, J.J.; Schunk, M.; Leroux-Roels, G.; Bosch, J.J.; Fendel, R.; et al. Safety and Immunogenicity of an mRNA-Lipid Nanoparticle Vaccine Candidate against SARS-CoV-2: A Phase 1 Randomized Clinical Trial. Wien. Klin. Wochenschr. 2021, 133, 931–941. [Google Scholar] [CrossRef]
| Variables | Round 1 n= 100 Frequency (%) | Round 2 n = 98 Frequency (%) | Round 3 n = 91 Frequency (%) | Round 4 n = 79 Frequency (%) |
|---|---|---|---|---|
| Residence Rural | 83 (83.0) | 82 (83.7) | 75 (82.4) | 68 (86.1) |
| Health care worker | 9 (9.0) | 8 (8.2) | 8 (8.8) | 5 (6.3) |
| Fever | 63 (63.0) | 16 (16.3) | 16 (17.6) | 13 (16.5) |
| Sore throat | 35 (35.0) | 10 (10.2) | 9 (9.9) | 8 (10.1) |
| Cough | 42 (42.0) | 13 (13.3) | 12 (13.2) | 11 (13.9) |
| Loss of taste | 24 (24.0) | 0 (0.0) | 2 (2.2) | 0 (0.0) |
| Running nose | 18 (18.0) | 9 (9.2) | 10 (11.0) | 6 (7.6) |
| Loss of appetite | 17 (17.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Loss of smell | 16 (16.0) | 1 (1.0) | 1 (1.1) | 0 (0.0) |
| Shortness of breath | 13 (13.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 12 (12.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Muscle ache | 12 (12.0) | 3 (3.1) | 0 (0.0) | 0 (0.0) |
| Headache | 11 (11.0) | 1 (1.0) | 0 (0.0) | 4 (5.1) |
| Joint ache | 10 (10.0) | 2 (2.0) | 1 (1.1) | 0 (0.0) |
| Nausea | 9 (9.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) |
| Chills | 8 (8.0) | 0 (0.0) | 2 (2.2) | 1 (1.3) |
| Vomiting | 7 (7.0) | 3 (3.1) | 1 (1.1) | 3 (3.8) |
| Diarrhoea | 4 (4.0) | 3 (3.1) | 1 (1.1) | 0 (0.0) |
| Conjunctivitis | 3 (3.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Rash | 2 (2.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Nose bleeding | 2 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Altered consciousness | 2 (2.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) |
| Seizure | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any symptoms | 70 (70.0) | 31 (31.6) | 23 (25.3) | 18 (22.8) |
| Sought medical treatment | 13 (13.0) | 2 (2.0) | 7 (7.7) | 0 (0.0) |
| Missed duty because of illness | 15 (15.0) | 2 (2.0) | 1 (1.1) | 1 (1.3) |
| Hospitalized | 10 (10.0) | 2 (2.0) | 5 (5.5) | 0 (0.0) |
| History of contact with COVID-19 case | 38 (38.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| COVID-19 vaccinated | 22 (22.0) | 61 (62.2) | 66 (72.5) | 74 (93.7) |
| COVID-19 testing | 100 (100) | 17 (17.4) | 2 (2.2) | 5 (6.3) |
| COVID-19 (+) report | 100 (100) | 15 (15.3) | 0 (0.0) | 1 (1.3) |
| RTPCR (+) | 76 (76) | 15 (15.3) | 0 (0.0) | 1 (1.3) |
| RAT (+) | 24 (24) | - | - | - |
| Test | Round of Follow-Up | n | Median (IQR) | p-Value(Friedman) | p-Value (Signed Rank) |
|---|---|---|---|---|---|
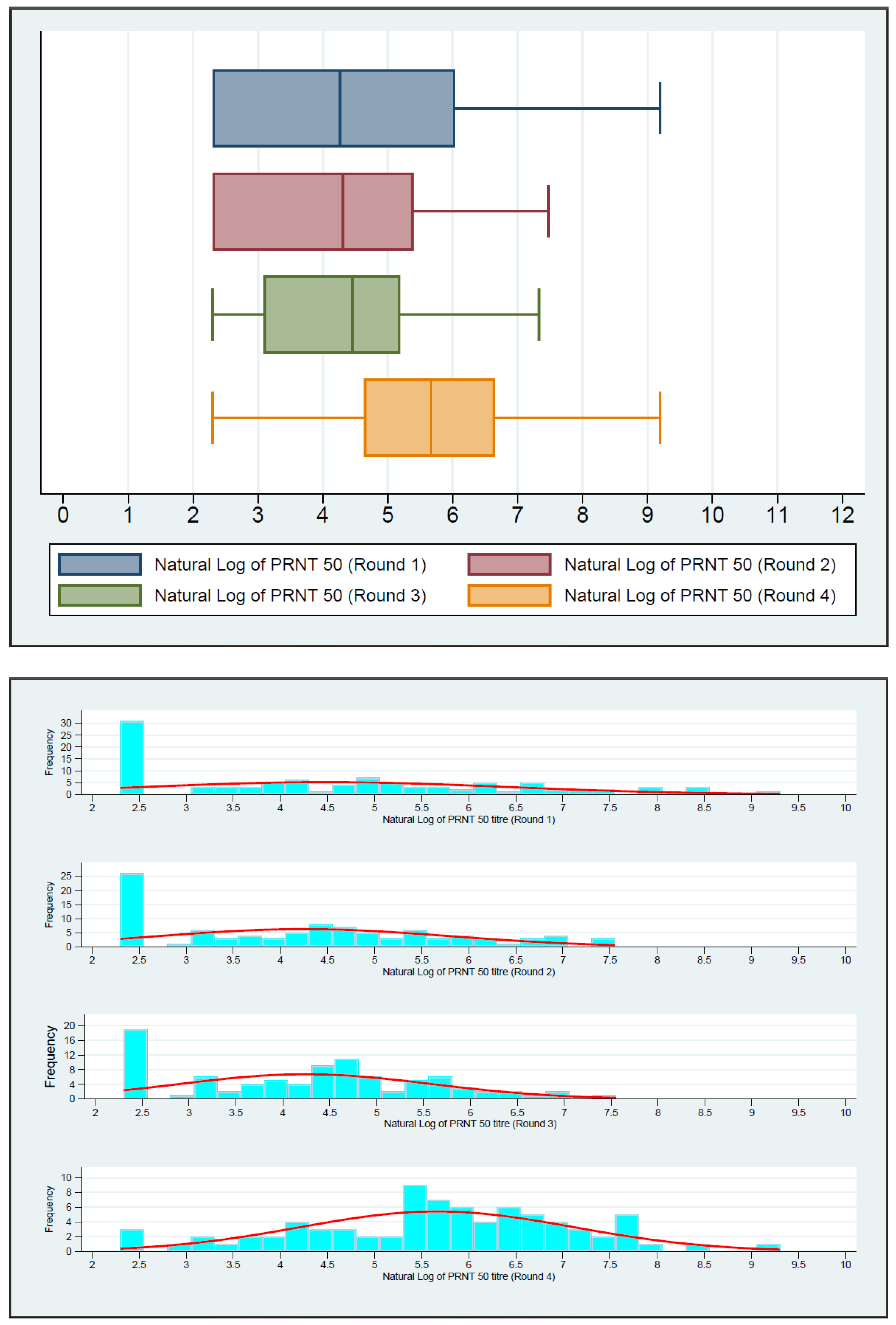
| PRNT50 titer | Round 1 | 100 | 71.00 (10.00–415.50) | <0.0001 | Ref. |
| Round 2 | 98 | 74.50 (10.00–219.00) | 0.0061 | ||
| Round 3 | 91 | 86.00 (22.00–180.00) | 0.0064 | ||
| Round 4 | 79 | 289.00 (103.00–770.00) | 0.0229 | ||
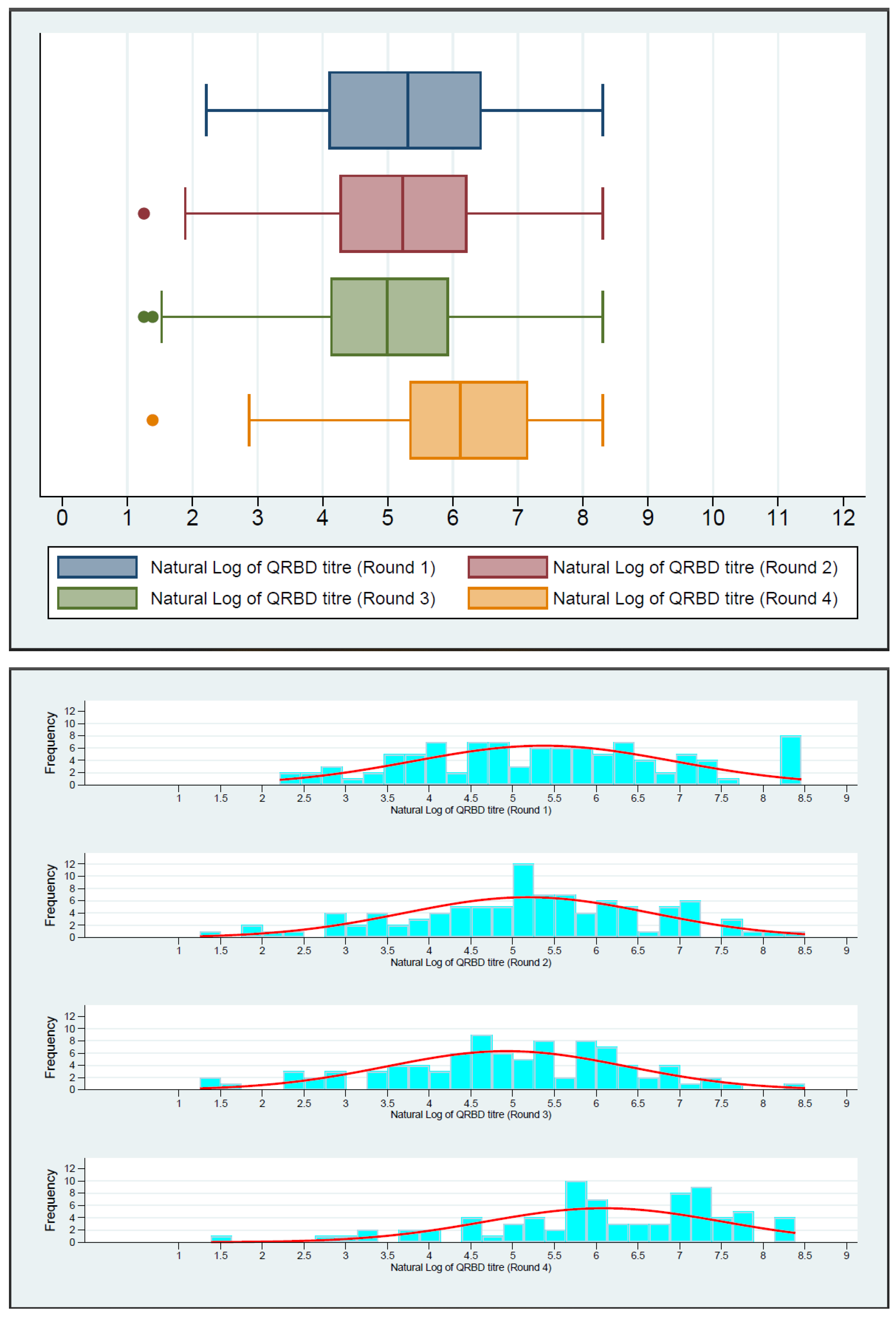
| QRBD | Round 1 | 100 | 201.97 (60.00–627.55) | <0.0001 | Ref. |
| Round 2 | 98 | 186.50 (70.96–503.80) | 0.0147 | ||
| Round 3 | 91 | 147.20 (61.50–378.80) | 0.0016 | ||
| Round 4 | 79 | 453.40 (206.80–1279.50) | 0.0089 | ||
| WANTAI | Round 1 | 100 | 3.45 (3.27–3.56) | 0.1448 | Ref. |
| Round 2 | 97 | 3.47 (3.41–3.54) | 0.0052 | ||
| Round 3 | 91 | 3.54 (3.50–3.63) | <0.0001 |
| Round 1 | Round 2 | Round 3 | Round 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency (%) | PRNT (Median (IQR)) | Frequency (%) | PRNT (Median (IQR)) | Frequency (%) | PRNT (Median (IQR)) | Frequency (%) | PRNT (Median (IQR)) | |
| Rural | 83 (83.0) | 67 (10–274) | 82 (83.7) | 63 (10–202) | 75 (82.4) | 75 (21–134) | 68 (86.1) | 272.5 (86–591) |
| Urban | 17 (17.0) | 218 (34–861) | 16 (17.6) | 103 (52.5–810) | 16 (17.6) | 114.5 (62.5–372) | 11 (13.9) | 796 (546–1695) |
| p-value | 0.0926 | 0.1301 | 0.0326 | 0.0065 | ||||
| Male | 64 (64.0) | 108 (10–497.5) | 62 (63.3) | 69.5 (26–241) | 56 (61.5) | 73.5 (24–216.5) | 49 (62.0) | 271 (91–770) |
| Female | 36 (36.0) | 54.5 (10–238) | 36 (36.7) | 75 (10–195) | 35 (38.5) | 95 (22–129) | 30 (38.0) | 359.5 (189–608) |
| p-value | 0.2179 | 0.5592 | 0.9282 | 0.3686 | ||||
| <40 years | 63 (63.0) | 45 (10–218) | 62 (63.3) | 61.5 (10–241) | 56 (61.5) | 76 (26–132) | 47 (59.5) | 342 (91–663) |
| ≥40 years | 37 (37.0) | 156 (45–770) | 36 (36.7) | 79 (17–207.5) | 35 (38.5) | 90 (10–204) | 32 (40.5) | 284 (145–1006) |
| p-value | 0.0023 | 0.8817 | 0.7963 | 0.8417 | ||||
| Health care worker (Yes) | 9 (9.0) | 861 (410–2922) | 8 (8.2) | 114 (79.5–1081.5) | 8 (8.8) | 114.5 (62.5–393.5) | 5 (6.3) | 796 (546–1695) |
| Health care worker (No) | 91 (91.0) | 58 (10–236) | 90 (91.8) | 63 (10–215) | 83 (91.2) | 80 (22–167) | 74 (93.7) | 284 (100–742) |
| p-value | 0.0003 | 0.0361 | 0.1319 | 0.1310 | ||||
| Hospitalized (+) | 90 (90.0) | 286 (10–770) | 96 (98.0) | 215 (74–791) | 86 (94.5) | 106 (90–272) | - | - |
| Hospitalized (−) | 10 (10.0) | 68.5 (10–279) | 2 (2.0) | 64 (10–200) | 5 (5.5) | 76 (22–167) | 79 (100.0) | 289 (103–770) |
| p-value | 0.3568 | 0.6756 | 0.7003 | - | ||||
| Any Symptoms (+) | 70 (70.0) | 71 (10–303) | 31 (31.6) | 62 (10–241) | 23 (25.3) | 99 (25–272) | 18 (22.8) | 347 (173–858) |
| Any Symptoms (−) | 30 (30.0) | 76 (10–494) | 67 (68.4) | 75 (24–219) | 68 (74.7) | 76 (22–141.5) | 61 (77.2) | 289 (103–749) |
| p-value | 0.7804 | 0.6027 | 0.3020 | 0.8123 | ||||
| COVID-19 Vaccinated (+) | 22 (22.0) | 590.0 (115.0–1204.0) | 60 (61.2) | 89.0 (28.5–295.0) | 66 (72.5) | 95.5 (44.0–257.0) | 74 (93.7) | 296.5 (135.0–749.0) |
| COVID-19 Vaccinated (−) | 78 (70.0) | 45.0 (10.0–197.0) | 38 (38.8) | 35.5 (10.0–107.0) | 25 (27.5) | 31.0 (10.0–97.0) | 5 (6.3) | 91.0 (43.0–1857.0) |
| p-value | 0.0001 | 0.0112 | 0.0043 | 0.6599 | ||||
| Round 1 | Round 2 | Round 3 | Round 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency (%) | QRBD (Median (IQR)) | Frequency (%) | QRBD (Median (IQR)) | Frequency (%) | QRBD (Median (IQR)) | Frequency (%) | QRBD (Median (IQR)) | |
| Rural | 83 (83.0) | 199.4 (55.4–612.1) | 82 (83.7) | 181.9 (64.8–487.9) | 75 (82.4) | 128.8 (46.6–357) | 68 (86.1) | 385.1 (199.8–1275.5) |
| Urban | 17 (17.0) | 434 (141.9–1183.4) | 16 (17.6) | 222.3 (122.4–714.3) | 16 (17.6) | 211.9 (117.3–899.7) | 11 (13.9) | 1005.8 (206.8–1690.3) |
| p-value | 0.0742 | 0.3817 | 0.0297 | 0.2602 | ||||
| Male | 64 (64.0) | 235.9 (53.8–664.2) | 62 (63.3) | 218.6 (84.1–487.9) | 56 (61.5) | 150.8 (62.4–377.6) | 49 (62.0) | 384 (181–1208.3) |
| Female | 36 (36.0) | 135.6 (74.3–562.4) | 36 (36.7) | 177.4 (45.6–518.6) | 35 (38.5) | 147.2 (50.1–430.2) | 30 (38.0) | 605.9 (311.7–1303.8) |
| p-value | 0.5709 | 0.7795 | 0.8162 | 0.2332 | ||||
| <40 years | 63 (63.0) | 129.5 (48.7–421.7) | 62 (63.3) | 175.7 (64.8–521.1) | 56 (61.5) | 151.6 (46.1–357.4) | 47 (59.5) | 375.5 (181.4–1272.8) |
| ≥40 years | 37 (37.0) | 414.8 (141.9–990.3) | 36 (36.7) | 218.6 (77.5–431.2) | 35 (38.5) | 146.7 (67.5– 430.2) | 32 (40.5) | 676.2 (263.7–1545.9) |
| p-value | 0.0042 | 0.8828 | 0.7784 | 0.3377 | ||||
| Health care worker (Yes) | 9 (9.0) | 798.4 (441.3–1415.9) | 8 (8.2) | 296.2 (187.6–639.4) | 8 (8.8) | 212.4 (136.4–749.8) | 5 (6.3) | 1204.9 (589.6–1976.3) |
| Health care worker (No) | 91 (91.0) | 184.7 (55.6–560.7) | 90 (91.8) | 178.3 (64.8–503.8) | 83 (91.2) | 140.6 (50.1–376.5) | 74 (93.7) | 405.1 (206.8–1278.3) |
| p-value | 0.0045 | 0.1466 | 0.1194 | 0.2745 | ||||
| Hospitalized (+) | 90 (90.0) | 566 (126.1–1183.4) | 96 (98.0) | 236 (26.1–445.9) | 86 (94.5) | 231.9 (50.1–357) | - | - |
| Hospitalized (−) | 10 (10.0) | 195.2 (59.9–589.8) | 2 (2.0) | 186.5 (75.9–506.2) | 5 (5.5) | 146.9 (63.3–378.8) | 79 (100.0) | 453.4 (206.8–1279.5) |
| p-value | 0.2082 | 0.7242 | 0.8191 | - | ||||
| Any Symptoms (+) | 70 (70.0) | 235.9 (55.6–612.1) | 31 (31.6) | 180.5 (47.8–503.8) | 23 (25.3) | 156 (50.1–452.5) | 18 (22.8) | 830.4 (300.7–1976.3) |
| Any Symptoms (−) | 30 (30.0) | 180.1 (63.0–692.6) | 67 (68.4) | 189.6 (84.1–508.7) | 68 (74.7) | 138 (62.4–375.1) | 61 (77.2) | 424.6 (181.4–1272.8) |
| p-value | 0.7152 | 0.9878 | 0.5837 | 0.3775 | ||||
| COVID-19 Vaccinated (+) | 22 (22.0) | 718.1 (441.3–1415.9) | 60 (61.2) | 284.1 (122.8–859.8) | 66 (72.5) | 197.5 (95.2–498.9) | 74 (93.7) | 488.0 (218.3–1278.3) |
| COVID-19 Vaccinated (−) | 78 (70.0) | 131.0 (52.3–372.3) | 38 (38.8) | 90.4 (29.3–180.5) | 25 (27.5) | 67.5 (26.2–140.6) | 5 (6.3) | 363.9 (46.4–1322) |
| p-value | <0.0001 | 0.0001 | 0.0004 | 0.6168 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misra, P.; Medigeshi, G.R.; Kant, S.; Jaiswal, A.; Ahmad, M.; Rahman, A.; Guleria, R.; Rai, S.K.; Deori, T.J.; Mandal, S.; et al. Long-Term Kinetics of SARS-CoV-2 Neutralizing and Anti-Receptor Binding Domain Antibodies among Laboratory-Confirmed COVID-19 Cases in Delhi National Capital Region, India: A Prospective, One-Year Follow-Up Study. J. Clin. Med. 2024, 13, 762. https://doi.org/10.3390/jcm13030762
Misra P, Medigeshi GR, Kant S, Jaiswal A, Ahmad M, Rahman A, Guleria R, Rai SK, Deori TJ, Mandal S, et al. Long-Term Kinetics of SARS-CoV-2 Neutralizing and Anti-Receptor Binding Domain Antibodies among Laboratory-Confirmed COVID-19 Cases in Delhi National Capital Region, India: A Prospective, One-Year Follow-Up Study. Journal of Clinical Medicine. 2024; 13(3):762. https://doi.org/10.3390/jcm13030762
Chicago/Turabian StyleMisra, Puneet, Guruprasad R. Medigeshi, Shashi Kant, Abhishek Jaiswal, Mohammad Ahmad, Anisur Rahman, Randeep Guleria, Sanjay Kumar Rai, Trideep Jyoti Deori, Suprakash Mandal, and et al. 2024. "Long-Term Kinetics of SARS-CoV-2 Neutralizing and Anti-Receptor Binding Domain Antibodies among Laboratory-Confirmed COVID-19 Cases in Delhi National Capital Region, India: A Prospective, One-Year Follow-Up Study" Journal of Clinical Medicine 13, no. 3: 762. https://doi.org/10.3390/jcm13030762
APA StyleMisra, P., Medigeshi, G. R., Kant, S., Jaiswal, A., Ahmad, M., Rahman, A., Guleria, R., Rai, S. K., Deori, T. J., Mandal, S., Gongal, G., Bairwa, M., Haldar, P., Kumar, R., & Garg, N. (2024). Long-Term Kinetics of SARS-CoV-2 Neutralizing and Anti-Receptor Binding Domain Antibodies among Laboratory-Confirmed COVID-19 Cases in Delhi National Capital Region, India: A Prospective, One-Year Follow-Up Study. Journal of Clinical Medicine, 13(3), 762. https://doi.org/10.3390/jcm13030762






