Assessment of Blood Loss during Neuroendovascular Procedures
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Sum of Squares | F Ratio | p-Value |
|---|---|---|---|
| Post Op Complications | 0.6210223 | 0.7029 | 0.4030 |
| Age at Encounter | 3.4334950 | 3.8862 | 0.0504 |
| Sex | 2.3726727 | 2.6855 | 0.1032 |
| BMI | 0.6659901 | 0.7538 | 0.3865 |
| Length of Surgery | 2.7038987 | 3.0604 | 0.0821 |
| Ethnicity | 2.4589830 | 1.3916 | 0.2516 |
| Treatment Type | 1.0127829 | 1.1463 | 0.2859 |
| Artery Access Site | 0.1161092 | 0.1314 | 0.7174 |
| Sheath Size | 0.3251763 | 0.1227 | 0.9466 |
| Antiplatelet/Anticoagulant Use | 6.6142992 | 7.4863 | 0.0069 |
| Post Op Disposition | 5.4447867 | 2.0542 | 0.1083 |
References
- Alakbarzade, V.; Pereira, A.C. Cerebral catheter angiography and its complications. Pract. Neurol. 2018, 18, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, S.M.; Caplan, L.R. Current role of cerebral angiography in the diagnosis of cerebrovascular diseases. Am. J. Roentgenol. 1992, 159, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fifi, J.T.; Meyers, P.M.; Lavine, S.D.; Cox, V.; Silverberg, L.; Mangla, S.; Pile-Spellman, J. Complications of Modern Diagnostic Cerebral Angiography in an Academic Medical Center. J. Vasc. Interv. Radiol. 2009, 20, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Guinn, N.R.; Broomer, B.W.; White, W.; Richardson, W.; Hill, S.E. Comparison of visually estimated blood loss with direct hemoglobin measurement in multilevel spine surgery. Transfusion 2013, 53, 2790–2794. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.S.; Vlok, A.J.; Esterhuizen, T.M.; van der Horst, A. Prediction of hematocrit decline and the impact of peri-operative fluid use in lumbar spinal fusion surgery. Eur. Spine. J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, S.; Montane-Muntane, M.; Gambus, P.L.; Capitan, D.; Navarro-Ripoll, R.; Blasi, A. Perioperative blood loss: Estimation of blood volume loss or haemoglobin mass loss? Blood Transfus. 2020, 18, 20–29. [Google Scholar] [PubMed]
- Muñoz, M.; Franchini, M.; Liumbruno, G.M. The post-operative management of anaemia: More efforts are needed. Blood Transfus. 2018, 16, 324–325. [Google Scholar] [PubMed]
- Kalra, S.K.; Thilagar, B.; Khambaty, M.; Manjarrez, E. Post-operative Anemia After Major Surgery: A Brief Review. Curr. Emerg. Hosp. Med. Rep. 2021, 9, 89–95. [Google Scholar] [CrossRef]
- Franchini, M.; Muñoz, M. Towards the implementation of patient blood management across Europe. Blood Transfus. 2017, 15, 292–293. [Google Scholar]
- Glance, L.G.; Dick, A.W.; Mukamel, D.B.; Fleming, F.J.; Zollo, R.A.; Wissler, R.; Salloum, R.; Meredith, U.W.; Osler, T.M. Association between Intraoperative Blood Transfusion and Mortality and Morbidity in Patients Undergoing Noncardiac Surgery. Anesthesiology 2011, 114, 283–292. [Google Scholar] [CrossRef]
- Rai, A.T.; Domico, J. Routine pre-procedure laboratory testing for patients undergoing outpatient cerebral angiography is not indicated. J. NeuroInterv. Surg. 2013, 5, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Karski, J.M.; Mathieu, M.; Cheng, D.; Carroll, J.; Scott, G.J. Etiology of preoperative anemia in patients undergoing scheduled cardiac surgery. Can. J. Anaesth. 1999, 46, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Ereth, M.H.; Nuttall, G.A.; Orszulak, T.A.; Santrach, P.J.; Cooney, W.P.; Oliver, W.C. Blood loss from coronary angiography increases transfusion requirements for coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2000, 14, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, E.S.; Chun, H.J.; Hwang, Y.J.; Lee, J.H.; Kang, S.H.; Yoo, I.K.; Kim, S.H.; Choi, H.S.; Keum, B.; et al. Discharge hemoglobin and outcome in patients with acute nonvariceal upper gastrointestinal bleeding. Endosc. Int. Open 2016, 4, E865–E869. [Google Scholar] [CrossRef]
- Wallis, J.P.; Wells, A.W.; Whitehead, S.; Brewster, N. Recovery from post-operative anaemia. Transfus. Med. 2005, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.M.; Stampfl, U.; Bellemann, N.; Ramsauer, S.; Loenard, B.M.; Haferkamp, A.; Hallscheidt, P.; Richter, G.M.; Kauczor, H.U.; Radeleff, B.A. Patients with Life-Threatening Arterial Renal Hemorrhage: CT Angiography and Catheter Angiography with Subsequent Superselective Embolization. CardioVasc. Interv. Radiol. 2010, 33, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Grayson, A.D.; McCarron, E.E.; Pullan, D.M.; Desmond, M.J. Reexploration for bleeding after coronary artery bypass surgery: Risk factors, outcomes, and the effect of time delay. Ann. Thorac. Surg. 2004, 78, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Shahi, V.; Brinjikji, W.; Murad, M.H.; Asirvatham, S.J.; Kallmes, D.F. Safety of Uninterrupted Warfarin Therapy in Patients Undergoing Cardiovascular Endovascular Procedures: A Systematic Review and Meta-Analysis. Radiology 2016, 278, 383–394. [Google Scholar] [CrossRef]
- Chongprasertpon, N.; Zebrauskaite, A.; Coughlan, J.J.; Ibrahim, A.; Arnous, S.; Hennessy, T.; Kiernan, T.J. Performing diagnostic radial access coronary angiography on uninterrupted direct oral anticoagulant therapy: A prospective analysis. Open Heart 2019, 6, e001026. [Google Scholar] [CrossRef]
- Troy, A.; Anderson, T.S. National Trends in Use of and Spending on Oral Anticoagulants among US Medicare Beneficiaries From 2011 to 2019. JAMA Health Forum 2021, 2, e211693. [Google Scholar] [CrossRef]
- Shaban, S.; Rastogi, A.; Phuyal, S.; Huasen, B.; Haridas, A.; Zelenak, K.; Iacobucci, M.; Martínez-Galdámez, M.; Jabbour, P.; Bhaskar, S.M.M. The association of transradial access and transfemoral access with procedural outcomes in acute ischemic stroke patients receiving endovascular thrombectomy: A meta-analysis. Clin. Neurol. Neurosurg. 2022, 215, 107209. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, E.; Biondi-Zoccai, G.; Sciahbasi, A.; Politi, L.; Rigattieri, S.; Pendenza, G.; Summaria, F.; Patrizi, R.; Borghi, A.; Di Russo, C.; et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: The RIFLE-STEACS (Radial versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J. Am. Coll. Cardiol. 2012, 60, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.K.; Cross, D.T., 3rd; Pilgram, T.K.; Moran, C.J.; Derdeyn, C.P.; Dacey, R.G., Jr. Effect of antiplatelet therapy on thromboembolic complications of elective coil embolization of cerebral aneurysms. AJNR Am. J. Neuroradiol. 2007, 28, 1778–1782. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value Categorical Variables: N (%) Continuous Variables: Mean ± SD |
|---|---|
| Age (years) | 60.1 ± 15.2 |
| Male Gender | 105 (52.5) |
| Ethnicity | |
| Not Hispanic | 190 (95) |
| Hispanic | 6 (3.0) |
| Refused/Unknown | 4 (2.0) |
| Race | |
| Asian | 1 (0.5) |
| Black | 24 (12.0) |
| Hispanic | 3 (1.5) |
| Other/Unknown | 8 (4.0) |
| White | 164 (82.0) |
| BMI | 27.8 ± 6.6 |
| Pre-Operative Anticoagulant or Antiplatelet Use | 96 (48.0) |
| Duration between Pre-Op and Post-Op Measurements (Hours) | 18.7 ± 10.5 |
| Length of Surgery (Minutes) | 80.5 ± 45.3 |
| Pre-Op Hemoglobin/Hematocrit (g/dL) | 12.6/37.4 ± 2.2/6.4 |
| Pre-Op RBCs/WBCs | 4.4/9.3 ± 2.7/4.2 |
| Post-Op Hemoglobin/Hematocrit (g/dL) | 11.6/34.6 ± 2.0/5.7 |
| Hemoglobin < 7 | 2 (1.0) |
| Post-Op RBCs/WBCs | 3.8/9.4 ± 0.7/4.0 |
| Change in Hemoglobin/Hematocrit (g/dL) | −1.0/−2.8 ± 1.0/3.2 |
| Treatment Type | |
| DSA/No Treatment | 62 (31.0) |
| Treatment | 138 (69.0) |
| Coiling | 17 (8.5) |
| Coiling, Flow Diversion | 3 (1.5) |
| AVM Embolization | 5 (2.5) |
| Fistula Embolization | 13 (6.5) |
| Flow Diversion | 16 (8.0) |
| Flow Diversion, Fistula Embolization | 1 (0.5) |
| Flow Diversion, Onyx Embolization | 1 (0.5) |
| MVP Embolization | 1 (0.5) |
| Onyx Embolization | 17 (8.5) |
| Stenting/Angioplasty | 16 (8.0) |
| Stenting/Angioplasty, WEB Embolization | 3 (1.5) |
| Thrombectomy | 27 (13.5) |
| Thrombectomy, Stenting/Angioplasty | 3 (1.5) |
| WEB Embolization | 15 (7.5) |
| Arterial Access | |
| Radial | 67 (33.5) |
| Femoral | 131 (65.5) |
| Sheath Size (French) | |
| 5 | 60 (30.0) |
| 6 | 51 (25.5) |
| 7 | 7 (3.5) |
| 8 | 71 (35.5) |
| Post-Op Disposition | |
| Floor | 9 (4.5) |
| Home | 11 (5.5) |
| ICU | 170 (85.0) |
| Intermediate | 8 (4.0) |
| Post-Op Complications | 5 (2.5) |
| Death | 1 (0.5) |
| Groin hematoma | 1 (0.5) |
| ICH | 3 (1.5) |
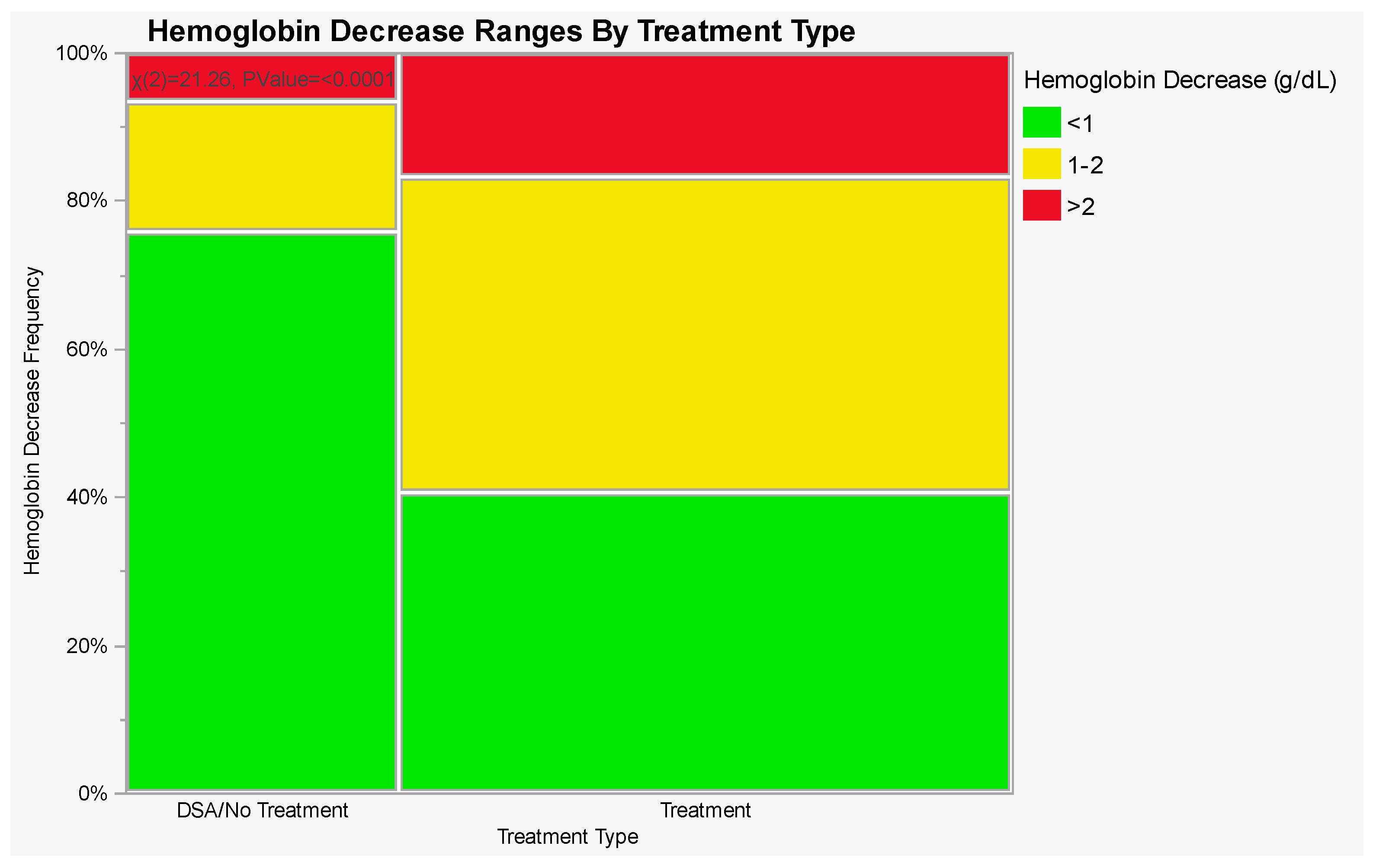
| Treatment Type | Hemoglobin Decrease (g/dL) | ||
|---|---|---|---|
| <1 | 1–2 | >2 | |
| Diagnostic Angiograms (% of Total) | 47 (75.8%) | 11 (17.7%) | 4 (6.5%) |
| Endovascular Interventions (% of Total) | 56 (40.6%) | 59 (42.8%) | 23 (16.6%) |
| Variable | Estimate | Std Error | t Ratio | p-Value |
|---|---|---|---|---|
| Age (years) | −0.0099 | 0.0050 | −1.97 | 0.050 |
| Gender | ||||
| Female | −0.12 | 0.073 | −1.64 | 0.10 |
| Ethnicity | 0.25 a | |||
| Hispanic | −0.36 | 0.31 | −1.16 | 0.25 |
| Not Hispanic | −0.22 | 0.23 | −0.94 | 0.35 |
| BMI | 0.0010 | 0.011 | 0.87 | 0.39 |
| Pre-Operative Antiplatelet/Anticoagulant | ||||
| None | 0.20 | 0.073 | 2.74 | 0.0069 |
| Length of Surgery (minutes) | −0.0032 | 0.0018 | −1.75 | 0.082 |
| Treatment Type | ||||
| DSA/No Treatment | 0.13 | 0.12 | 1.07 | 0.29 |
| Femoral Access | 0.033 | 0.091 | 0.36 | 0.72 |
| Sheath Size | 0.95 a | |||
| 5 French | −0.013 | 0.18 | −0.07 | 0.94 |
| 6 French | 0.044 | 0.15 | 0.29 | 0.78 |
| 7 French | 0.052 | 0.29 | 0.18 | 0.86 |
| Post-Op Disposition | 0.11 a | |||
| Floor | 0.59 | 0.29 | 2.05 | 0.042 |
| Home | −0.25 | 0.29 | −0.87 | 0.39 |
| ICU | −0.29 | 0.16 | −1.79 | 0.075 |
| Post Op Complications | ||||
| None | 0.19 | 0.23 | 0.84 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goutnik, M.; Nguyen, A.; Fleeting, C.; Patel, A.; Lucke-Wold, B.; Laurent, D.; Wahbeh, T.; Amini, S.; Al Saiegh, F.; Koch, M.; et al. Assessment of Blood Loss during Neuroendovascular Procedures. J. Clin. Med. 2024, 13, 677. https://doi.org/10.3390/jcm13030677
Goutnik M, Nguyen A, Fleeting C, Patel A, Lucke-Wold B, Laurent D, Wahbeh T, Amini S, Al Saiegh F, Koch M, et al. Assessment of Blood Loss during Neuroendovascular Procedures. Journal of Clinical Medicine. 2024; 13(3):677. https://doi.org/10.3390/jcm13030677
Chicago/Turabian StyleGoutnik, Michael, Andrew Nguyen, Chance Fleeting, Aashay Patel, Brandon Lucke-Wold, Dimitri Laurent, Tamara Wahbeh, Shawna Amini, Fadi Al Saiegh, Matthew Koch, and et al. 2024. "Assessment of Blood Loss during Neuroendovascular Procedures" Journal of Clinical Medicine 13, no. 3: 677. https://doi.org/10.3390/jcm13030677
APA StyleGoutnik, M., Nguyen, A., Fleeting, C., Patel, A., Lucke-Wold, B., Laurent, D., Wahbeh, T., Amini, S., Al Saiegh, F., Koch, M., Hoh, B., & Chalouhi, N. (2024). Assessment of Blood Loss during Neuroendovascular Procedures. Journal of Clinical Medicine, 13(3), 677. https://doi.org/10.3390/jcm13030677







