Screening for Occult Transthyretin Amyloidosis in Patients with Severe Aortic Stenosis and Amyloid Red Flags
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Procedures
2.3. Statistical Analysis
3. Results
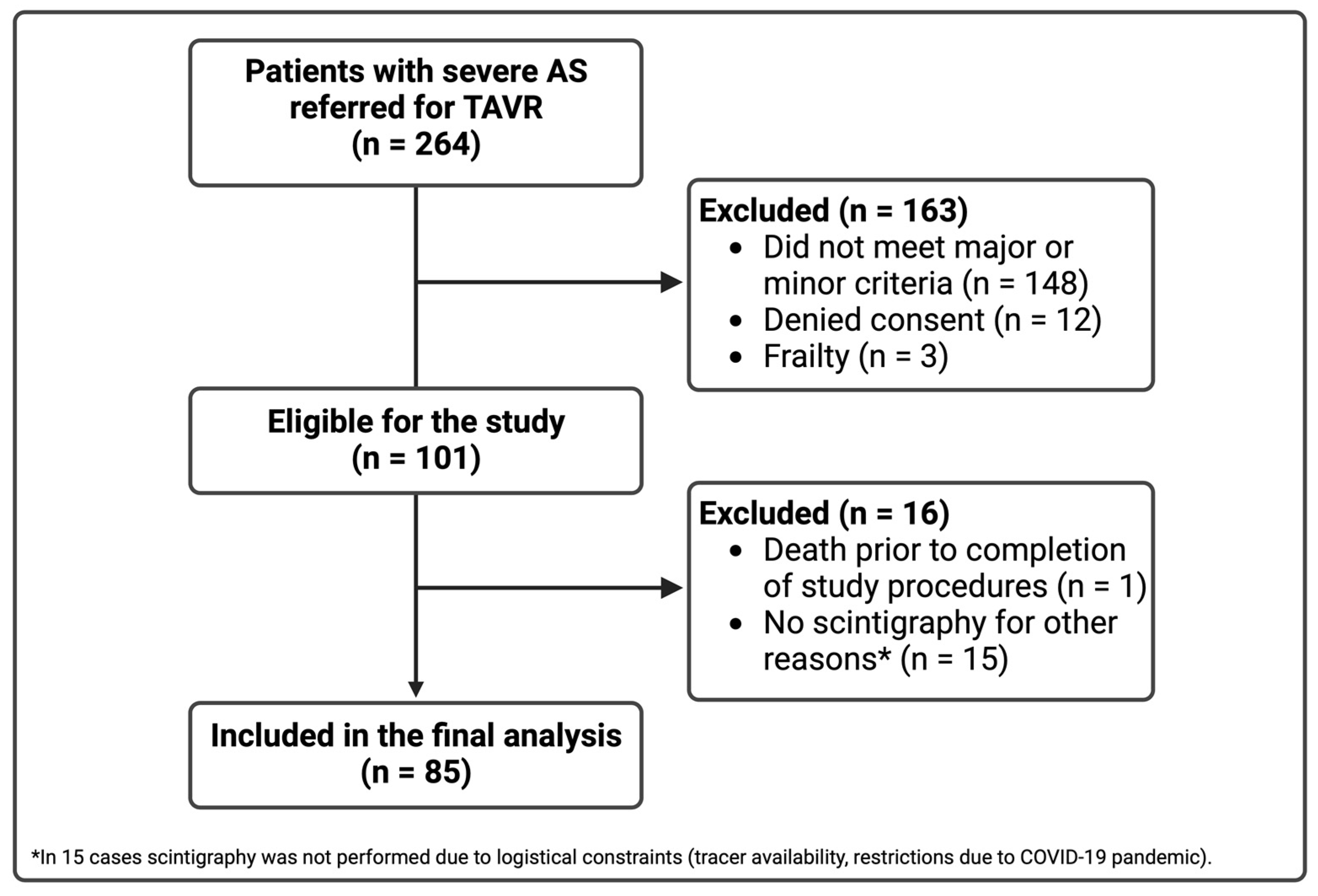
3.1. Study Population
3.2. Baseline Characteristics
3.3. The Prevalence of CA
3.4. Clinical Features of Lone AS vs. AS-ATTR
3.5. Periprocedural Complications and Mortality
4. Discussion
4.1. The Prevalence of ATTR-CA in Patients with Severe AS
4.2. The Predictive Value of Amyloid Red Flags
4.3. Prognostic Implications of Dual Pathology: AS and ATTR-CA
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS-ATTR | (dual pathology of) aortic stenosis and transthyretin-type cardiac amyloidosis |
| ATTR-CA | transthyretin-type cardiac amyloidosis |
| DPD | 99mtechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid |
| hs-cTnI | high-sensitivity cardiac troponin I |
| NT-proBNP | N-terminal pro B-type natriuretic peptide |
| SAVR | surgical aortic valve replacement |
| TAVR | transcatheter aortic valve replacement |
| TTR | transthyretin |
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.; Thourani, V.H.; Waksman, R. Transcatheter Aortic Valve Replacement in Intermediate- and Low-Risk Patients. J. Am. Heart Assoc. 2018, 7, e007147. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; Fontana, M.; Gilbertson, J.A.; Castelletti, S.; White, S.K.; Scully, P.R.; Roberts, N.; Hutt, D.F.; Rowczenio, D.M.; Whelan, C.J.; et al. Occult Transthyretin Cardiac Amyloid in Severe Calcific Aortic Stenosis: Prevalence and Prognosis in Patients Undergoing Surgical Aortic Valve Replacement. Circ. Cardiovasc. Imaging 2016, 9, e005066. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Narotsky, D.L.; Hamid, N.; Khalique, O.K.; Morgenstern, R.; DeLuca, A.; Rubin, J.; Chiuzan, C.; Nazif, T.; Vahl, T.; et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017, 38, 2879–2887. [Google Scholar] [CrossRef]
- Nitsche, C.; Aschauer, S.; Kammerlander, A.A.; Schneider, M.; Poschner, T.; Duca, F.; Binder, C.; Koschutnik, M.; Stiftinger, J.; Goliasch, G.; et al. Light-chain and transthyretin cardiac amyloidosis in severe aortic stenosis: Prevalence, screening possibilities, and outcome. Eur. J. Heart Fail. 2020, 22, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Patel, K.P.; Treibel, T.A.; Thornton, G.D.; Hughes, R.K.; Chadalavada, S.; Katsoulis, M.; Hartman, N.; Fontana, M.; Pugliese, F.; et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Scully, P.R.; Patel, K.P.; Kammerlander, A.A.; Koschutnik, M.; Dona, C.; Wollenweber, T.; Ahmed, N.; Thornton, G.D.; Kelion, A.D.; et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 128–139. [Google Scholar] [CrossRef]
- Patel, K.P.; Scully, P.R.; Nitsche, C.; Kammerlander, A.A.; Joy, G.; Thornton, G.; Hughes, R.; Williams, S.; Tillin, T.; Captur, G.; et al. Impact of afterload and infiltration on coexisting aortic stenosis and transthyretin amyloidosis. Heart 2022, 108, 67–72. [Google Scholar] [CrossRef]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I.L.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P.J.; et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann. Med. 2008, 40, 232–239. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Writing, C.; Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Sperry, B.W.; Jones, B.M.; Vranian, M.N.; Hanna, M.; Jaber, W.A. Recognizing Transthyretin Cardiac Amyloidosis in Patients With Aortic Stenosis: Impact on Prognosis. JACC Cardiovasc. Imaging 2016, 9, 904–906. [Google Scholar] [CrossRef]
- Geller, H.I.; Singh, A.; Alexander, K.M.; Mirto, T.M.; Falk, R.H. Association between ruptured distal biceps tendon and wild-type transthyretin cardiac amyloidosis. JAMA 2017, 318, 962–963. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Qin, L.; Shen, K.; Guan, H.; Ren, H.; Zhao, Y.; Guan, Y.; Zhou, D.; Peng, B.; Li, J.; et al. Light-Chain Amyloidosis With Peripheral Neuropathy as an Initial Presentation. Front. Neurol. 2021, 12, 707134. [Google Scholar] [CrossRef] [PubMed]
- Caponetti, A.G.; Rapezzi, C.; Gagliardi, C.; Milandri, A.; Dispenzieri, A.; Kristen, A.V.; Wixner, J.; Maurer, M.S.; Garcia-Pavia, P.; Tournev, I.; et al. Sex-Related Risk of Cardiac Involvement in Hereditary Transthyretin Amyloidosis: Insights from THAOS. JACC Heart Fail. 2021, 9, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 1 of 2-Evidence Base and Standardized Methods of Imaging. Circ. Cardiovasc. Imaging 2021, 14, e000029. [Google Scholar] [CrossRef]
- Okin, P.M.; Roman, M.J.; Devereux, R.B.; Pickering, T.G.; Borer, J.S.; Kligfield, P. Time-voltage QRS area of the 12-lead electrocardiogram: Detection of left ventricular hypertrophy. Hypertension 1998, 31, 937–942. [Google Scholar] [CrossRef][Green Version]
- Cipriani, A.; De Michieli, L.; Porcari, A.; Licchelli, L.; Sinigiani, G.; Tini, G.; Zampieri, M.; Sessarego, E.; Argiro, A.; Fumagalli, C.; et al. Low QRS Voltages in Cardiac Amyloidosis: Clinical Correlates and Prognostic Value. JACC CardioOncol. 2022, 4, 458–470. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef]
- Scully, P.R.; Treibel, T.A.; Fontana, M.; Lloyd, G.; Mullen, M.; Pugliese, F.; Hartman, N.; Hawkins, P.N.; Menezes, L.J.; Moon, J.C. Prevalence of Cardiac Amyloidosis in Patients Referred for Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Bansal, R.; Singh, A.; Dorbala, S.; Sharma, G.; Gupta, K.; Saxena, A.; Bhargava, B.; Karthikeyan, G.; Ramakrishnan, S.; et al. Concomitant Transthyretin Amyloidosis and Severe Aortic Stenosis in Elderly Indian Population: A Pilot Study. JACC CardioOncol. 2021, 3, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Makivic, N.; Stollberger, C.; Nakuz, T.; Schneider, B.; Schmid, C.; Hasun, M.; Weidinger, F. Reversible myocardial oedema due to acute myocardial infarction as differential diagnosis of cardiac transthyretin amyloidosis. ESC Heart Fail. 2020, 7, 1987–1991. [Google Scholar] [CrossRef] [PubMed]
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Sperry, B.W.; Reyes, B.A.; Ikram, A.; Donnelly, J.P.; Phelan, D.; Jaber, W.A.; Shapiro, D.; Evans, P.J.; Maschke, S.; Kilpatrick, S.E.; et al. Tenosynovial and Cardiac Amyloidosis in Patients Undergoing Carpal Tunnel Release. J. Am. Coll. Cardiol. 2018, 72, 2040–2050. [Google Scholar] [CrossRef]
- Fosbol, E.L.; Rorth, R.; Leicht, B.P.; Schou, M.; Maurer, M.S.; Kristensen, S.L.; Kober, L.; Gustafsson, F. Association of Carpal Tunnel Syndrome With Amyloidosis, Heart Failure, and Adverse Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2019, 74, 15–23. [Google Scholar] [CrossRef]
- Cappelli, F.; Zampieri, M.; Fumagalli, C.; Nardi, G.; Del Monaco, G.; Matucci Cerinic, M.; Allinovi, M.; Taborchi, G.; Martone, R.; Gabriele, M.; et al. Tenosynovial complications identify TTR cardiac amyloidosis among patients with hypertrophic cardiomyopathy phenotype. J. Intern. Med. 2021, 289, 831–839. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Rijal, S.; Abdelkarim, I.; Althouse, A.D.; Sharbaugh, M.S.; Fridman, Y.; Soman, P.; Forman, D.E.; Schindler, J.T.; Gleason, T.G.; et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J. Cardiovasc. Magn. Reson. 2017, 19, 98. [Google Scholar] [CrossRef]
- Rosenblum, H.; Masri, A.; Narotsky, D.L.; Goldsmith, J.; Hamid, N.; Hahn, R.T.; Kodali, S.; Vahl, T.; Nazif, T.; Khalique, O.K.; et al. Unveiling outcomes in coexisting severe aortic stenosis and transthyretin cardiac amyloidosis. Eur. J. Heart Fail. 2021, 23, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, T.; Jaswaney, R.; Arora, S.; Jain, A.; Arora, N.; Augusto Palma Dallan, L.; Yoon, S.; Najeeb Osman, M.; Filby, S.J.; Attizzani, G.F. Transcatheter aortic valve replacement in patients with aortic stenosis and cardiac amyloidosis. Int. J. Cardiol. Heart Vasc. 2022, 40, 101008. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Koschutnik, M.; Dona, C.; Radun, R.; Mascherbauer, K.; Kammerlander, A.; Heitzinger, G.; Dannenberg, V.; Spinka, G.; Halavina, K.; et al. Reverse Remodeling Following Valve Replacement in Coexisting Aortic Stenosis and Transthyretin Cardiac Amyloidosis. Circ. Cardiovasc. Imaging 2022, 15, e014115. [Google Scholar] [CrossRef] [PubMed]
- Kristen, A.V.; Schnabel, P.A.; Winter, B.; Helmke, B.M.; Longerich, T.; Hardt, S.; Koch, A.; Sack, F.U.; Katus, H.A.; Linke, R.P.; et al. High prevalence of amyloid in 150 surgically removed heart valves—A comparison of histological and clinical data reveals a correlation to atheroinflammatory conditions. Cardiovasc. Pathol. 2010, 19, 228–235. [Google Scholar] [CrossRef]
- Park, J.K.; Petrazzini, B.O.; Saha, A.; Vaid, A.; Vy, H.M.T.; Marquez-Luna, C.; Chan, L.; Nadkarni, G.N.; Do, R. Machine Learning Identifies Plasma Metabolites Associated With Heart Failure in Underrepresented Populations With the TTR V122I Variant. J. Am. Heart Assoc. 2023, 12, e027736. [Google Scholar] [CrossRef]
- Di Stefano, V.; Prinzi, F.; Luigetti, M.; Russo, M.; Tozza, S.; Alonge, P.; Romano, A.; Sciarrone, M.A.; Vitali, F.; Mazzeo, A.; et al. Machine Learning for Early Diagnosis of ATTRv Amyloidosis in Non-Endemic Areas: A Multicenter Study from Italy. Brain Sci. 2023, 13, 805. [Google Scholar] [CrossRef]
- Schrutka, L.; Anner, P.; Agibetov, A.; Seirer, B.; Dusik, F.; Rettl, R.; Duca, F.; Dalos, D.; Dachs, T.M.; Binder, C.; et al. Machine learning-derived electrocardiographic algorithm for the detection of cardiac amyloidosis. Heart 2022, 108, 1137–1147. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Zheng, J.-L.; Kuang, L.; Yan, H. Machine learning algorithms to automate differentiating cardiac amyloidosis from hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2023, 39, 339–348. [Google Scholar] [CrossRef]


| All n = 85 | Lone AS n = 79 | AS and ATTR-CA n = 6 | p-Value | |
|---|---|---|---|---|
| Major criteria | ||||
| CTS, n (%) | 24 (28) | 19 (24) | 5 (83) | 0.003 |
| Biceps tendon rupture, n (%) | 16 (19) | 15 (19) | 1 (17) | 0.887 |
| Spinal stenosis, n (%) | 16 (19) | 14 (18) | 2 (33) | 0.379 |
| NT-proBNP > 1000 pg/mL | 71 (84) | 65 (82) | 6 (100) | 0.134 |
| Hs-cTnI > 99th percentile | 20 (24) | 17 (22) | 3 (50) | 0.142 |
| Minor Criteria | ||||
| AF, n (%) | 50 (58.8) | 45 (57) | 5 (83) | 0.206 |
| Diastolic dysfunction ≥2 grade or lateral e’ <10 cm/s | 40 (52) | 35 (49) | 5 (83) | 0.094 |
| Atrioventricular block or previously implanted pacemaker | 25 (29) | 22 (28) | 3 (50) | 0.272 |
| Inclusion reason (criteria) | ||||
| 1 major | 21 (24.7) | 21 (26.6) | 0 (0) | 0.059 |
| 2–3 major | 24 (28.2) | 23 (29.1) | 1 (16.7) | 0.493 |
| 4–5 major | 1 (1.2) | 1 (1.3) | 0 (0) | 0.701 |
| 2 minor only | 3 (3.5) | 3 (3.8) | 0 (0) | 0.504 |
| 2 minor + ≥1 major | 36 (42.4) | 31 (39.2) | 5 (83.3) | 0.032 |
| All n = 85 | Lone AS n = 79 | AS and ATTR-CA n = 6 | p-Value | |
|---|---|---|---|---|
| Age, years | 81.7 ± 5.2 | 81.5 ± 5.2 | 84.8 ± 4.2 | 0.123 |
| Male sex, n (%) | 51 (60) | 45 (57) | 6 (100) | 0.011 |
| BMI, kg/m2 | 27.1 ± 4.9 | 27.3 ± 0.6 | 24.2 ± 1.5 | 0.138 |
| Euroscore II | 5.3 (3.9–8.4) | 5.3 (3.8–8.4) | 5.1 (4.4–15.6) | 0.498 |
| NYHA class, n (%) | 0.593 | |||
| I | 1 (1) | 1 (1) | 0 (0) | |
| II | 9 (11) | 9 (11) | 0 (0) | |
| III | 73 (86) | 67 (85) | 6 (100) | |
| IV | 2 (2) | 2 (3) | 0 (0) | |
| History of syncope, n (%) | 8 (9) | 5 (6) | 3 (50) | 0.010 |
| AS phenotype | ||||
| High gradient, n (%) | 54 (64) | 51 (65) | 3 (50) | 0.483 |
| LFLG with reduced EF, n (%) | 17 (20) | 15 (19) | 2 (33) | 0.425 |
| LFLG with preserved EF, n (%) | 13 (15) | 12 (15) | 1 (17) | 0.924 |
| Normal-flow low-gradient with preserved EF, n (%) | 1 (1) | 1 (1) | 0 (0) | 0.701 |
| Comorbidities | ||||
| Peripheral neuropathy, n (%) | 9 (11) | 8 (10) | 1 (17) | 0.638 |
| Pre-interventional pacemaker, n (%) | 11 (13) | 9 (11) | 2 (33) | 0.177 |
| Hypertension, n (%) | 76 (89) | 70 (89) | 6 (100) | 0.237 |
| Diabetes mellitus, n (%) | 19 (22) | 18 (23) | 1 (17) | 0.720 |
| Dyslipidaemia, n (%) | 51 (60) | 46 (58) | 5 (83) | 0.201 |
| Atrial fibrillation, n (%) | 50 (59) | 45 (57) | 5 (83) | 0.206 |
| Coronary artery disease, n (%) | 62 (73) | 57 (72) | 5 (83) | 0.534 |
| Previous stroke, n (%) | 6 (7) | 6 (8) | 0 (0) | 0.340 |
| Chronic kidney disease, n (%) | 36 (42) | 33 (42) | 3 (50) | 0.696 |
| Anemia, n (%) | 30 (35) | 27 (34) | 3 (50) | 0.444 |
| All n = 85 | Lone AS n = 79 | AS and ATTR-CA n = 6 | p-Value | |
|---|---|---|---|---|
| Laboratory testing | ||||
| NT-proBNP (pg/mL) | 2518 (1340.5–5319.5) | 2449 (1256–5274) | 4081.5 (1868.8–18771.5) | 0.181 |
| BNP (pg/mL) | 265.7 (163.3–420) | 251.6 (153–386) | 589.9 (294.1–1007.4) | 0.035 |
| Hs-cTnI (ng/L) | 18 (11–42.8) | 18 (10.8–41.3) | 47 (10.8–62.5) | 0.305 |
| eGFR (mL/min/1.73 m2) | 56.5 ± 20.8 | 56.7 ± 20.8 | 53.7 ± 21.6 | 0.739 |
| Creatinine (mg/dL) | 1.3 ± 0.7 | 1.3 ± 0.7 | 1.4 ± 0.5 | 0.807 |
| Haemoglobin (g/dL) | 11.9 ± 1.9 | 11.9 ± 1.9 | 11.3 ± 2.1 | 0.463 |
| Albumin (mg/L) | 3.6 ± 0.4 | 3.6 ± 0.5 | 3.8 ± 0.2 | 0.374 |
| Monoclonal immunoglobulin, n (%) | 17 (20) | 14 (18) | 3 (50) | 0.093 |
| Echocardiography | ||||
| LVEF (%) | 52 ± 8.8 | 52 ± 9.1 | 52 ± 5.1 | 0.992 |
| GLS (%) | −12.9 ± 3.6 | −13 ± 3.7 | −12.1 ± 2.4 | 0.624 |
| MCF (%) | 28.3 ± 13.3 | 27.4 ± 12 | 24.1 ± 12.7 | 0.524 |
| Pericardial effusion, n (%) | 10 (12) | 6 (8) | 4 (67) | <0.001 |
| LVDd (cm) | 4.8 ± 0.8 | 4.8 ± 0.9 | 4.7 ± 0.5 | 0.772 |
| RVDd (mm) | 4.10 ± 0.73 | 3.91 ± 0.71 | 4.5 ± 0.74 | 0.029 |
| IVSd (mm) | 16 ± 2.5 | 16 ± 2.4 | 18 ± 3.5 | 0.075 |
| LVMI (g/m2) | 150.6 ± 40.3 | 149.7 ± 40.5 | 162.1 ± 40.1 | 0.473 |
| LAVI (ml/m2) | 55.1 ± 23.4 | 55.5 ± 24.1 | 50.2 ± 11.7 | 0.590 |
| RA dimension (mm) | 4.4 ± 0.8 | 4.3 ± 0.7 | 5 ± 1.4 | 0.052 |
| SV (mL) | 69.9 ± 23.3 | 70.1 ± 23.4 | 67 ± 23.1 | 0.749 |
| Aortic valve area, cm2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.301 |
| AV MPG, mmHg | 39.9 ± 16.7 | 40.5 ± 17.1 | 31.1 ± 7.2 | 0.184 |
| AV Vmax, m/s | 4 ± 0.8 | 4.1 ± 0.8 | 3.4 ± 1 | 0.062 |
| E velocity (cm/s) | 102.3 ± 40.7 | 102 ± 41.5 | 106.1 ± 33.3 | 0.813 |
| E’ (cm/s) | 8.6 ± 2.8 | 8.6 ± 2.9 | 8.2 ± 1.5 | 0.756 |
| Lateral E/e’ | 11.9 ± 4.7 | 11.8 ± 4.8 | 13.6 ± 1.5 | 0.471 |
| Peak TR velocity, (m/s) | 2.9 (2.5–3.4) | 2.9 (2.6–3.4) | 2.7 (2.3–3.6) | 0.557 |
| sPAP (mmHg) | 42 ± 15.7 | 42.1 ± 14.9 | 41.7 ± 24.2 | 0.969 |
| TAPSE (mm) | 20.2 ± 5.4 | 20.2 ± 5.5 | 19.5 ± 4.7 | 0.749 |
| Right ventricular s’ (cm/s) | 10.2 ± 2.1 | 10.4 ± 2.3 | 9.4 ± 0.9 | 0.403 |
| ECG | ||||
| Atrial fibrillation/flutter, n (%) | 29 (34) | 28 (35) | 1 (17) | 0.323 |
| Heart rate (beats/min) | 74 ± 12 | 74 ± 13 | 72 ± 10 | 0.693 |
| AV block ≥2 grade, n (%) | 17 (20) | 15 (19) | 2 (33) | 0.425 |
| PQ duration, ms * | 191 ± 42 | 188 ± 39 | 235 ± 69 | 0.062 |
| QRS duration, ms * | 110 ± 36 | 108 ± 33 | 139 ± 56 | 0.038 |
| RBBB, n (%) * | 13 (15) | 12 (15) | 1 (17) | 0.780 |
| LBBB, n (%) * | 10 (12) | 10 (13) | 0 (0) | 0.252 |
| LAFB, n (%) * | 15 (18) | 15 (19) | 0 (0) | 0.154 |
| Low QRS voltage, n (%) * | 9 (11) | 9 (13) | 0 (0) | 0.376 |
| Sokolow–Lyon index, mV * | 2.1 ± 0.8 | 2.1 ± 0.8 | 2.6 ± 0.2 | 0.259 |
| RWT/SaVR index * | 0.07 (0.05–0.1) | 0.07 (0.05–0.1) | 0.08 (0.06–0.1) | 0.398 |
| VMR, mV/g/m2 × 10−2 * | 1.5 ± 0.6 | 1.5 ± 0.7 | 1.7 ± 0.3 | 0.709 |
| All n = 85 | Lone AS n = 79 | AS and ATTR-CA n = 6 | p-Value | |
|---|---|---|---|---|
| DPD scintigraphy parameters | ||||
| Perugini grade, n (%) | <0.001 | |||
| 0 | 76 (89.4) | 76 (96.2) | 0 (0) | |
| 1 | 2 (2.4) | 2 (2.5) | 0 (0) | |
| 2 | 2 (2.4) | 1 (1.3) | 1 (16.7) | |
| 3 | 5 (5.9) | 0 (0) | 5 (83.3) | |
| H/CL ratio | 1.4 ± 0.6 | 1.2 ± 0.4 | 2.7 ± 0.9 | <0.001 |
| Radioactivity (MBq) | 567.5 ± 83.7 | 565.8 ± 85.8 | 589.7 ± 48.9 | 0.505 |
| All n = 85 | Lone AS n = 79 | AS and ATTR-CA n = 6 | p-Value | |
|---|---|---|---|---|
| New-onset conduction disorders warranting pacemaker implantation, n (%) * | 8/74 (10.8) | 8/70 (11.3) | 0/4 (0) | 0.332 |
| Access-site bleeding, n (%) | 6 (7.1) | 6 (7.7) | 0 (0) | 0.336 |
| Acute kidney injury, n (%) | 11 (12.9) | 11 (14.1) | 0 (0) | 0.186 |
| Stroke/TIA, n (%) | 1 (1.2) | 1 (1.3) | 0 (0) | 0.699 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakstaite, A.M.; Vogel, J.K.; Luedike, P.; Jánosi, R.A.; Carpinteiro, A.; Rischpler, C.; Herrmann, K.; Rassaf, T.; Papathanasiou, M. Screening for Occult Transthyretin Amyloidosis in Patients with Severe Aortic Stenosis and Amyloid Red Flags. J. Clin. Med. 2024, 13, 671. https://doi.org/10.3390/jcm13030671
Jakstaite AM, Vogel JK, Luedike P, Jánosi RA, Carpinteiro A, Rischpler C, Herrmann K, Rassaf T, Papathanasiou M. Screening for Occult Transthyretin Amyloidosis in Patients with Severe Aortic Stenosis and Amyloid Red Flags. Journal of Clinical Medicine. 2024; 13(3):671. https://doi.org/10.3390/jcm13030671
Chicago/Turabian StyleJakstaite, Aiste Monika, Julia Kirsten Vogel, Peter Luedike, Rolf Alexander Jánosi, Alexander Carpinteiro, Christoph Rischpler, Ken Herrmann, Tienush Rassaf, and Maria Papathanasiou. 2024. "Screening for Occult Transthyretin Amyloidosis in Patients with Severe Aortic Stenosis and Amyloid Red Flags" Journal of Clinical Medicine 13, no. 3: 671. https://doi.org/10.3390/jcm13030671
APA StyleJakstaite, A. M., Vogel, J. K., Luedike, P., Jánosi, R. A., Carpinteiro, A., Rischpler, C., Herrmann, K., Rassaf, T., & Papathanasiou, M. (2024). Screening for Occult Transthyretin Amyloidosis in Patients with Severe Aortic Stenosis and Amyloid Red Flags. Journal of Clinical Medicine, 13(3), 671. https://doi.org/10.3390/jcm13030671








