Intensification with Intravenous Ustekinumab in Refractory Crohn’s Disease
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, R.; Hu, P.J. The Future of IBD Therapy: Where Are We and Where Should We Go Next? Dig. Dis. 2016, 34, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J Crohns Colitis. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Gravina, A.G.; Cuomo, A.; Mucherino, C.; Sgambato, D.; Facchiano, A.; Granata, L.; Priadko, K.; Pellegrino, R.; de Filippo, F.R.; et al. Efficacy of ustekinumab in the treatment of patients with Crohn’s disease with failure to previous conventional or biologic therapy: A prospective observational real-life study. J. Physiol. Pharmacol. 2021, 72, 537–543. [Google Scholar] [CrossRef]
- Singh, S.; Murad, M.H.; Fumery, M.; Sedano, R.; Jairath, V.; Panaccione, R.; Sandborn, W.J.; Ma, C. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Colombel, J.F.; Ungaro, R. Review article: Predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 47, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Chateau, T.; Peyrin-Biroulet, L. Two cases of inflammatory bowel disease patients treated with ustekinumab 90 mg every 3 weeks. Inflamm. Bowel Dis 2020, 26, e7. [Google Scholar] [CrossRef]
- Fumery, M.; Peyrin-Biroulet, L.; Nancey, S.; Altwegg, R.; Gilletta, C.; Veyrard, P.; Bouguen, G.; Viennot, S.; Poullenot, F.; Filippi, J.; et al. Effectiveness and Safety of Ustekinumab Intensification At 90 Mg Every Four Weeks In Crohn’s Disease: A Multicenter Study. J. Crohn’s Colitis 2020, 15, 222–227. [Google Scholar] [CrossRef]
- Sedano, R.; Guizzetti, L.; McDonald, C.; Jairath, V. Intravenous Ustekinumab Reinduction Is Effective in Prior Biologic Failure Crohn’s Disease Patients Already on Every-4-Week Dosing. Clin. Gastroenterol. Hepatol. 2021, 19, 1497–1498.e1. [Google Scholar] [CrossRef]
- Ollech, J.E.; Normatov, I.; Peleg, N.; Wang, J.; Patel, S.A.; Rai, V.; Yi, Y.; Singer, J.; Dalal, S.R.; Sakuraba, A.; et al. Effectiveness of ustekinumab dose escalation in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2020, 19, 104–110. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef] [PubMed]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Afif, W.; Drobne, D.; Dubinsky, M.C.; Ferrante, M.; Irving, P.M.; Kamperidis, N.; Kobayashi, T.; Kotze, P.G.; Lambert, J.; et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: Unmet needs and future perspectives. Lancet Gastroenterol. Hepatol. 2022, 7, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, B.; Nitschmann, S. Ustekinumab als Therapeutikum bei Morbus Crohn: UNITI-IM-UNITI [Ustekinumab for the treatment of Crohn’s disease: UNITI-IM-UNITI]. Internist 2017, 58, 424–426. Erratum in Internist 2017, 58, 426. (In Germany) [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Yung, D.E.; Ma, C.; Pariente, B.; Wils, P.; Eliakim, R.; Ungar, B.; Ben-Horin, S.; Kopylov, U. Effectiveness and safety of Ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig Liver Dis. 2019, 51, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, S.; Ginard, D.; Marín-Jiménez, I.; Chaparro, M.; Sierra, M.; Aguas, M.; Sicilia, B.; García-Sánchez, V.; Suarez, C.; Villoria, A.; et al. Ustekinumab for the Treatment of Refractory Crohn’s Disease: The Spanish Experience in a Large Multicentre Open-label Cohort. Inflamm. Bowel Dis. 2016, 22, 1662–1669. [Google Scholar] [CrossRef]
- Eberl, A.; Hallinen, T.; Björkesten, C.-G.A.; Heikkinen, M.; Hirsi, E.; Kellokumpu, M.; Koskinen, I.; Moilanen, V.; Nielsen, C.; Nuutinen, H.; et al. Ustekinumab for Crohn’s disease: A nationwide real-life cohort study from Finland (FINUSTE). Scand. J. Gastroenterol. 2019, 54, 718–725. [Google Scholar] [CrossRef]
- Wils, P.; Bouhnik, Y.; Michetti, P.; Flourie, B.; Brixi, H.; Bourrier, A.; Allez, M.; Duclos, B.; Serrero, M.; Buisson, A.; et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: A multicentre experience. Aliment. Pharmacol. Ther. 2018, 47, 588–595. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rutgeerts, P.; Gasink, C.; Jacobstein, D.; Zou, B.; Johanns, J.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment. Pharmacol. Ther. 2018, 48, 65–77. [Google Scholar] [CrossRef]
- Meserve, J.; Ma, C.; Dulai, P.S.; Jairath, V.; Singh, S. Effectiveness of Reinduction and/or Dose Escalation of Ustekinumab in Crohn’s Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2728–2740.e1. [Google Scholar] [CrossRef]
- Vermeire, S.; Gils, A. Value of drug level testing and antibody assays in optimising biological therapy. Frontline Gastroenterol. 2013, 4, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Morita, Y.; Imai, T.; Takahashi, K.; Yoshida, A.; Bamba, S.; Inatomi, O.; Andoh, A. Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn’s disease. BMC Gastroenterol. 2022, 22, 195. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Jacobstein, D.; Szapary, P.; Johanns, J.; Gao, L.L.; Davis, H.M.; Hanauer, S.B.; Feagan, B.G.; et al. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients with Crohn’s Disease. Gastroenterology 2018, 154, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Pauwels, R.W.M.; van der Woude, C.J.; Doukas, M.; Oudijk, L.; Peppelenbosch, M.P.; Grohmann, U.; Crombag, M.B.S.; de Vries, A.C.; Fuhler, G.M. Ustekinumab Tissue and Serum Levels in Patients with Crohn’s Disease Are Closely Correlated Though Not Consistently Associated with Objective Response After Induction. Inflamm. Bowel Dis. 2022, 29, 1038–1046. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; D’Haens, G.; Panés, J.; Dignass, A.; Magro, F.; Nazar, M.; Le Bars, M.; Lahaye, M.; Ni, L.; et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): An open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol. Hepatol. 2022, 7, 294–306, Erratum in Lancet Gastroenterol. Hepatol. 2022, 7, e8. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Gasink, C.; Chan, D.; Lang, Y.; Pollack, P.; Colombel, J.-F.; Wolf, D.C.; Jacobstein, D.; Johanns, J.; Szapary, P.; et al. Efficacy of Ustekinumab for Inducing Endoscopic Healing in Patients with Crohn’s Disease. Gastroenterology 2018, 155, 1045–1058. [Google Scholar] [CrossRef]
- Kucharzik, T.; Wilkens, R.; Maconi, G.; A D’agostino, M.; Le Bars, M.; Nazar, M.; Sloan, S.; Lahaye, M.; Ni, L.; Ercole, E.; et al. DOP10 Intestinal ultrasound response and transmural healing after ustekinumab induction in Crohn’s disease: Week 16 interim analysis of the STARDUST trial substudy. J. Crohn’s Colitis 2020, 14, S046–S048. [Google Scholar] [CrossRef]
| Variables | Number of Patients, % | |||||
|---|---|---|---|---|---|---|
| Sex | Women | Men | ||||
| (12) 44.4% | (15) 55.5% | |||||
| Tobacco | No | Yes | Former smoker | |||
| (16) 59.3% | (6) 22.2% | (5) 18.5% | ||||
| Extraintestinal manifestations | No | Yes | ||||
| (22) 81.5% | (5) 18.5% | |||||
| Location (Montreal) | L1 | L2 | L3 | L4 | ||
| (18) 66.6% | (0), 0% | (6), 22.2% | (3), 11.1% | |||
| Phenotype (Montreal) | B1 | B2 | B3 | |||
| (6) 22.2% | (15) 55.5% | (6) 22.2% | ||||
| Perianal involvement | No | Yes | ||||
| (18) 66.7% | (9) 33.3% | |||||
| Previous surgeries | No | 1 | 2 | 3 | ||
| (18), 66.6% | (4), 14.8% | (4), 14.8% | (1), 3.7% | |||
| Concomitant immunosuppression | No | Yes | ||||
| (22), 81.4% | (5), 18.5% | |||||
| Variables | Basal | 12 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|
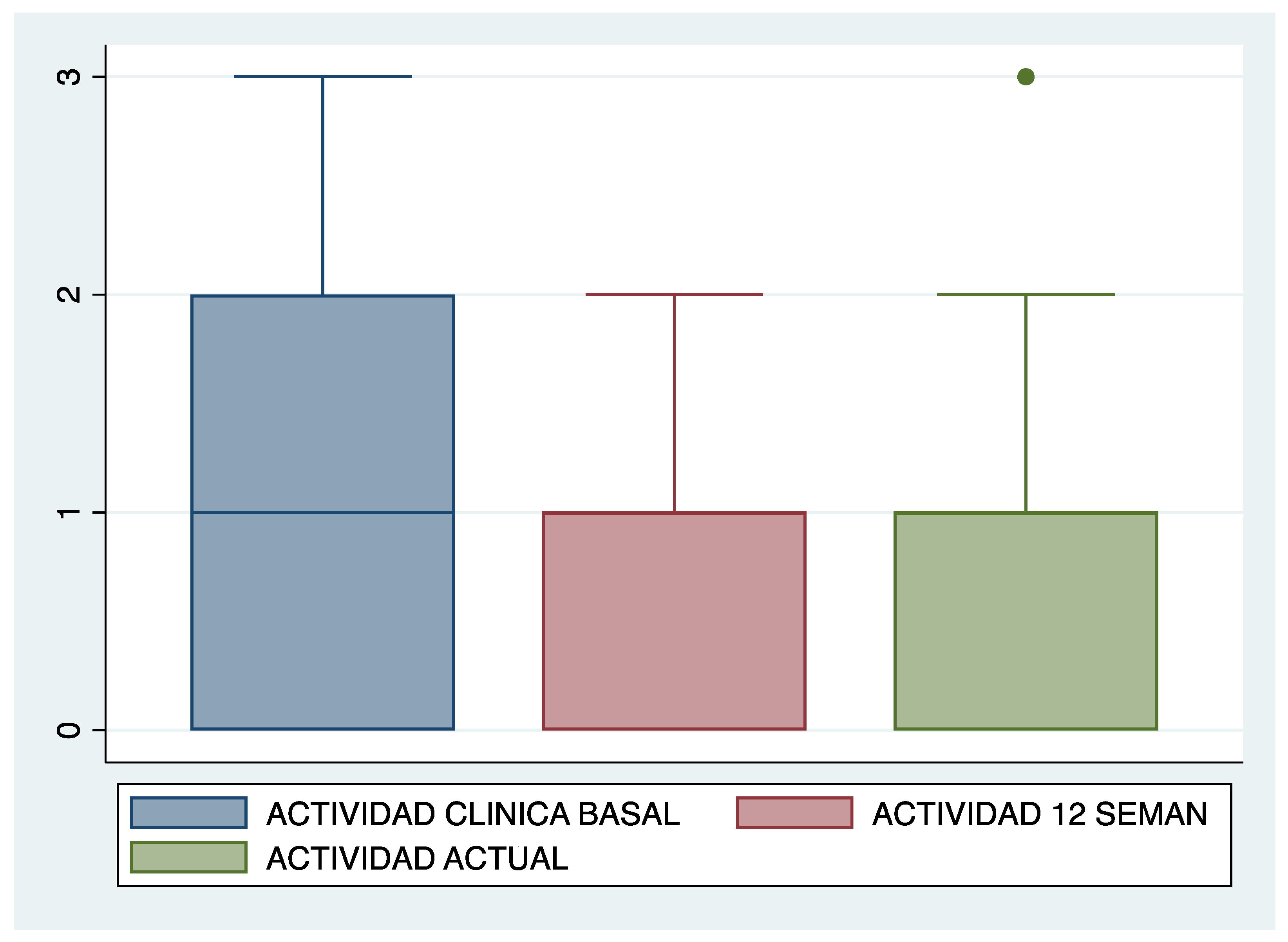
| Clinical activity (n, %) | No | Mild | Moderate | Serious | No | Mild | Moderate | Serious |
| 8 29.6% | 9 33.3% | 8 29.6% | 2 7.4% | 9 33.3% | 13 48.1% | 5 18.5% | 0 0% | |
| PCR | Median | I.Q.R. | Median | I.Q.R. | ||||
| 6.6 | 10.7 | 4.1 | 6.7 | |||||
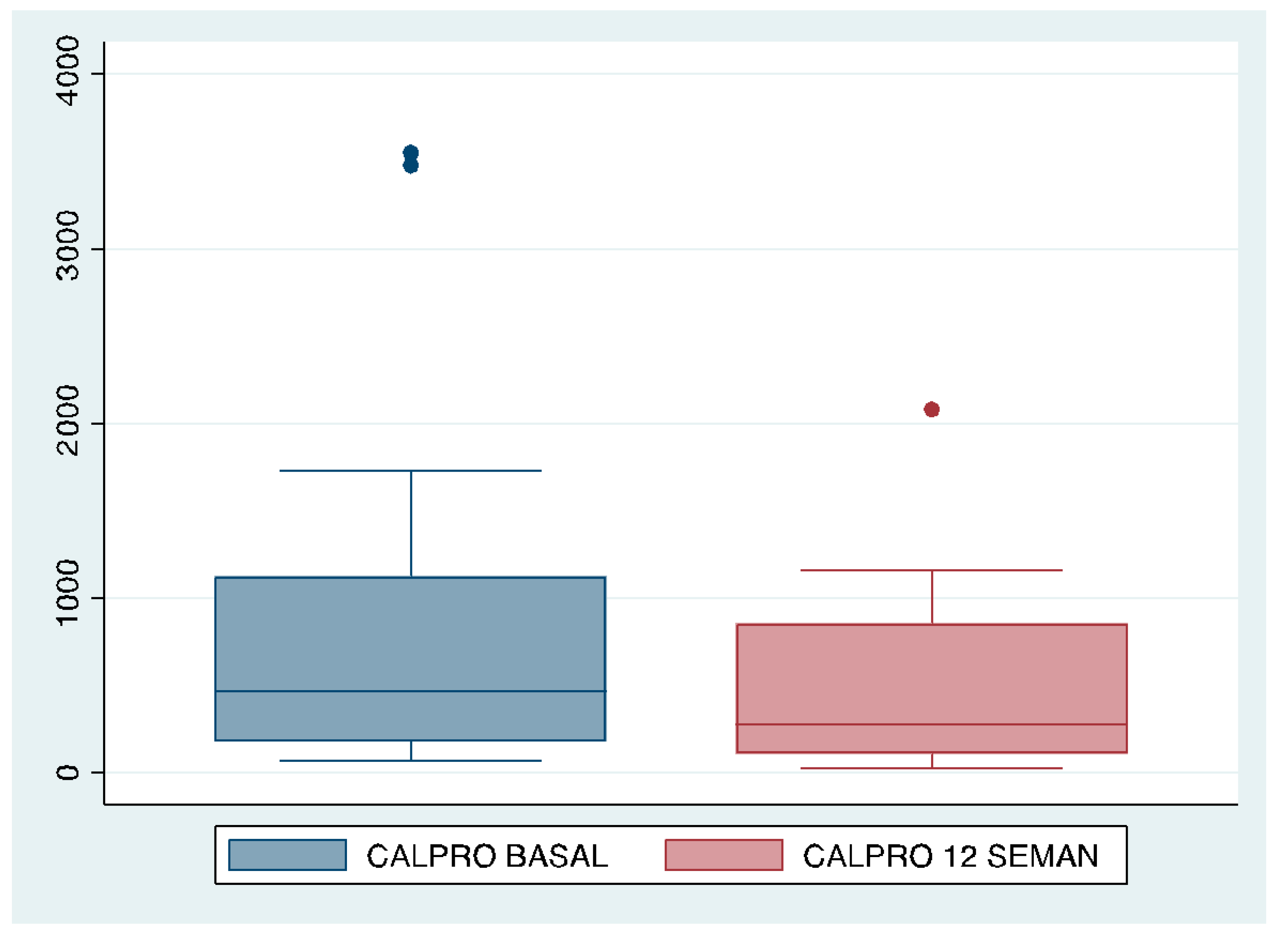
| Calprotectin | Median | I.Q.R. | Median | I.Q.R. | ||||
| 463 | 946.5 | 272.5 | 749.5 | |||||
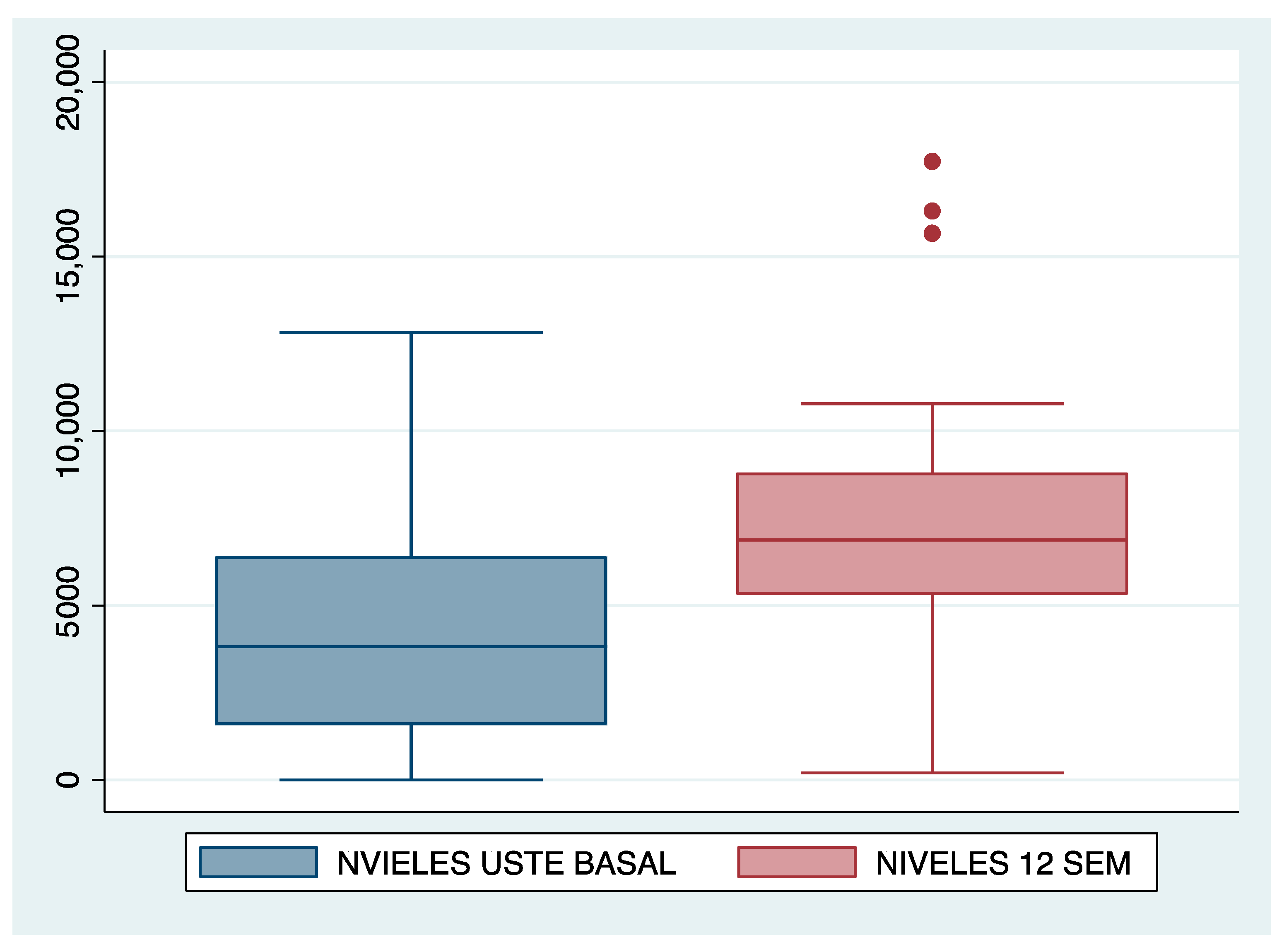
| UST levels | Median | I.Q.R. | Median | I.Q.R. | ||||
| 3810 | 4840 | 6870 | 3510 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez Ferrer, C.; Arroyo Argüelles, J.; Rueda García, J.L.; García Ramírez, L.; Martin Arranz, E.; Sánchez Azofra, M.; Poza Cordón, J.; Noci Belda, J.; Martin-Arranz, M.D. Intensification with Intravenous Ustekinumab in Refractory Crohn’s Disease. J. Clin. Med. 2024, 13, 669. https://doi.org/10.3390/jcm13030669
Suárez Ferrer C, Arroyo Argüelles J, Rueda García JL, García Ramírez L, Martin Arranz E, Sánchez Azofra M, Poza Cordón J, Noci Belda J, Martin-Arranz MD. Intensification with Intravenous Ustekinumab in Refractory Crohn’s Disease. Journal of Clinical Medicine. 2024; 13(3):669. https://doi.org/10.3390/jcm13030669
Chicago/Turabian StyleSuárez Ferrer, Cristina, José Arroyo Argüelles, Jose Luis Rueda García, Laura García Ramírez, Eduardo Martin Arranz, María Sánchez Azofra, Joaquín Poza Cordón, Jesús Noci Belda, and Maria Dolores Martin-Arranz. 2024. "Intensification with Intravenous Ustekinumab in Refractory Crohn’s Disease" Journal of Clinical Medicine 13, no. 3: 669. https://doi.org/10.3390/jcm13030669
APA StyleSuárez Ferrer, C., Arroyo Argüelles, J., Rueda García, J. L., García Ramírez, L., Martin Arranz, E., Sánchez Azofra, M., Poza Cordón, J., Noci Belda, J., & Martin-Arranz, M. D. (2024). Intensification with Intravenous Ustekinumab in Refractory Crohn’s Disease. Journal of Clinical Medicine, 13(3), 669. https://doi.org/10.3390/jcm13030669






