Regional Variations in the Incidence of Lichen Sclerosus in Sweden: Insights from a Nationwide Register Study (2001–2021)
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Study Population and Data Source
2.3. Swedish National Patient Register
2.4. Statistical Analysis
3. Results
3.1. Incidence by Region
3.2. Distribution of LSc Diagnoses by Medical Speciality
3.3. Marital Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Ghatage, P. Etiology, Clinical Features, and Diagnosis of Vulvar Lichen Sclerosus: A Scoping Review. Obstet. Gynecol. Int. 2020, 2020, 7480754. [Google Scholar] [CrossRef] [PubMed]
- Kirtschig, G. Lichen sclerosus: Symptoms, diagnosis, therapeutic procedures. Hautarzt 2018, 69, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kirtschig, G. Lichen Sclerosus-Presentation, Diagnosis and Management. Dtsch. Arztebl. Int. 2016, 113, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Beazley, R.; Uppal, E.; Kravvas, G.; Bunker, C. The age-related incidence of male genital lichen sclerosus is triphasic. Skin Health Dis. 2024, 4, e447. [Google Scholar] [CrossRef]
- Jerkovic Gulin, S.; Lundin, F.; Eriksson, O.; Seifert, O. Lichen Sclerosus—Incidence and Comorbidity: A Nationwide Swedish Register Study. J. Clin. Med. 2024, 13, 2761. [Google Scholar] [CrossRef]
- Lewis, F.M.; Tatnall, F.M.; Velangi, S.S.; Bunker, C.B.; Kumar, A.; Brackenbury, F.; Mohd Mustapa, M.F.; Exton, L.S.; McHenry, P.M.; Leslie, T.A.; et al. British Association of Dermatologists guidelines for the management of lichen sclerosus, 2018. Br. J. Dermatol. 2018, 178, 839–853. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D.A.; Papara, C.; Vorobyev, A.; Staiger, H.; Bieber, K.; Thaçi, D.; Ludwig, R.J. Lichen sclerosus: The 2023 update. Front. Med. 2023, 10, 1106318. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Barnardo, M.C.; Winsey, S.; Ahmad, T.; Cook, J.; Agudelo, J.D.; Zhai, N.; Powell, J.J.; Fuggle, S.V.; Wojnarowska, F. The association between HLA DR, DQ antigens, and vulval lichen sclerosus in the UK: HLA DRB1*12 and its associated DRB1*12/DQB1*0301/04/ 09/010 haplotype confers susceptibility to vulval lichen sclerosus, and HLA DRB1*0301/04 and its associated DRB1*0301/04/DQB1*0201/02/03 haplotype protects from vulval lichen sclerosus. J. Investig. Dermatol. 2005, 125, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Hasegawa, M. Lichen Sclerosus: A Current Landscape of Autoimmune and Genetic Interplay. Diagnostics 2022, 12, 3070. [Google Scholar] [CrossRef]
- Sherman, V.; McPherson, T.; Baldo, M.; Salim, A.; Gao, X.; Wojnarowska, F. The high rate of familial lichen sclerosus suggests a genetic contribution: An observational cohort study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1031–1034. [Google Scholar] [CrossRef]
- Dear, K.; Kravvas, G.; Sim, S.; Mastoraki, E.; James, M.; Watchorn, R.; Haider, A.; Ellery, P.; Freeman, A.; Alnajjar, H.M.; et al. Primary penile melanoma and genital lichen sclerosus. Skin Health Disease 2023, 3, e274. [Google Scholar] [CrossRef]
- Leis, M.; Singh, A.; Li, C.; Ahluwalia, R.; Fleming, P.; Lynde, C.W. Risk of Vulvar Squamous Cell Carcinoma in Lichen Sclerosus and Lichen Planus: A Systematic Review. J. Obstet. Gynaecol. Can. 2022, 44, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Lichen sclerosus and risk of cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, M.C.G.; Visser, P.J.; Overbeek, L.I.H.; Van Beurden, M.; Berkhof, J. Lichen sclerosus: Incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.M.; Ali, I.; Baldo, M.; Wojnarowska, F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: A case-control study. Arch. Dermatol. 2008, 144, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Andersson, E.; Ekbom, A.; Feychting, M.; Kim, J.L.; Reuterwall, C.; Heurgren, M.; Olausson, P.O. External review and validation of the Swedish national inpatient register. BMC Public Health 2011, 11, 450. [Google Scholar] [CrossRef]
- Kumar, K.S.; Morrel, B.; van Hees, C.L.M.; van der Toorn, F.; van Dorp, W.; Mendels, E.J. Comparison of lichen sclerosus in boys and girls: A systematic literature review of epidemiology, symptoms, genetic background, risk factors, treatment, and prognosis. Pediatr. Dermatol. 2022, 39, 400–408. [Google Scholar] [CrossRef]
- Murphy, R. Lichen sclerosus. Dermatol. Clin. 2010, 28, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Kerminen, S.; Martin, A.R.; Koskela, J.; Ruotsalainen, S.E.; Havulinna, A.S.; Surakka, I.; Palotie, A.; Perola, M.; Salomaa, V.; Daly, M.J.; et al. Geographic Variation and Bias in the Polygenic Scores of Complex Diseases and Traits in Finland. Am. J. Hum. Genet. 2019, 104, 1169–1181. [Google Scholar] [CrossRef]
- Svensson, D.; Rentoft, M.; Dahlin, A.M.; Lundholm, E.; Olason, P.I.; Sjödin, A.; Nylander, C.; Melin, B.S.; Trygg, J.; Johansson, E. A whole-genome sequenced control population in northern Sweden reveals subregional genetic differences. PLoS ONE 2020, 15, e0237721. [Google Scholar] [CrossRef]
- Ameur, A.; Dahlberg, J.; Olason, P.; Vezzi, F.; Karlsson, R.; Martin, M.; Viklund, J.; Kähäri, A.K.; Lundin, P.; Che, H.; et al. SweGen: A whole-genome data resource of genetic variability in a cross-section of the Swedish population. Eur. J. Hum. Genet. 2017, 25, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Allentoft, M.E.; Sikora, M.; Refoyo-Martínez, A.; Irving-Pease, E.K.; Fischer, A.; Barrie, W.; Ingason, A.; Stenderup, J.; Sjögren, K.G.; Pearson, A.; et al. Population genomics of post-glacial western Eurasia. Nature 2024, 625, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.A.; Tan, X.; Macri, C.J.; Goldstein, A.T.; Fu, S.W. Lichen sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int. J. Biol. Sci. 2019, 15, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Marren, P.; Jell, J.; Charnock, F.M.; Bunce, M.; Welsh, K.; Wojnarowska, F. The association between lichen sclerosus and antigens of the HLA system. Br. J. Dermatol. 1995, 132, 197–203. [Google Scholar] [CrossRef]
- Lipscombe, T.K.; Wayte, J.; Wojnarowska, F.; Marren, P.; Luzzi, G. A study of clinical and aetiological factors and possible associations of lichen sclerosus in males. Australas. J. Dermatol. 1997, 38, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Browning, S.R.; Browning, B.L. Population structure can inflate SNP-based heritability estimates. Am. J. Hum. Genet. 2011, 89, 191–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosenberg, N.A.; Pritchard, J.K.; Weber, J.L.; Cann, H.M.; Kidd, K.K.; Zhivotovsky, L.A.; Feldman, M.W. Genetic structure of human populations. Science 2002, 298, 2381–2385. [Google Scholar] [CrossRef]
- Hardy, J.; Singleton, A.; Gwinn-Hardy, K. Ethnic differences and disease phenotypes [3] (multiple letters). Science 2003, 300, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Angers, B.; Perez, M.; Menicucci, T.; Leung, C. Sources of epigenetic variation and their applications in natural populations. Evol. Appl. 2020, 13, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic Gulin, S.; Liljeberg, L.; Seifert, O. The impact of genital lichen sclerosus in men and women on quality of life: A prospective cohort study. Int. J. Womens Dermatol. 2024, 10, e131. [Google Scholar] [CrossRef] [PubMed]
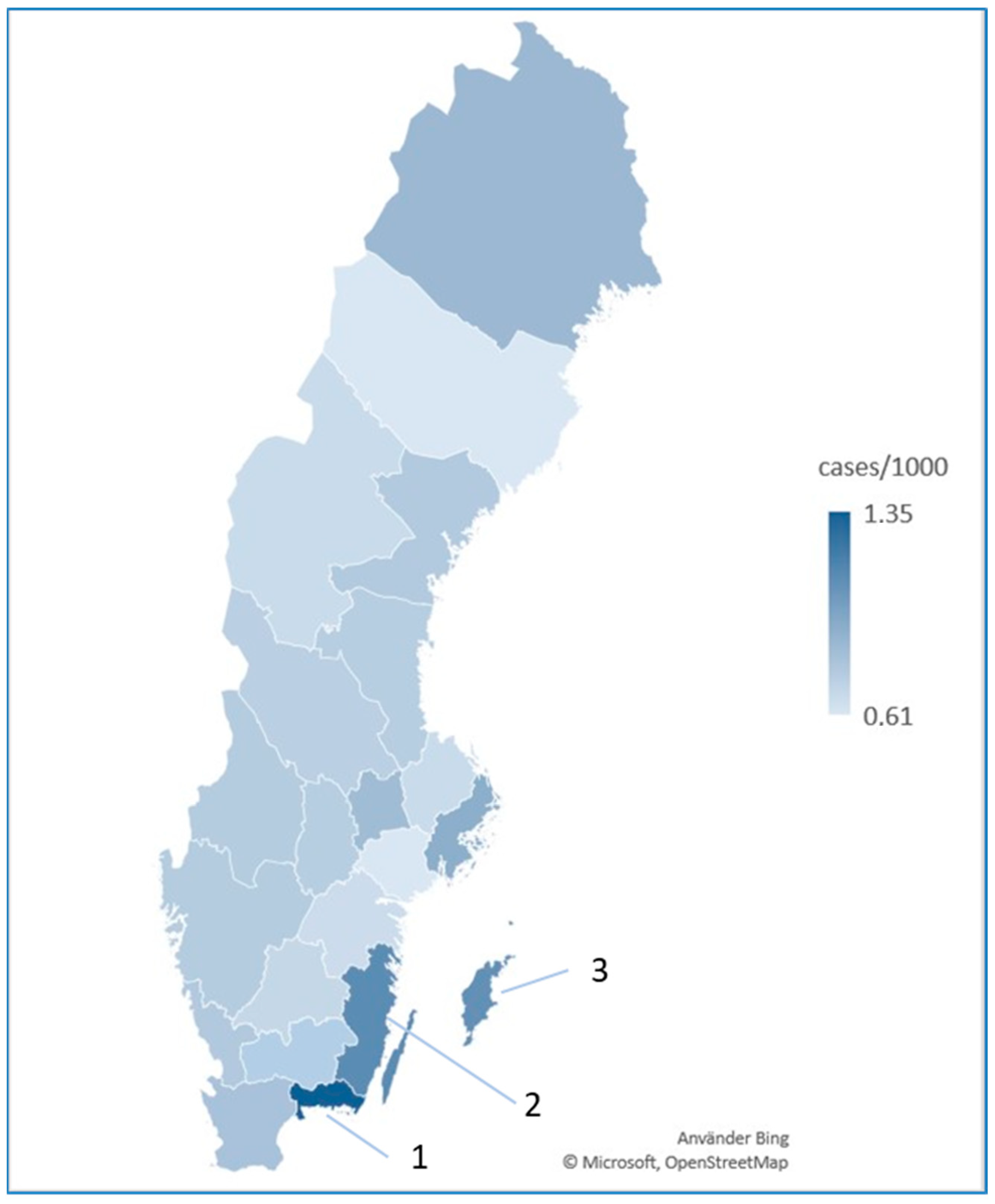
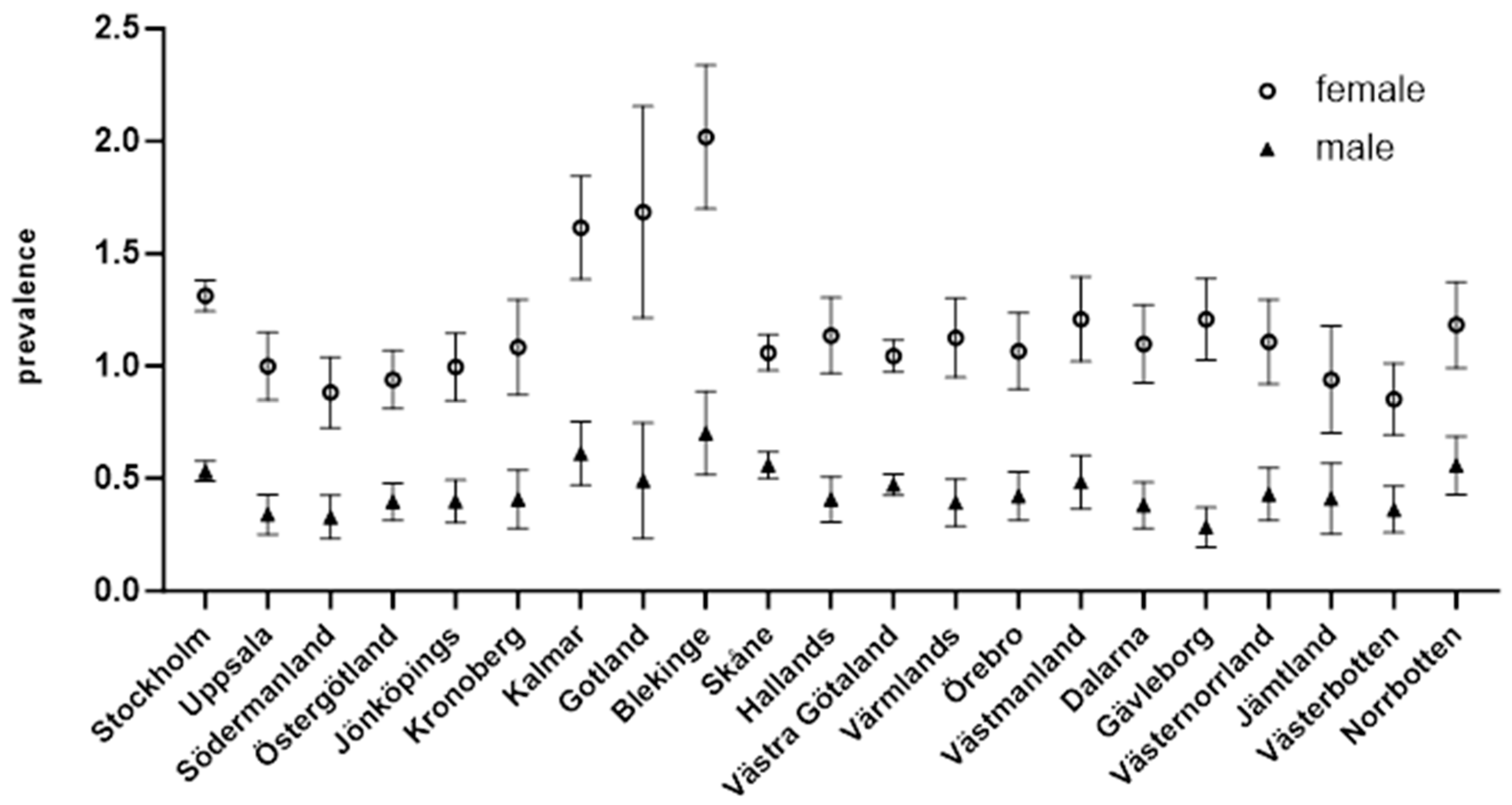
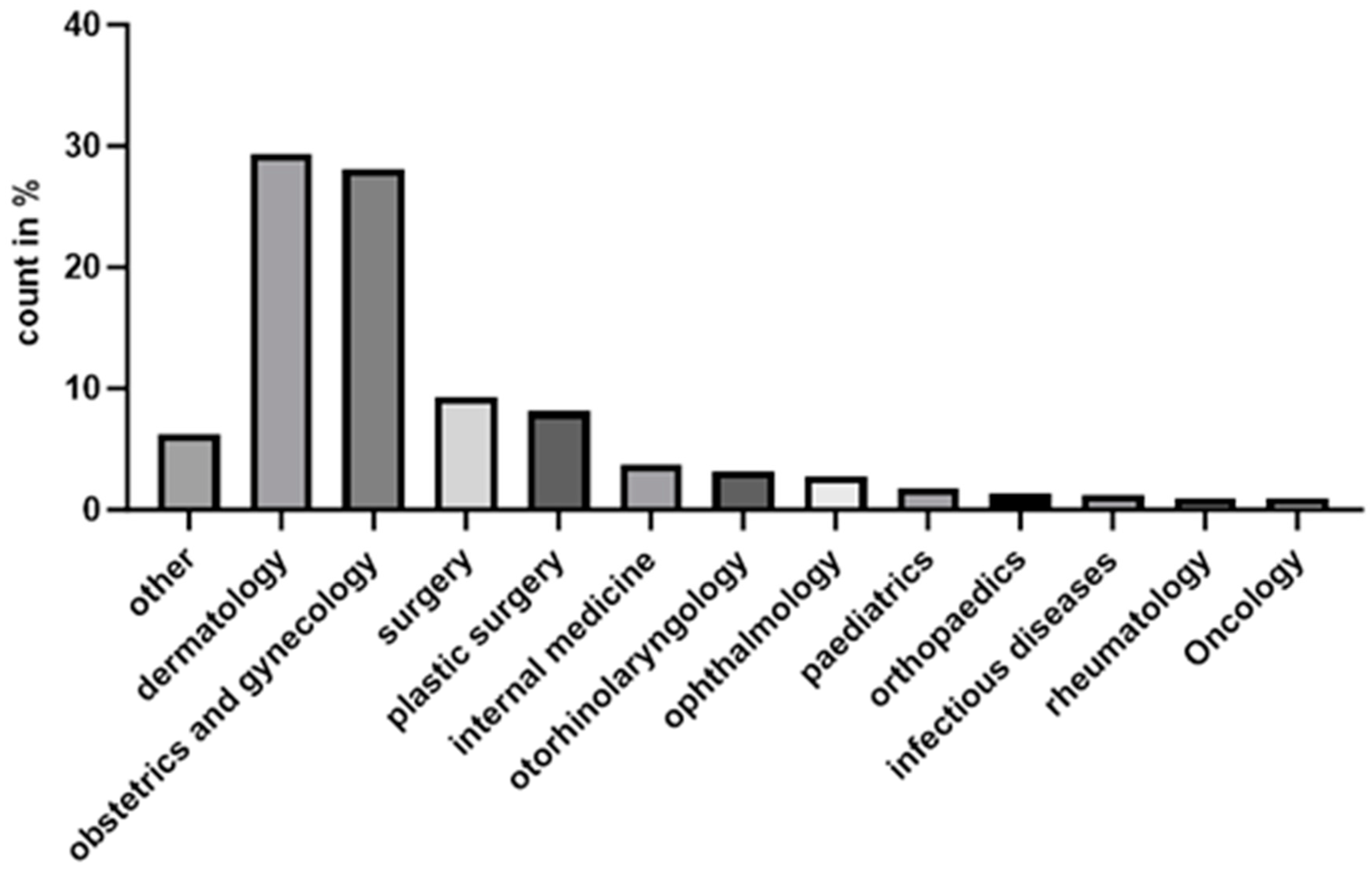
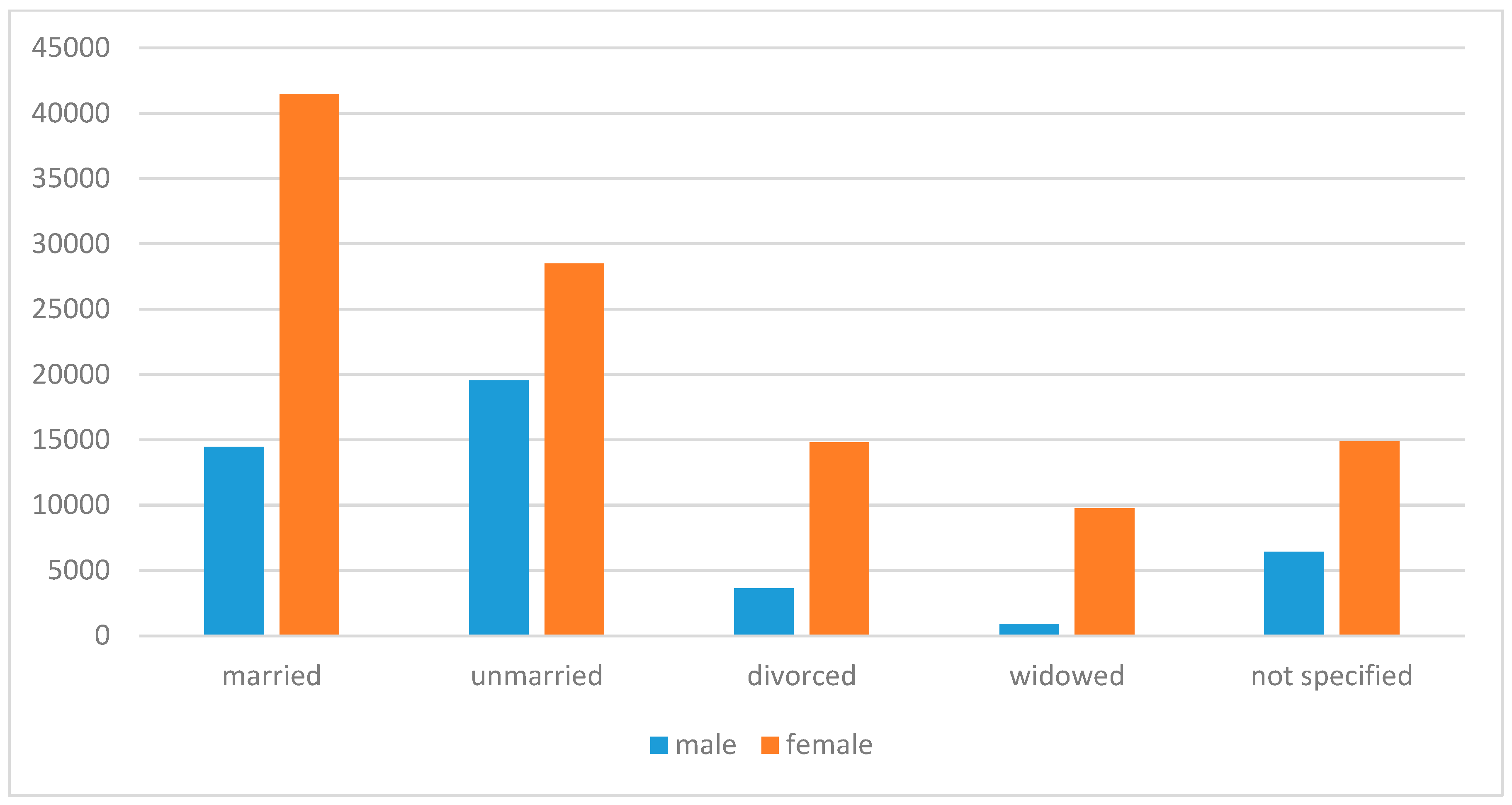
| Swedish County | Incidence |
|---|---|
| Stockholm | 0.93 (0.88–0.97) |
| Uppsala | 0.67 (0.58–0.76) |
| Södermanland | 0.61 (0.51–0.70) |
| Östergötland | 0.67 (0.59–0.74) |
| Jönköping | 0.70 (0.61–0.78) |
| Kronoberg | 0.74 (0.62–0.86) |
| Kalmar | 1.11 (0.98–1.15) |
| Gotland | 1.09 (0.82–1.36) |
| Blekinge | 1.35 (1.17–1.53) |
| Skåne | 0.81 (0.76–0.86) |
| Halland | 0.77 (0.67–0.87) |
| Västra Götaland | 0.76 (0.72–0.80) |
| Värmland | 0.76 (0.66–0.86) |
| Örebro | 0.74 (0.64–0.84) |
| Västmanland | 0.85 (0.73–0.96) |
| Dalarna | 0.74 (0.64–0.84) |
| Gävleborg | 0.74 (0.64–0.85) |
| Västernorrland | 0.77 (0.66–0.88) |
| Jämtland | 0.67 (0.53–0.82) |
| Västerbotten | 0.61 (0.51–0.70) |
| Norrbotten | 0.86 (0.75–0.98) |
| 1 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 12 | 13 | 14 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stockholm (1) | * | * | * | * | * | * | ns | * | * | * | * | * | * | ns | * | * | * | * | * | ns | |
| Uppsala (3) | ns | ns | ns | ns | * | * | * | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ||
| Södermanland (4) | ns | ns | ns | * | * | * | * | ns | * | ns | ns | * | ns | ns | ns | ns | ns | * | |||
| Östergötland (5) | ns | ns | * | * | * | * | ns | ns | ns | ns | * | ns | ns | ns | ns | ns | * | ||||
| Jönköping (6) | ns | * | * | * | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | |||||
| Kronoberg (7) | * | * | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||||||
| Kalmar (8) | ns | ns | * | * | * | * | * | * | * | * | * | * | * | * | |||||||
| Gotland (9) | ns | * | * | * | * | * | ns | * | * | * | * | * | ns | ||||||||
| Blekinge (10) | * | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Skåne (12) | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | ||||||||||
| Halland (13) | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | |||||||||||
| Västra Götaland (14) | ns | ns | ns | ns | ns | ns | ns | * | ns | ||||||||||||
| Värmland (17) | ns | ns | ns | ns | ns | ns | * | ns | |||||||||||||
| Örebro (18) | * | ns | |||||||||||||||||||
| Västmanland (19) | * | ns | |||||||||||||||||||
| Dalarna (20) | ns | ns | |||||||||||||||||||
| Gävleborg (21) | * | ns | |||||||||||||||||||
| Västernorrland (22) | * | ns | |||||||||||||||||||
| Jämtland (23) | ns | ns | |||||||||||||||||||
| Västerbotten (24) | * | ||||||||||||||||||||
| Norrbotten (25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerkovic Gulin, S.; Kravvas, G.; Seifert, O. Regional Variations in the Incidence of Lichen Sclerosus in Sweden: Insights from a Nationwide Register Study (2001–2021). J. Clin. Med. 2024, 13, 7836. https://doi.org/10.3390/jcm13247836
Jerkovic Gulin S, Kravvas G, Seifert O. Regional Variations in the Incidence of Lichen Sclerosus in Sweden: Insights from a Nationwide Register Study (2001–2021). Journal of Clinical Medicine. 2024; 13(24):7836. https://doi.org/10.3390/jcm13247836
Chicago/Turabian StyleJerkovic Gulin, Sandra, Georgios Kravvas, and Oliver Seifert. 2024. "Regional Variations in the Incidence of Lichen Sclerosus in Sweden: Insights from a Nationwide Register Study (2001–2021)" Journal of Clinical Medicine 13, no. 24: 7836. https://doi.org/10.3390/jcm13247836
APA StyleJerkovic Gulin, S., Kravvas, G., & Seifert, O. (2024). Regional Variations in the Incidence of Lichen Sclerosus in Sweden: Insights from a Nationwide Register Study (2001–2021). Journal of Clinical Medicine, 13(24), 7836. https://doi.org/10.3390/jcm13247836






