Impact of Postoperative Norepinephrine Administration on Free Flap Flow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population
2.3. Outcomes and Data Collection
2.4. Monitoring of Blood Pressure and Norepinephrine Infusion Rates
2.5. Microcirculation
2.6. Statistical Analysis
3. Results
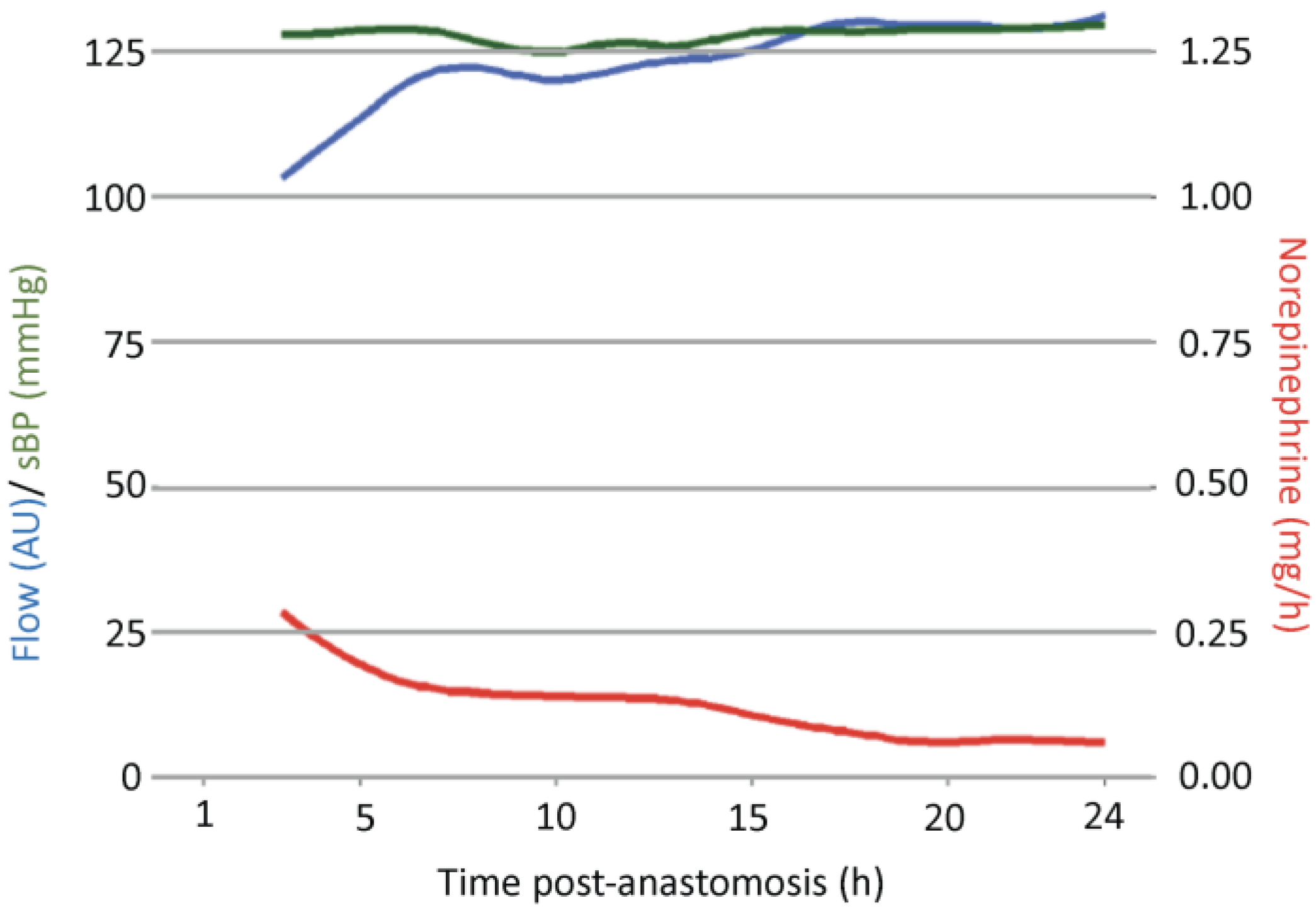
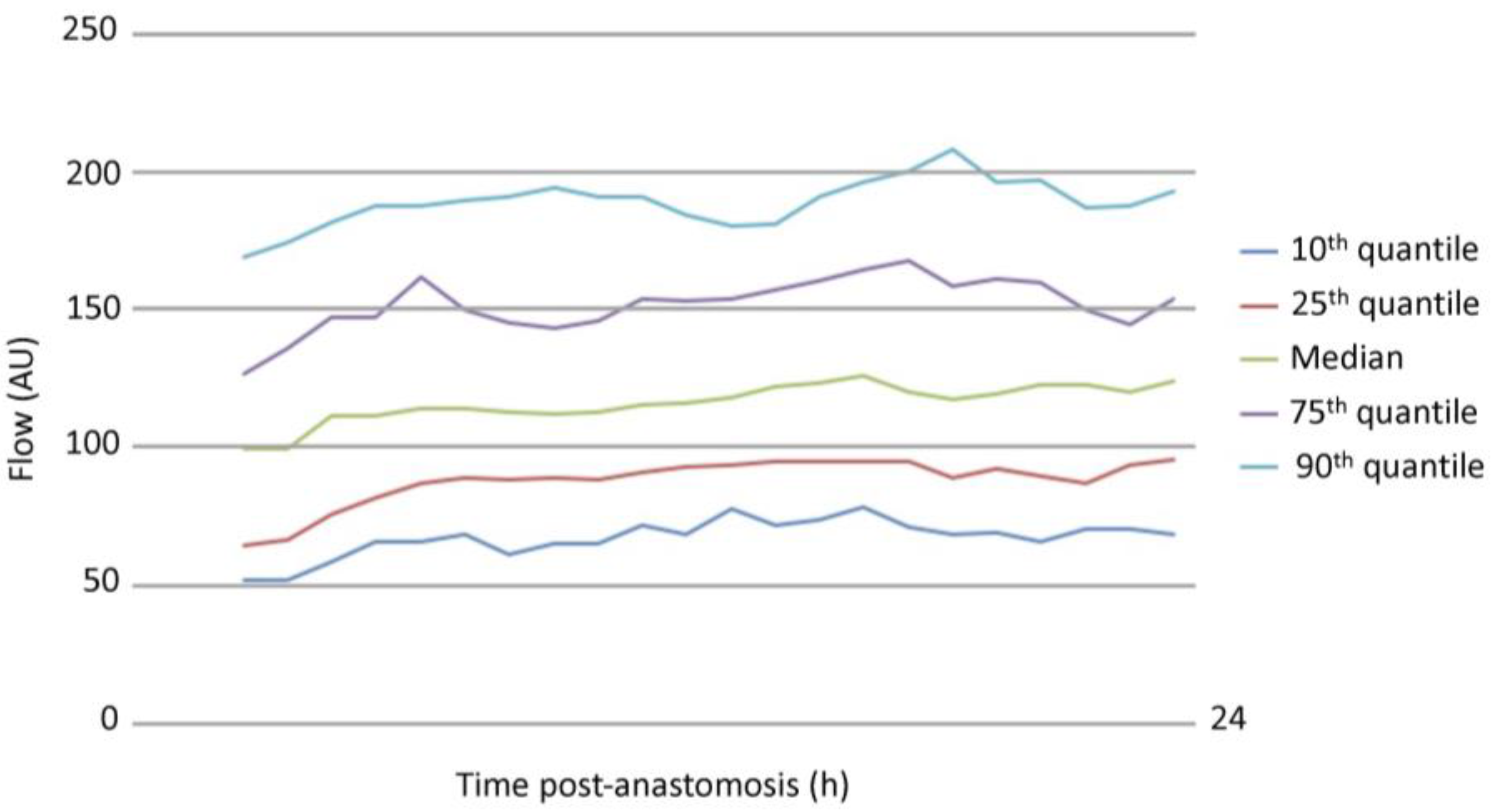
3.1. Analysis of Free Flap Microcirculation over Time
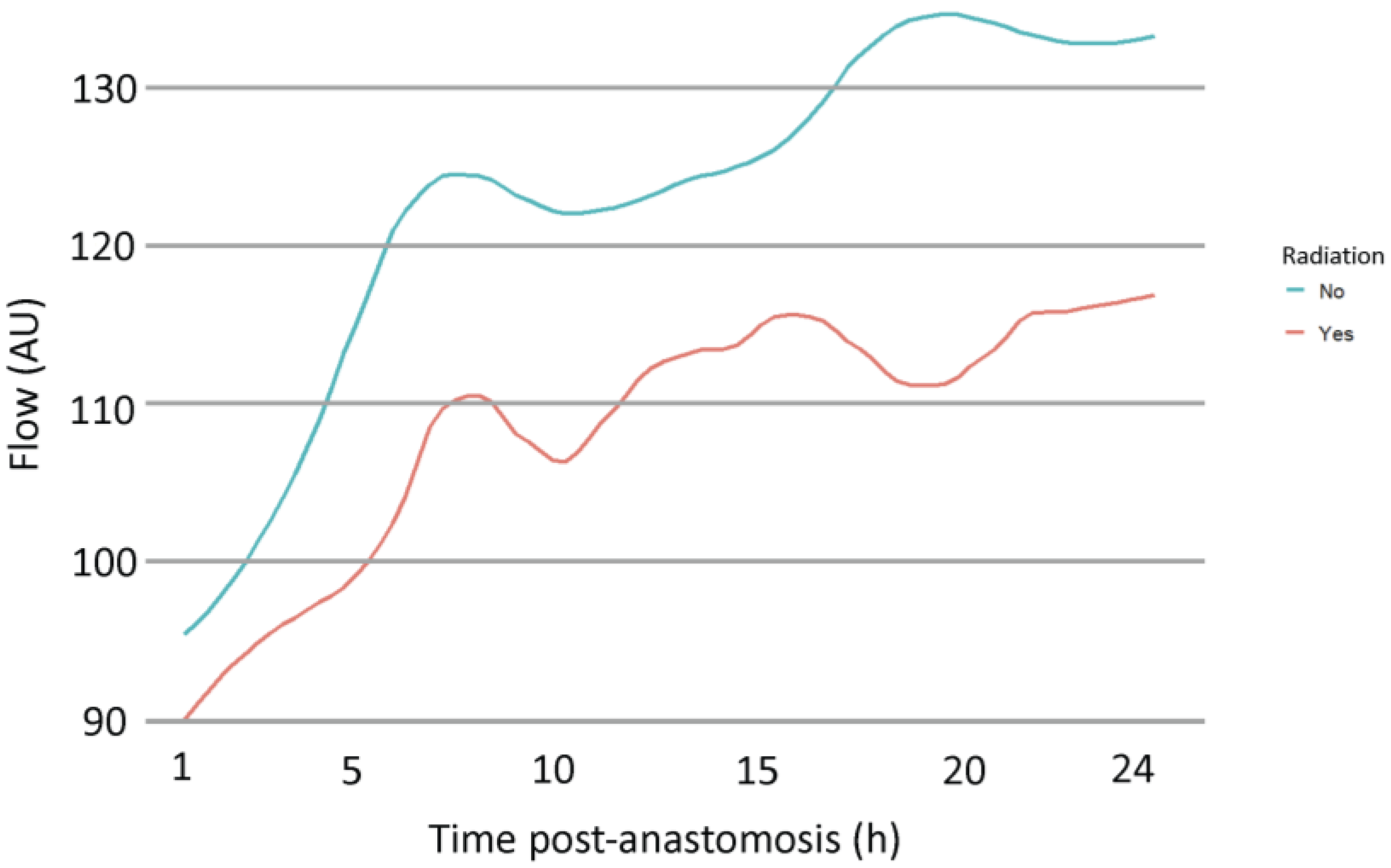
3.2. Multivariate Analysis of Variables Potentially Affecting Microvascular Flow Post-Anastomosis
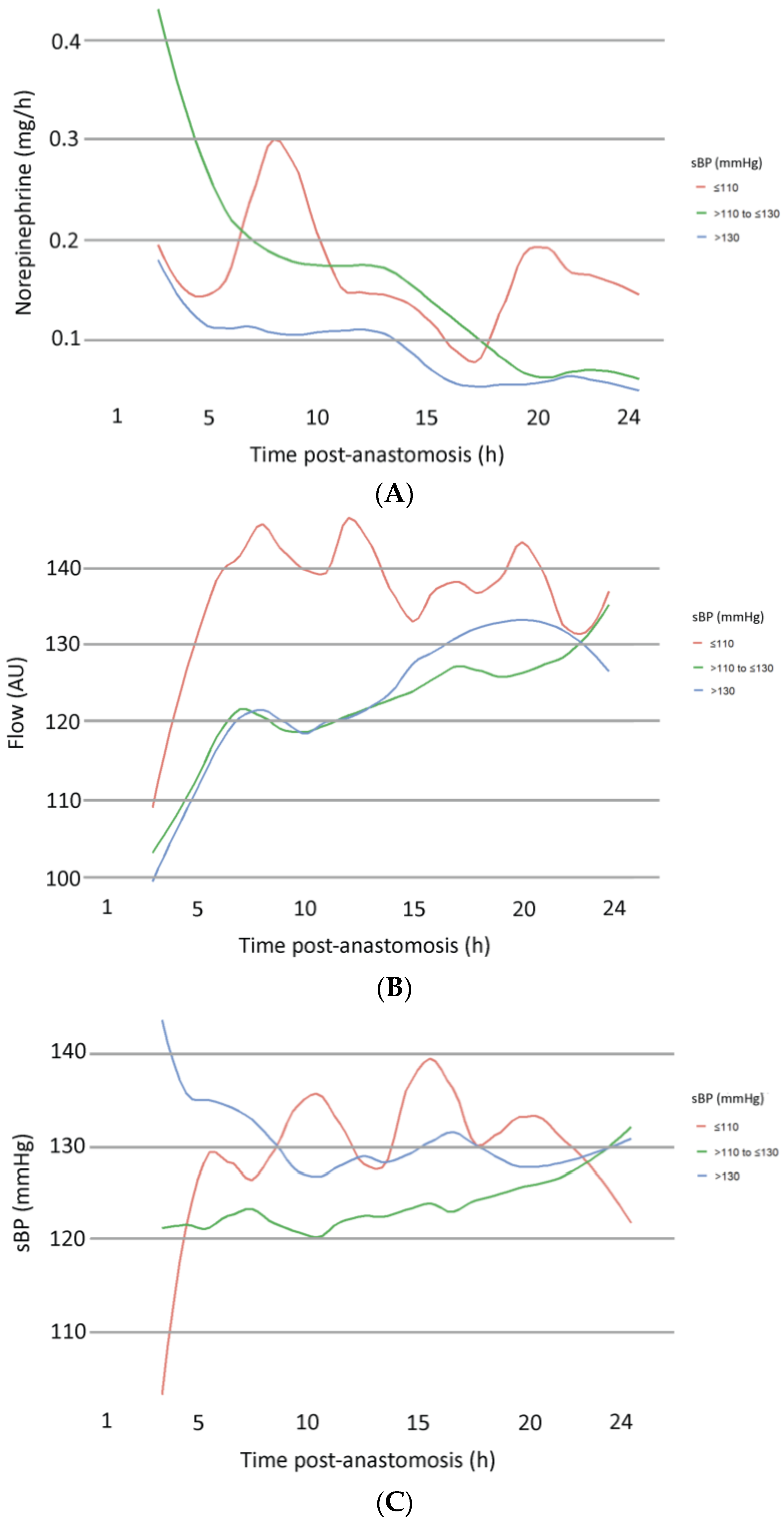
3.3. Subgroup Analysis According to sBP
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, V.A.; Vidouris, A.; Cecconi, M. Effects of Fluids on the Macro- and Microcirculations. Crit. Care 2018, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N.; Rabbitts, J.A. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin. Proc. 2009, 84, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Vasopressor therapy in critically ill patients with shock. Intensive Care Med. 2019, 45, 1503–1517. [Google Scholar] [CrossRef]
- Cordeiro, P.G.; Santamaria, E.; Hu, Q.Y.; Heerdt, P. Effects of vasoactive medications on the blood flow of island musculocutaneous flaps in swine. Ann. Plast. Surg. 1997, 39, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Goldstein, D.; Hofer, S.; Gilbert, R. Impact of vasopressors on outcomes in head and neck free tissue transfer. Microsurgery 2012, 32, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.; Pugh, S.; Fardy, M.; Shafik, M.; Hall, J.E. The effect of dobutamine on blood flow of free tissue transfer flaps during head and neck reconstructive surgery. Anaesthesia 2009, 64, 1089–1093. [Google Scholar] [CrossRef]
- Naik, A.N.; Freeman, T.; Li, M.M.; Marshall, S.; Tamaki, A.; Ozer, E.; Agrawal, A.; Kang, S.Y.; Old, M.O.; Seim, N.B. The Use of Vasopressor Agents in Free Tissue Transfer for Head and Neck Reconstruction: Current Trends and Review of the Literature. Front. Pharmacol. 2020, 11, 1248. [Google Scholar] [CrossRef]
- Tapia, B.; Garrido, E.; Cebrian, J.L.; Del Castillo, J.L.; Gonzalez, J.; Losantos, I.; Gilsanz, F. Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications. Cancers 2021, 13, 1545. [Google Scholar] [CrossRef]
- Moellhoff, N.; Heidekrueger, P.I.; Frank, K.; Pistek, S.; Alt, V.; Giunta, R.E.; Ehrl, D. Comparing the Time-Dependent Evolution of Microcirculation in Gracilis vs. ALT Flaps Using Laser-Doppler Flowmetry and Tissue-Spectrometry. J. Clin. Med. 2022, 11, 2425. [Google Scholar] [CrossRef]
- Moellhoff, N.; Gernert, C.; Frank, K.; Giunta, R.E.; Ehrl, D. The 72-Hour Microcirculation Dynamics in Viable Free Flap Reconstructions. J. Reconstr. Microsurg. 2022, 38, 637–646. [Google Scholar] [CrossRef]
- Moellhoff, N.; Taha, S.; Wachtel, N.; Hirschmann, M.; Hellweg, M.; Giunta, R.E.; Ehrl, D. Analysis of Factors Determining Patient Survival after Receiving Free-Flap Reconstruction at a Single Center-A Retrospective Cohort Study. Diagnostics 2022, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.S.L.; Ng, M.J.M.; Song, D.H.; Ooi, A.S.H. Perioperative Vasopressor Use in Free Flap Surgery: A Systematic Review and Meta-Analysis. J. Reconstr. Microsurg. 2019, 35, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.; Gatherwright, J.; Gurunluoglu, R. A literature review and meta-analysis of outcomes in microsurgical reconstruction using vasopressors. Microsurgery 2019, 39, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, A.; Turner, M.W.; Anand, R.; Puxeddu, R.; Brennan, P.A. Cardiac output monitoring to guide fluid replacement in head and neck microvascular free flap surgery-what is current practice in the UK? Br. J. Oral. Maxillofac. Surg. 2012, 50, 500–503. [Google Scholar] [CrossRef]
- Eley, K.A.; Young, J.D.; Watt-Smith, S.R. Epinephrine, norepinephrine, dobutamine, and dopexamine effects on free flap skin blood flow. Plast. Reconstr. Surg. 2012, 130, 564–570. [Google Scholar] [CrossRef]
- Lee, S.; Ju, J.W.; Yoon, S.; Lee, H.J.; Ha, J.H.; Hong, K.Y.; Jin, U.S.; Chang, H.; Cho, Y.J. Norepinephrine preserved flap blood flow compared to phenylephrine in free transverse rectus abdominis myocutaneous flap breast reconstruction surgery: A randomized pilot study. J. Plast. Reconstr. Aesthet. Surg. 2023, 83, 438–447. [Google Scholar] [CrossRef]
- Figus, A.; Ramakrishnan, V.; Rubino, C. Hemodynamic changes in the microcirculation of DIEP flaps. Ann. Plast. Surg. 2008, 60, 644–648. [Google Scholar] [CrossRef]
- Mucke, T.; Rau, A.; Merezas, A.; Kanatas, A.; Mitchell, D.A.; Wagenpfeil, S.; Wolff, K.D.; Steiner, T. Changes of perfusion of microvascular free flaps in the head and neck: A prospective clinical study. Br. J. Oral Maxillofac. Surg. 2014, 52, 810–815. [Google Scholar] [CrossRef]
- Lorenzetti, F.; Salmi, A.; Ahovuo, J.; Tukiainen, E.; Asko-Seljavaara, S. Postoperative changes in blood flow in free muscle flaps: A prospective study. Microsurgery 1999, 19, 196–199. [Google Scholar] [CrossRef]
- Schraven, S.P.; Kossack, B.; Struder, D.; Jung, M.; Skopnik, L.; Gross, J.; Hilsmann, A.; Eisert, P.; Mlynski, R.; Wisotzky, E.L. Continuous intraoperative perfusion monitoring of free microvascular anastomosed fasciocutaneous flaps using remote photoplethysmography. Sci. Rep. 2023, 13, 1532. [Google Scholar] [CrossRef]
- Ehrl, D.; Heidekrueger, P.I.; Haas, E.M.; Coenen, M.; Giunta, R.; Ninkovic, M.; Broer, P.N. Does Cigarette Smoking Harm Microsurgical Free Flap Reconstruction? J. Reconstr. Microsurg. 2018, 34, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Moellhoff, N.; Fritschen, U.V.; Germann, G.; Giunta, R.E.; Zeman, F.; Kehrer, A.; Lonic, D.; Broer, P.N.; Ehrl, D.; et al. Impact of Smoking Status in Free Deep Inferior Epigastric Artery Perforator Flap Breast Reconstruction: A Multicenter Study. J. Reconstr. Microsurg. 2020, 36, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Garip, M.; Van Dessel, J.; Grosjean, L.; Politis, C.; Bila, M. The impact of smoking on surgical complications after head and neck reconstructive surgery with a free vascularised tissue flap: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, e79–e98. [Google Scholar] [CrossRef] [PubMed]
- Booi, D.I.; Debats, I.B.; Boeckx, W.D.; van der Hulst, R.R. Risk factors and blood flow in the free transverse rectus abdominis (TRAM) flap: Smoking and high flap weight impair the free TRAM flap microcirculation. Ann. Plast. Surg. 2007, 59, 364–371. [Google Scholar] [CrossRef]
- Ooms, M.; Puladi, B.; Houschyar, K.S.; Heitzer, M.; Rashad, A.; Bickenbach, J.; Holzle, F.; Modabber, A. Smoking and microvascular free flap perfusion in head and neck reconstruction: Radial free forearm flaps and anterolateral thigh flaps. Sci. Rep. 2022, 12, 13902. [Google Scholar] [CrossRef]
- Tasch, C.; Pattiss, A.; Maier, S.; Lanthaler, M.; Pierer, G. Free Flap Outcome in Irradiated Recipient Sites: A Systematic Review and Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4216. [Google Scholar] [CrossRef]
- Borghini, A.; Gianicolo, E.A.; Picano, E.; Andreassi, M.G. Ionizing radiation and atherosclerosis: Current knowledge and future challenges. Atherosclerosis 2013, 230, 40–47. [Google Scholar] [CrossRef]
- Barry, M.; Kell, M.R. Radiotherapy and breast reconstruction: A meta-analysis. Breast Cancer Res. Treat. 2011, 127, 15–22. [Google Scholar] [CrossRef]
- Fracol, M.E.; Basta, M.N.; Nelson, J.A.; Fischer, J.P.; Wu, L.C.; Serletti, J.M.; Fosnot, J. Bilateral Free Flap Breast Reconstruction After Unilateral Radiation: Comparing Intraoperative Vascular Complications and Postoperative Outcomes in Radiated Versus Nonradiated Breasts. Ann. Plast. Surg. 2016, 76, 311–314. [Google Scholar] [CrossRef]
- Fosnot, J.; Fischer, J.P.; Smartt, J.M., Jr.; Low, D.W.; Kovach, S.J., 3rd; Wu, L.C.; Serletti, J.M. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plast. Reconstr. Surg. 2011, 127, 496–504. [Google Scholar] [CrossRef]
- Foerster, Y.; Baumann, L.; Kafantari, I.; Olmos, M.; Wehrhan, F.; Kesting, M.R.; Preidl, R.H. Recipient bed perfusion as a predictor for postoperative complications in irradiated patients with microvascular free tissue transfer of the head and neck area: A clinical analysis of 191 microvascular free flaps. Oral Maxillofac. Surg. 2023, 27, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Holzle, F.; Rau, A.; Loeffelbein, D.J.; Mucke, T.; Kesting, M.R.; Wolff, K.D. Results of monitoring fasciocutaneous, myocutaneous, osteocutaneous and perforator flaps: 4-year experience with 166 cases. Int. J. Oral Maxillofac. Surg. 2010, 39, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ooms, M.; Heitzer, M.; Winnand, P.; Bock, A.; Katz, M.; Bickenbach, J.; Holzle, F.; Modabber, A. Impacts of vascular comorbidities on free flap perfusion in microvascular head and neck reconstruction. Eur. Arch. Otorhinolaryngol. 2023, 280, 3375–3382. [Google Scholar] [CrossRef]
- Fracol, M.; Teven, C.M.; Selimos, B.; Wier, S.; Stockslager, C.; Schoenfeldt, J.; Connors, P.; Monahan, D.; Dumanian, G.A.; Howard, M.A. Pushing the DIEP Envelope with ERAS: 24 Hour Discharge is Safe in Appropriately Selected Patients. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5070. [Google Scholar] [CrossRef]
| Variable | |
|---|---|
| Age (y), mean ± SD | 61.46 ± 16.29 |
| Gender, n (%) | |
| Male | 50 (47.62%) |
| Female | 55 (52.38%) |
| BMI (kg/m2), mean ± SD | 25.11 ± 5.76 |
| ASA classification, n (%) | |
| 1 | 3 (2.86%) |
| 2 | 23 (21.90%) |
| 3 | 70 (66.67%) |
| 4 | 9 (8.57%) |
| Co-morbidities, n (%) | |
| Hypertension | 71 (67.62%) |
| PAD | 9 (8.57%) |
| Diabetes | 21 (20%) |
| History of thrombosis | 23 (21.90%) |
| History of myocardial infarction | 20 (19.05%) |
| Hospitalization (d), mean ± SD | 17.19 ± 7.84 |
| Days on IMC/ICU | 3.97 ± 12.97 |
| Defect etiology, n (%) | |
| Tumor | 57 (54.29%) |
| Trauma | 14 (13.33%) |
| Infection | 12 (11.43%) |
| Chronic wound | 16 (15.24%) |
| Other | 6 (5.71%) |
| Defect location, n (%) | |
| Head and neck | 36 (34.29%) |
| Trunk | 32 (30.48%) |
| Upper extremity | 14 (13.33%) |
| Lower extremity | 23 (21.90%) |
| Previous radiation therapy, n (%) | |
| Yes | 29 (27.62%) |
| No | 76 (72.38%) |
| Smoker | |
| Yes | 18 (17.14%) |
| No | 10 (9.52%) |
| Ex | 11 (10.48%) |
| NR | 66 (62.86%) |
| Variable | |
|---|---|
| Type of free flap, n (%) | |
| GM | 15 (14.29%) |
| LDM | 37 (35.24%) |
| ALT/MVL | 34 (32.38%) |
| DIEP | 10 (9.52%) |
| Others | 9 (8.57%) |
| Time of surgery (min), mean ± SD | 354.42 ± 110.58 |
| Flap ischemia (min), mean ± SD | 48.66 ± 20.39 |
| Recipient vessel, n (%) | |
| Internal mammary | 22 (20.95%) |
| Superior thyroid | 10 (9.52%) |
| Temporal | 19 (18.10%) |
| Facial | 10 (9.52%) |
| Anterior tibial | 8 (7.62%) |
| Posterior tibial | 8 (7.62%) |
| Fibular | 2 (1.90%) |
| Radial | 5 (4.76%) |
| Ulnar | 4 (3.81%) |
| Others | 17 (16.19%) |
| Number of venous anastomoses, n (%) | |
| 1 | 69 (65.71%) |
| 2 | 36 (34.29%) |
| Hour | Flow (AU) | p-Value | SO2 (%) | p-Value | rHb (AU) | p-Value | MAD (mmHg) | p-Value | sBP (mmHg) | p-Value | dBP (mmHg) | p-Value | Norepinephrine (mg/h) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 104.27 (44.85) | 43.62 (22.51) | 37.30 (12.90) | 85.14 (9.64) | 128.36 (15.26) | 61.67 (8.44) | 0.28 (0.37) | |||||||
| 4 | 106.63 (46.39) | 1 | 42.65 (21.95) | 1 | 37.91 (13.67) | 1 | 83.66 (10.77) | 1 | 127.20 (16.92) | 1 | 60.63 (9.28) | 1 | 0.24 (0.37) | 0.34 |
| 5 | 113.96 (47.72) | 0.37 | 41.51 (22.02) | 1 | 36.16 (12.01) | 1 | 85.02 (10.79) | 1 | 129.07 (17.13) | 1 | 61.09 (9.15) | 1 | 0.20 (0.37) | 0.02 |
| 6 | 118.46 (48.47) | 0.48 | 42.80 (21.10) | 1 | 37.28 (12.53) | 1 | 84.21 (11.01) | 1 | 129.24 (16.25) | 1 | 60.39 (10.08) | 1 | 0.16 (0.29) | 0.02 |
| 7 | 122.58 (49.32) | 0.12 | 43.34 (20.57) | 1 | 36.56 (12.43) | 1 | 82.75 (11.71) | 1 | 127.60 (16.59) | 1 | 59.33 (10.34) | 1 | 0.16 (0.25) | 0.04 |
| 8 | 121.96 (48.38) | 0.02 | 43.44 (20.98) | 1 | 36.16 (11.95) | 1 | 82.93 (10.19) | 1 | 128.30 (16.72) | 1 | 59.43 (8.66) | 1 | 0.14 (0.20) | 0.01 |
| 9 | 120.98 (49.46) | 0.12 | 42.45 (20.38) | 1 | 35.811 (12.39) | 1 | 79.06 (10.20) | 0.05 | 124.08 (15.17) | 1 | 56.65 (9.42) | <0.001 | 0.14 (0.21) | 0.02 |
| 10 | 120.28 (49.28) | 0.04 | 41.67 (20.43) | 1 | 35.88 (12.52) | 1 | 81.06 (10.65) | 0.96 | 125.76 (15.86) | 1 | 57.87 (9.48) | 0.07 | 0.14 (0.20) | 0.05 |
| 11 | 120.46 (48.51) | 0.01 | 41.55 (20.27) | 1 | 35.96 (12.10) | 1 | 80.39 (10.13) | 0.25 | 125.92 (16.37) | 1 | 56.44 (8.25) | <0.001 | 0.14 (0.19) | 0.03 |
| 12 | 123.49 (47.74) | <0.001 | 41.51 (20.56) | 1 | 35.43 (12.19) | 1 | 80.68 (9.87) | 1 | 126.88 (17.93) | 1 | 56.81 (8.41) | 0.01 | 0.14 (0.12) | 0.05 |
| 13 | 123.04 (45.79) | <0.001 | 41.60 (20.57) | 1 | 35.54 (12.41) | 1 | 80.04 (8.77) | 0.15 | 126.23 (15.10) | 1 | 56.11 (7.60) | <0.001 | 0.13 (0.19) | 0.03 |
| Hour | Flow (AU) | p-value | SO2 (%) | p-value | rHb (AU) | p-value | MBP (mmHg) | p-value | sBP (mmHg) | p-value | dBP (mmHg) | p-value | Norepinephrine (mg/h) | p-value |
| 14 | 124.20 (44.71) | <0.001 | 40.98 (21.14) | 1 | 35.84 (12.77) | 1 | 79.48 (9.26) | 0.11 | 125.56 (14.95) | 1 | 55.58 (8.66) | <0.001 | 0.12 (0.17) | 0.01 |
| 15 | 125.28 (45.12) | <0.001 | 41.48 (20.87) | 1 | 36.08 (12.99) | 1 | 84.16 (21.20) | 1 | 130.52 (22.42) | 1 | 59.91 (23.19) | 0.37 | 0.11 (0.16) | <0.001 |
| 16 | 127.44 (47.34) | <0.001 | 41.94 (20.68) | 1 | 36.49 (12.80) | 1 | 80.33 (10.16) | 0.25 | 127.00 (18.51) | 1 | 56.68 (8.27) | <0.001 | 0.09 (0.15) | <0.001 |
| 17 | 130.09 (48.03) | <0.001 | 42.40 (21.47) | 1 | 35.82 (12.67) | 1 | 82.85 (11.89) | 1 | 129.16 (19.36) | 1 | 58.22 (10.63) | 0.69 | 0.08 (0.16) | <0.001 |
| 18 | 130.45 (51.92) | <0.001 | 42.49 (22.25) | 1 | 35.60 (12.45) | 1 | 84.95 (10.86) | 1 | 129.29 (18.15) | 1 | 59.39 (8.88) | 1 | 0.08 (0.14) | <0.001 |
| 19 | 128.83 (54.34) | <0.001 | 40.53 (21.63) | 1 | 36.23 (12.63) | 1 | 82.06 (12.55) | 1 | 127.22 (17.08) | 1 | 58.26 (11.43) | 1 | 0.06 (0.12) | <0.001 |
| 20 | 129.84 (54.59) | <0.001 | 37.87 (20.54) | 1 | 36.26 (12.18) | 1 | 83.19 (15.12) | 1 | 129.27 (20.51) | 1 | 57.70 (12.35) | 1 | 0.06 (0.13) | <0.001 |
| 21 | 129.83 (54.00) | <0.001 | 38.86 (20.71) | 1 | 37.19 (14.15) | 1 | 82.03 (11.14) | 1 | 130.55 (17.22) | 1 | 57.61 (10.30) | 1 | 0.07 (0.16) | <0.001 |
| 22 | 128.31 (60.08) | <0.001 | 40.01 (22.46) | 1 | 39.18 (19.42) | 1 | 81.37 (11.37) | 1 | 126.15 (16.60) | 1 | 58.93 (12.10) | 1 | 0.07 (0.17) | <0.001 |
| 23 | 129.15 (56.76) | <0.001 | 40.01 (21.45) | 1 | 39.59 (19.04) | 1 | 83.40 (16.10) | 1 | 131.75 (21.36) | 1 | 60.65 (15.86) | 1 | 0.06 (0.17) | <0.001 |
| 24 | 131.63 (59.06) | <0.001 | 39.36 (21.43) | 1 | 37.59 (12.26) | 1 | 81.70 (11.80) | 1 | 128.87 (15.48) | 1 | 58.55 (11.92) | 1 | 0.06 (0.18) | <0.001 |
| Value | Std. Error | DF | t-Value | p-Value | |
|---|---|---|---|---|---|
| (Intercept) | 43.27 | 23.78 | 413 | 1.82 | 0.07 |
| rHb | −0.79 | 0.16 | 413 | −5.04 | <0.001 |
| SO2 | 0.55 | 0.13 | 413 | 4.27 | <0.001 |
| Smoker no | 21.39 | 15.69 | 26 | 1.36 | 0.18 |
| Smoker ex | 2.5 | 14.76 | 26 | 0.17 | 0.87 |
| ASA L | −28.56 | 22.78 | 26 | −1.25 | 0.22 |
| ASA Q | 12.34 | 17.11 | 26 | 0.72 | 0.48 |
| ASA C | −1.1 | 13.27 | 26 | −0.08 | 0.93 |
| Norepinephrine | 6.54 | 9.91 | 413 | 0.66 | 0.51 |
| sBP | 0.06 | 0.05 | 413 | 1.11 | 0.27 |
| Time (h) | 3.76 | 1.24 | 413 | 3.03 | <0.001 |
| Time_quad | −0.11 | 0.05 | 413 | −2.16 | 0.03 |
| Age > 65 | −9.19 | 12.88 | 26 | −0.71 | 0.48 |
| Gender female | 29.25 | 11.78 | 26 | 2.48 | 0.02 |
| Radiation no | 41.21 | 18.53 | 26 | 2.22 | 0.04 |
| Value | Std. Error | DF | t-Value | p-Value | |
|---|---|---|---|---|---|
| (Intercept) | 99.156 | 15.823 | 432.000 | 6.267 | <0.001 |
| rHb | −0.396 | 0.162 | 432.000 | −2.440 | 0.015 |
| SO2 | 0.307 | 0.105 | 432.000 | 2.919 | 0.004 |
| Smoker | 1.900 | 4.358 | 87.000 | 0.436 | 0.664 |
| ASA 3–4 | −19.859 | 10.439 | 87.000 | −1.902 | 0.060 |
| Norepinephrine | 3.998 | 5.992 | 432.000 | 0.667 | 0.505 |
| sBP | 0.013 | 0.058 | 432.000 | 0.219 | 0.827 |
| Time (h) | 9.333 | 3.468 | 432.000 | 2.691 | 0.007 |
| Time_quad | −0.368 | 0.241 | 432.000 | −1.525 | 0.128 |
| sBP >110 to ≤130 | −2.251 | 1.946 | 432.000 | −1.157 | 0.248 |
| sBP > 130 | −2.754 | 1.959 | 432.000 | −1.406 | 0.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehrl, D.; Pistek, S.; Rieder, C.; Irlbeck, M.; Hofmann-Kiefer, K.; Braig, D.; Klein, F.; Groene, P.; Giunta, R.E.; Moellhoff, N. Impact of Postoperative Norepinephrine Administration on Free Flap Flow. J. Clin. Med. 2024, 13, 7816. https://doi.org/10.3390/jcm13247816
Ehrl D, Pistek S, Rieder C, Irlbeck M, Hofmann-Kiefer K, Braig D, Klein F, Groene P, Giunta RE, Moellhoff N. Impact of Postoperative Norepinephrine Administration on Free Flap Flow. Journal of Clinical Medicine. 2024; 13(24):7816. https://doi.org/10.3390/jcm13247816
Chicago/Turabian StyleEhrl, Denis, Svenja Pistek, Clemens Rieder, Michael Irlbeck, Klaus Hofmann-Kiefer, David Braig, Frederic Klein, Philipp Groene, Riccardo E. Giunta, and Nicholas Moellhoff. 2024. "Impact of Postoperative Norepinephrine Administration on Free Flap Flow" Journal of Clinical Medicine 13, no. 24: 7816. https://doi.org/10.3390/jcm13247816
APA StyleEhrl, D., Pistek, S., Rieder, C., Irlbeck, M., Hofmann-Kiefer, K., Braig, D., Klein, F., Groene, P., Giunta, R. E., & Moellhoff, N. (2024). Impact of Postoperative Norepinephrine Administration on Free Flap Flow. Journal of Clinical Medicine, 13(24), 7816. https://doi.org/10.3390/jcm13247816







