Method Evaluation of the QuidelOrtho Diagnostics Vitros NT-proBNP II Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurement of NT-proBNP
2.3. Validation Procedures
3. Results
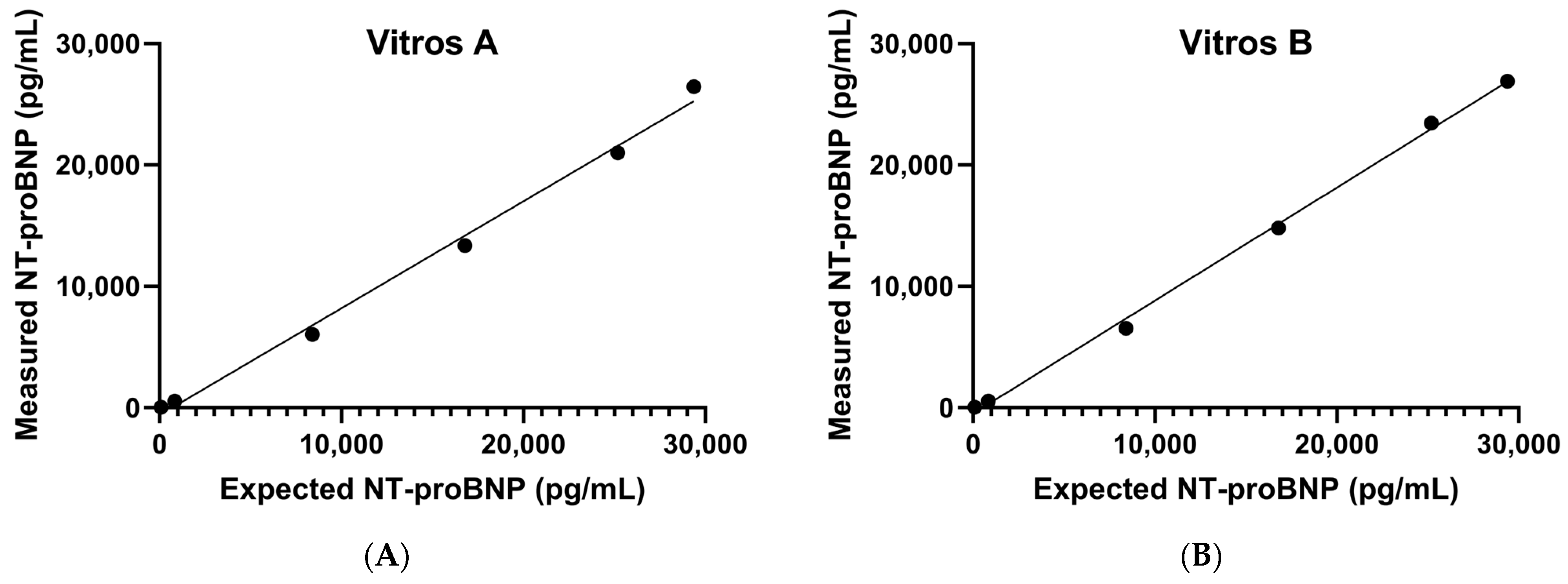
3.1. Precision, AMR, and CRR
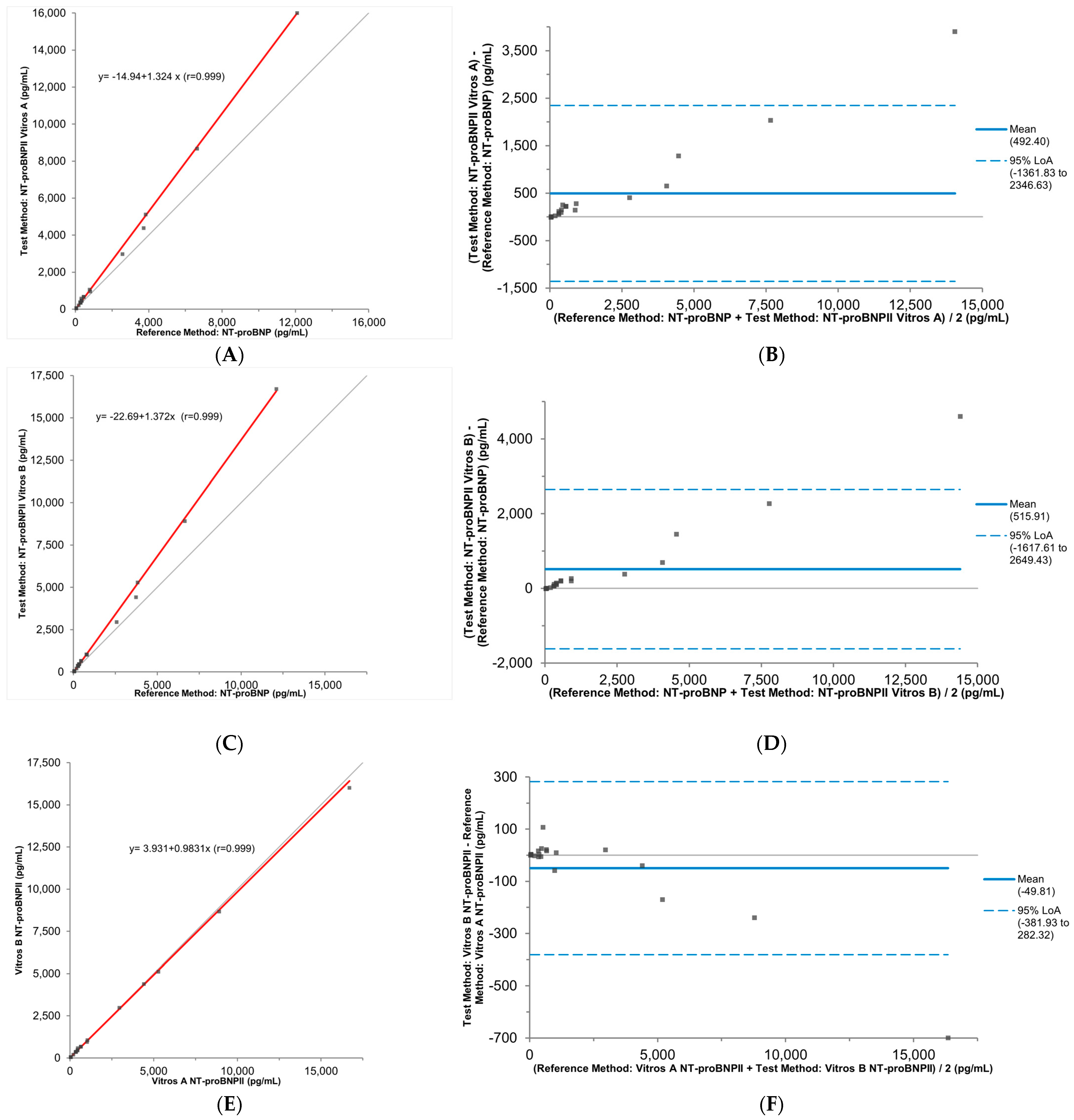
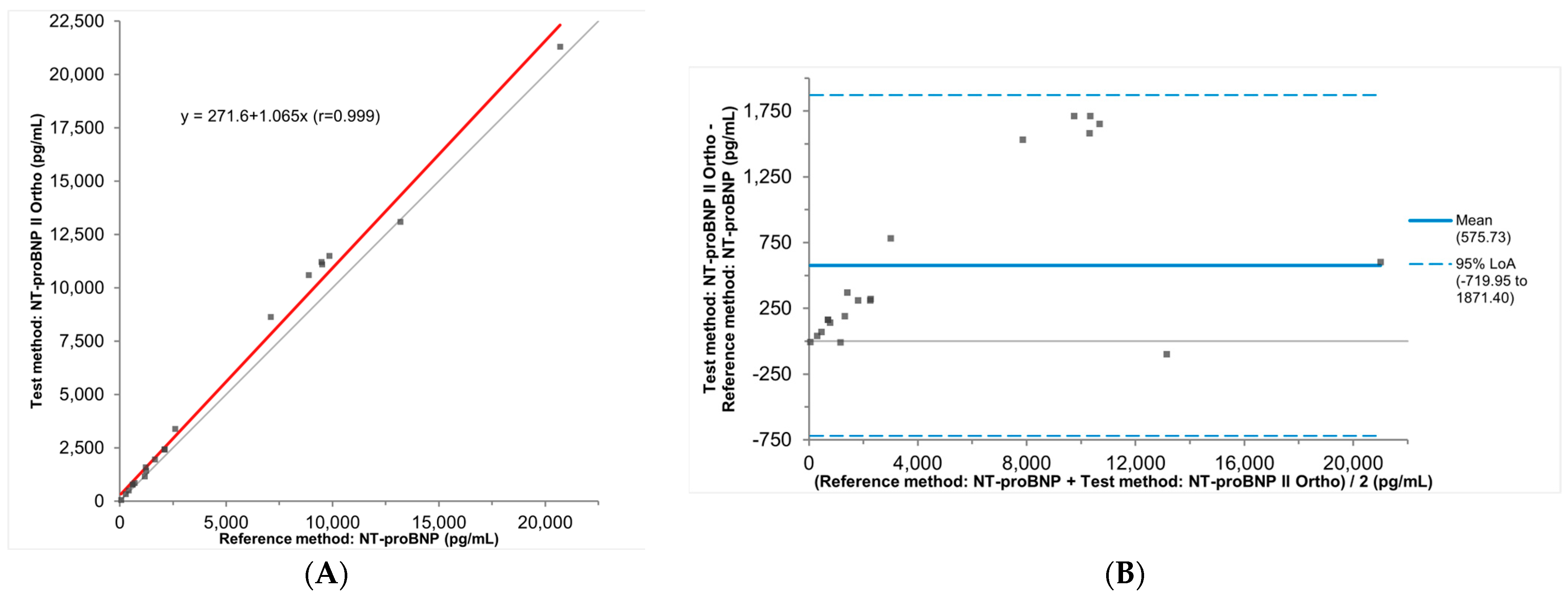
3.2. Accuracy
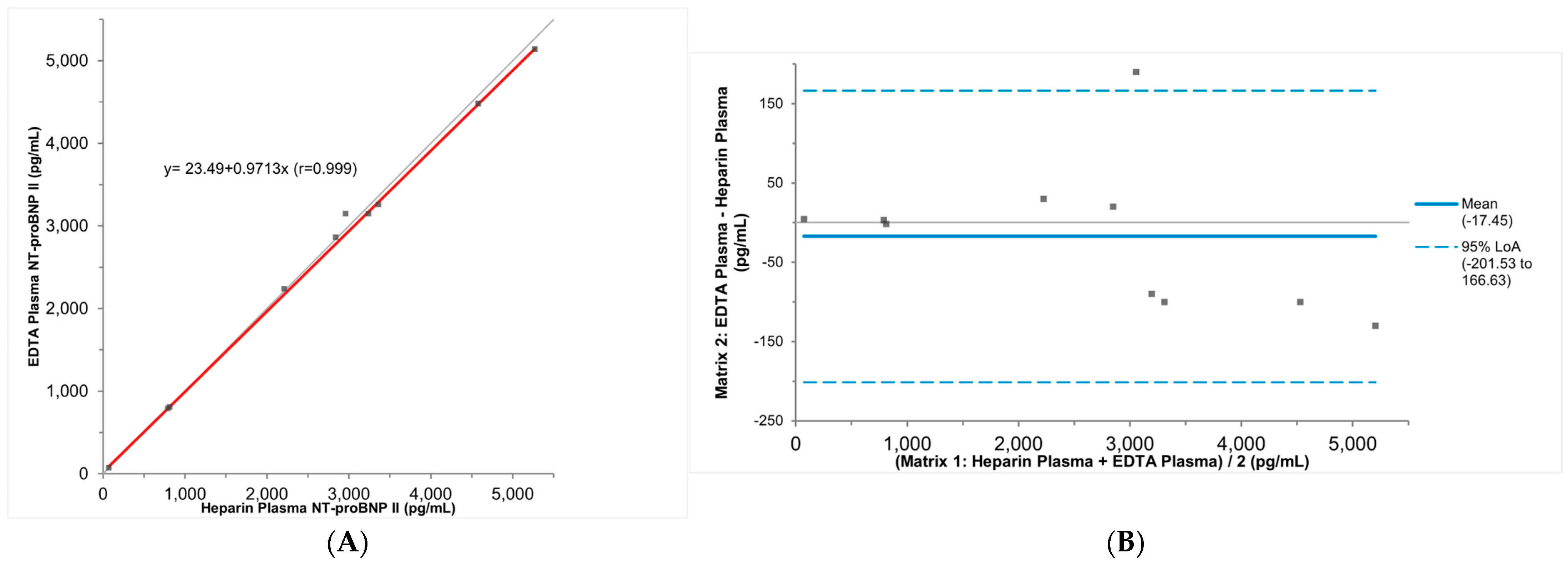
3.3. Stability and Specimen Type Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Michelle, M.K.; Khadijah, B.; Boback, Z.; David, A.; Vanessa, B.; Biykem, B.; Rebecca, L.D.; Michael, P.D.; Paul, A.H.; Corrine, Y.J.; et al. 2024 Update to the 2020 ACC/AHA Clinical Performance and Quality Measures for Adults With Heart Failure: A Report of the American Heart Association/American College of Cardiology Joint Committee on Performance Measures. J. Am. Coll. Cardiol. 2024, 84, 1123–1143. [Google Scholar] [CrossRef]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Balla, C.; Fucili, A. Heart failure: An historical perspective. Eur. Heart J. Suppl. 2016, 18, G3–G10. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.G.; Tamm, N.N.; Seferian, K.R.; Postnikov, A.B.; Karpova, N.S.; Serebryanaya, D.V.; Koshkina, E.V.; Krasnoselsky, M.I.; Katrukha, A.G. Processing of pro-B-type natriuretic peptide: Furin and corin as candidate convertases. Clin. Chem. 2010, 56, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Srisawasdi, P.; Vanavanan, S.; Charoenpanichkit, C.; Kroll, M.H. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am. J. Clin. Pathol. 2010, 133, 14–23. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Su, X.; Lei, T.; Yu, H.; Zhang, L.; Feng, Z.; Shuai, T.; Guo, H.; Liu, J. NT-proBNP in Different Patient Groups of COPD: A Systematic Review and Meta-Analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 811–825. [Google Scholar] [CrossRef]
- Li, L.H.; Kao, W.F.; Chiu, Y.H.; Hou, S.K.; Meng, C.; How, C.K. Impact of renin-angiotensin-aldosterone system activation and body weight change on N-terminal pro-B-type natriuretic peptide variation in 100-km ultramarathon runners. J. Chin. Med. Assoc. 2020, 83, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mihailovici, A.R.; Donoiu, I.; Gheonea, D.I.; Mirea, O.; Tartea, G.C.; Buse, M.; Calborean, V.; Obleaga, C.; Padureanu, V.; Istratoaie, O. NT-proBNP and Echocardiographic Parameters in Liver Cirrhosis—Correlations with Disease Severity. Med. Princ. Pract. 2019, 28, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Gegenhuber, A.; Mueller, T.; Firlinger, F.; Lenz, K.; Poelz, W.; Haltmayer, M. Time course of B-type natriuretic peptide (BNP) and N-terminal proBNP changes in patients with decompensated heart failure. Clin. Chem. 2004, 50, 454–456. [Google Scholar] [CrossRef]
- Noveanu, M.; Breidthardt, T.; Potocki, M.; Reichlin, T.; Twerenbold, R.; Uthoff, H.; Socrates, T.; Arenja, N.; Reiter, M.; Meissner, J.; et al. Direct comparison of serial B-type natriuretic peptide and NT-proBNP levels for prediction of short- and long-term outcome in acute decompensated heart failure. Crit. Care 2011, 15, R1. [Google Scholar] [CrossRef]
- Yeo, K.T.; Wu, A.H.; Apple, F.S.; Kroll, M.H.; Christenson, R.H.; Lewandrowski, K.B.; Sedor, F.A.; Butch, A.W. Multicenter evaluation of the Roche NT-proBNP assay and comparison to the Biosite Triage BNP assay. Clin. Chim. Acta 2003, 338, 107–115. [Google Scholar] [CrossRef]
- Claggett, B.; Packer, M.; McMurray, J.J.; Swedberg, K.; Rouleau, J.; Zile, M.R.; Jhund, P.; Lefkowitz, M.; Shi, V.; Solomon, S.D.; et al. Estimating the Long-Term Treatment Benefits of Sacubitril-Valsartan. N. Engl. J. Med. 2015, 373, 2289–2290. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef]
- Miller, W.L.; Hartman, K.A.; Burritt, M.F.; Borgeson, D.D.; Burnett, J.C., Jr.; Jaffe, A.S. Biomarker responses during and after treatment with nesiritide infusion in patients with decompensated chronic heart failure. Clin. Chem. 2005, 51, 569–577. [Google Scholar] [CrossRef]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Halfinger, B.; Hammerer-Lercher, A.; Amplatz, B.; Sarg, B.; Kremser, L.; Lindner, H.H. Unraveling the Molecular Complexity of O-Glycosylated Endogenous (N-Terminal) pro-B-Type Natriuretic Peptide Forms in Blood Plasma of Patients with Severe Heart Failure. Clin. Chem. 2017, 63, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Seferian, K.R.; Tamm, N.N.; Semenov, A.G.; Tolstaya, A.A.; Koshkina, E.V.; Krasnoselsky, M.I.; Postnikov, A.B.; Serebryanaya, D.V.; Apple, F.S.; Murakami, M.M.; et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin. Chem. 2008, 54, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Panteghini, M.; Ravkilde, J.; Mair, J.; Wu, A.H.; Tate, J.; Pagani, F.; Christenson, R.H.; Jaffe, A.S.; Committee on Standardization of Markers of Cardiac Damage of the IFCC. Quality specifications for B-type natriuretic peptide assays. Clin. Chem. 2005, 51, 486–493. [Google Scholar] [CrossRef]
- Luckenbill, K.N.; Christenson, R.H.; Jaffe, A.S.; Mair, J.; Ordonez-Llanos, J.; Pagani, F.; Tate, J.; Wu, A.H.; Ler, R.; Apple, F.S. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: Preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin. Chem. 2008, 54, 619–621. [Google Scholar] [CrossRef]
- NT-proBNP Calibrators and Reagent Pack V11.0 [Package Insert]; QuidelOrtho: Raritan, NJ, USA, 2017.
- (NBNP2) NT-proBNP II Reagent Pack and Calibrators V 2.0 [Package Insert]; QuidelOrtho: Raritan, NJ, USA, 2022.
- Diagnostics, O.C. Clinical Cutoffs for the Aid in Diagnosis of Heart Failure With NT-proBNP. Ortho Clin. Diagn. Appl. Notes 2022. [Google Scholar]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Bohm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Campbell, R.T.; Mooney, L.; Kimenai, D.M.; Hayward, C.; Campbell, A.; Porteous, D.; Mills, N.L.; Lang, N.N.; Petrie, M.C.; et al. Reference Ranges for NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) and Risk Factors for Higher NT-proBNP Concentrations in a Large General Population Cohort. Circ. Heart Fail. 2022, 15, e009427. [Google Scholar] [CrossRef] [PubMed]
| Repeatability (n = 20) | Reproducibility (n = 20) | ||||
|---|---|---|---|---|---|
| Mean (pg/mL) | CV (%) | Mean (pg/mL) | CV (%) | ||
| Vitros A | QC1 | 48 | 4 | 45 | 5 |
| QC2 | 2895 | 2 | 2829 | 3 | |
| Vitros B | QC1 | 51 | 5 | 47 | 10 |
| QC2 | 3015 | 2 | 2825 | 6 | |
| Specimen ID # | Dilution | Results from the Vitros NT-proBNP Assay (pg/mL). Results from Duplicated Measurements Are Documented When Available | Results from the Vitros NT-proBNP II Assay (pg/mL). Results from Duplicated Measurements Are Documented When Available | % Recovery of the Result (Vitros NT-proBNP II Assay vs. Vitros NT-proBNP Assay). Results Are Averaged When Duplicated Measurements Were Performed | ||
|---|---|---|---|---|---|---|
| 1 | Neat | 16,400 | 18,800 | 115% | ||
| 1:4 | 4130 | 4090 | 4740 | 4760 | 116% | |
| 1:10 | 1470 | 1480 | 1730 | 1700 | 116% | |
| 2 | Neat | 9910 | 11,000 | 111% | ||
| 1:4 | 2460 | 2440 | 2710 | 2730 | 111% | |
| 1:10 | 919 | 926 | 1050 | 1030 | 113% | |
| 3 | Neat | 2590 | 3350 | 129% | ||
| 1:4 | 667 | 661 | 798 | 824 | 122% | |
| 1:10 | 274 | 270 | 324 | 322 | 119% | |
| 4 | 1:4 | 2470 | 2470 | 2750 | 2790 | 112% |
| 1:10 | 945 | 937 | 1040 | 964 | 106% | |
| Specimen ID # | Sample Type | Sample Preparation | Results from the Vitros NT-proBNP Assay (pg/mL) | Results from the Vitros NT-proBNP II Assay (pg/mL) | % Recovery of the Result (Vitros NT-proBNP II Assay vs. Vitros NT-proBNP Assay) |
|---|---|---|---|---|---|
| 5 | K2EDTA plasma | N/A | 4350 | 5650 | 130% |
| Double centrifugation | 4290 | 5640 | 131% | ||
| One freeze–thaw cycle | 4300 | 5570 | 130% | ||
| One freeze–thaw cycle with a single centrifugation at 4000× g for 5 min | 4410 | 5670 | 129% | ||
| 6 | K2EDTA plasma | N/A | 5320 | 6210 | 117% |
| Double centrifugation | 5320 | 6510 | 122% | ||
| One freeze–thaw cycle | 5200 | 6410 | 123% | ||
| One freeze–thaw cycle with a single centrifugation at 4000× g for 5 min | 5240 | 6430 | 123% | ||
| 7 | Serum | N/A | 7100 | 8630 | 122% |
| One freeze–thaw cycle | 5760 | 6840 | 119% | ||
| 8 | Serum | N/A | 1220 | 1590 | 130% |
| One freeze–thaw cycle | 1120 | 1480 | 132% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Sun, C.; Tsan, J.; Leung, E.K.Y. Method Evaluation of the QuidelOrtho Diagnostics Vitros NT-proBNP II Assay. J. Clin. Med. 2024, 13, 7751. https://doi.org/10.3390/jcm13247751
Xiao Y, Sun C, Tsan J, Leung EKY. Method Evaluation of the QuidelOrtho Diagnostics Vitros NT-proBNP II Assay. Journal of Clinical Medicine. 2024; 13(24):7751. https://doi.org/10.3390/jcm13247751
Chicago/Turabian StyleXiao, Yi, Chao Sun, Justin Tsan, and Edward Ki Yun Leung. 2024. "Method Evaluation of the QuidelOrtho Diagnostics Vitros NT-proBNP II Assay" Journal of Clinical Medicine 13, no. 24: 7751. https://doi.org/10.3390/jcm13247751
APA StyleXiao, Y., Sun, C., Tsan, J., & Leung, E. K. Y. (2024). Method Evaluation of the QuidelOrtho Diagnostics Vitros NT-proBNP II Assay. Journal of Clinical Medicine, 13(24), 7751. https://doi.org/10.3390/jcm13247751






