The Role of Radioiodine Therapy in Differentiated Thyroid Cancer Arising from Struma Ovarii: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
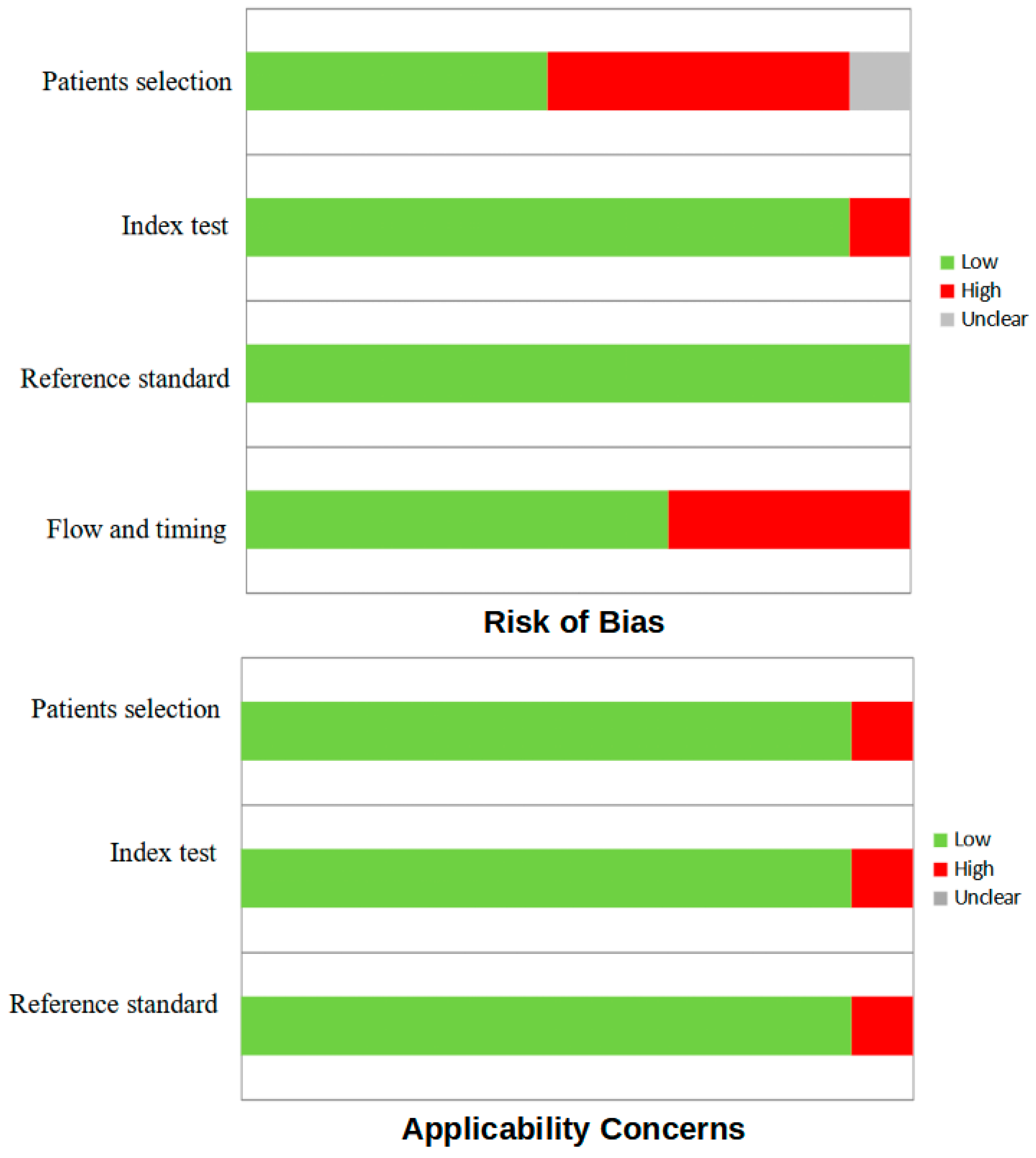
2.3. Quality Assessment
2.4. Data Extraction
2.5. Literature Search Findings
3. Results
Study Synthesis: RAI Applications and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gottschalk, S. Ein neuer typus einer kleincystischen bosartigen eierstockgeschwulst. Arch. Gynak. 1899, 59, 676–698. [Google Scholar] [CrossRef]
- Kondi-Pafiti, A.; Mavrigiannaki, P.; Grigoriadis, C.; Kontogianni-Katsarou, K.; Mellou, A.; Kleanthis, C.K.; Liapis, A. Monodermal teratomas (struma ovarii). Clinicopathological characteristics of 11 cases and literature review. Eur. J. Gynaecol. Oncol. 2011, 32, 657–659. [Google Scholar]
- Wei, S.; Baloch, Z.W.; LiVolsi, V.A. Pathology of Struma Ovarii: A Report of 96 Cases. Endocr. Pathol. 2015, 26, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yao, J.; Wang, S.; Zhao, J.; Dong, J.; Liao, L. The Clinical and Pathological Characteristics of Malignant Struma Ovarii: An Analysis of 144 Published Patients. Front. Oncol. 2021, 11, 645156. [Google Scholar] [CrossRef]
- Li, S.; Yang, T.; Xiang, Y.; Li, X.; Zhang, L.; Deng, S. Clinical characteristics and survival outcomes of malignant struma ovarii confined to the ovary. BMC Cancer 2021, 21, 383. [Google Scholar] [CrossRef]
- Goffredo, P.; Sawka, A.M.; Pura, J.; Adam, M.A.; Roman, S.A.; Sosa, J.A. Malignant struma ovarii: A population-level analysis of a large series of 68 patients. Thyroid 2015, 25, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Soslow, R.A.; Rivera, M.; Tuttle, M.R.; Ghossein, R.A. Histologically bland “extremely well differentiated” thyroid carcinomas arising in struma ovarii can recur and metastasize. Int. J. Gynecol. Pathol. 2009, 28, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Robboy, S.J.; Shaco-Levy, R.; Peng, R.Y.; Snyder, M.J.; Donahue, J.; Bentley, R.C.; Bean, S.; Krigman, H.R.; Roth, L.M.; Young, R.H. Malignant struma ovarii: An analysis of 88 cases, including 27 with extraovarian spread. Int. J. Gynecol. Pathol. 2009, 28, 405–422. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Petranović Ovčariček, P.; Kreissl, M.C.; Campenni, A.; de Keizer, B.; Tuncel, M.; Vrachimis, A.; Deandreis, D.; Giovanella, L. SNMMI/EANM practice guideline vs. ETA Consensus Statement: Differences and similarities in approaching differentiated thyroid cancer management—The EANM perspective. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.Q.; Wang, C.; Zhang, X.; Li, X.; Lin, Y.S. Delayed initial radioiodine therapy related to incomplete response in low- to intermediate-risk differentiated thyroid cancer. Clin. Endocrinol. 2018, 88, 601–606. [Google Scholar] [CrossRef]
- Steinschneider, M.; Pitaro, J.; Koren, S.; Mizrakli, Y.; Benbassat, C.; Muallem Kalmovich, L. Differentiated Thyroid Cancer with Biochemical Incomplete Response: Clinico-Pathological Characteristics and Long Term Disease Outcomes. Cancers 2023, 13, 5422. [Google Scholar] [CrossRef]
- Bellini, P.; Dondi, F.; Gatta, E.; Zilioli, V.; Albano, D.; Cappelli, C.; Bertagna, F. Prognostic role and characteristics of the indeterminate response in differentiated thyroid cancer: A systematic review. Endocrine 2024, 84, 812–821. [Google Scholar] [CrossRef]
- Gambale, C.; Prete, A.; Contartese, L.; Torregrossa, L.; Bianchi, F.; Molinaro, E.; Materazzi, G.; Elisei, R.; Matrone, A. Usefulness of second 131I treatment in biochemical persistent differentiated thyroid cancer patients. Eur. Thyroid. J. 2023, 12, e230052. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, C.; Feng, F.; Wu, S.; Wang, H.; Fu, H. The value of [18F]FDG PET/CT in avoiding overtreatment of 131l avidity pulmonary metastasis of differentiated thyroid cancer. Endokrynol. Pol. 2023, 74, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Dondi, F.; Mazzoletti, A.; Bellini, P.; Rodella, C.; Bertagna, F. Prognostic Role of 2-[18F]FDG PET/CT Metabolic Volume Parameters in Patients Affected by Differentiated Thyroid Carcinoma with High Thyroglobulin Level, Negative 131I WBS and Positive 2-[18F]-FDG PET/CT. Diagnostics 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Verburg, F.A.; Hänscheid, H.; Luster, M. Radioactive iodine (RAI) therapy for metastatic differentiated thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Piccardo, A.; Rizzo, A.; Cuzzocrea, M.; Bottoni, G.; Bellini, P.; Bertagna, F.; Treglia, G. Diagnostic performance of 2-[18F]FDG PET/CT in recurrent differentiated thyroid cancer and elevated antithyroglobulin antibodies: An updated systematic review and bivariate meta-analysis. Endocrine 2024. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, K.; Cegla, P.; Dedecjus, M. Role of [18F]FDG PET/CT in the management of follicular cell-derived thyroid carcinoma. Cancer Imaging 2024, 24, 147. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Leem, D.E.; Yoo, J.H.; Kim, T.H.; Kim, S.W.; Chung, J.H. Clinical Manifestations of Malignant Struma Ovarii: A Retrospective Case Series in a Tertiary Hospital in Korea. Endocrinol. Metab. 2024, 39, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Giuliani, D.; Cosio, S.; Lissoni, A.; Ferrero, A.M.; Landoni, F. Clinical Outcome of Patients with Malignant Tumors Associated With Mature Cystic Teratomas of the Ovary: A Retrospective Multicenter Italian Study. Anticancer Res. 2019, 39, 2513–2517. [Google Scholar] [CrossRef]
- Li, S.; Hong, R.; Yin, M.; Zhang, T.; Zhang, X.; Yang, J. Incidence, clinical characteristics, and survival outcomes of ovarian strumal diseases: A retrospective cohort study. BMC Womens Health 2023, 23, 497. [Google Scholar] [CrossRef] [PubMed]
- Addley, S.; Mihai, R.; Alazzam, M.; Dhar, S.; Soleymani Majd, H. Malignant struma ovarii: Surgical, histopathological and survival outcomes for thyroid-type carcinoma of struma ovarii with recommendations for standardising multi-modal management. A retrospective case series sharing the experience of a single institution over 10 years. Arch. Gynecol. Obstet. 2021, 303, 863–870. [Google Scholar] [CrossRef]
- Devaney, K.; Snyder, R.; Norris, H.J.; Tavassoli, F.A. Proliferative and histologically malignant struma ovarii: A clinicopathologic study of 54 cases. Int. J. Gynecol. Pathol. 1993, 12, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, T.; Li, X.; Zhang, L.; Shi, H.; Cheng, N.; Lang, J. FIGO Stage IV and Age Over 55 Years as Prognostic Predicators in Patients with Metastatic Malignant Struma Ovarii. Front. Oncol. 2020, 10, 584917. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.M.; Miller, A.W., 3rd; Talerman, A. Typical thyroid-type carcinoma arising in struma ovarii: A report of 4 cases and review of the literature. Int. J. Gynecol. Pathol. 2008, 27, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.L.; Clark, V.E.; Harper, H.; Chhieng, D.C.; Sosa, J.A.; Roman, S.A. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: A series of 4 patients and a review of 53 reported cases. Thyroid 2012, 22, 400–406. [Google Scholar] [CrossRef]
- Poli, R.; Scatolini, M.; Grosso, E.; Maletta, F.; Gallo, M.; Liscia, D.; Nelva, A.; Cesario, F.; Forte, G.; Metovic, J.; et al. Malignant struma ovarii: Next-generation sequencing of six cases revealed Nras, Braf, and Jak3 mutations. Endocrine 2021, 71, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.C.; Chang, K.H.; Lyu, M.O.; Chang, S.J.; Ryu, H.S.; Kim, H.S. Clinical characteristics of struma ovarii. J. Gynecol. Oncol. 2008, 19, 135–138. [Google Scholar] [CrossRef]
- Violetis, O.; Konstantakou, P.; Spyroglou, A.; Xydakis, A.; Kekis, P.B.; Tseleni, S.; Kolomodi, D.; Konstadoulakis, M.; Mastorakos, G.; Theochari, M.; et al. The Long Journey towards Personalized Targeted Therapy in Poorly Differentiated Thyroid Carcinoma (PDTC): A Case Report and Systematic Review. J. Pers. Med. 2024, 14, 654. [Google Scholar] [CrossRef]
- Giovanella, L.; Tuncel, M.; Aghaeel, A.; Campenni, A.; De Virgilio, A.; Petranović Ovčariček, P. Theranostics of Thyroid Cancer. Semin. Nucl. Med. 2024, 54, 470–487. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, C.P.; Lele, S.M.; Modesitt, S.C. Malignant struma ovarii: A case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol. Oncol. 2003, 89, 543–548. [Google Scholar] [CrossRef]
- Batog, I.W.; Riain, C.Ó.; Abu Saadeh, F. The dilemma of managing thyroid gland after incidental diagnosis of malignant struma Ovarii. Is radical thyroidectomy and radioactive iodine Necessary? A case report and literature review. Gynecol. Oncol. Rep. 2023, 47, 101189. [Google Scholar] [CrossRef] [PubMed]
- Shaco-Levy, R.; Bean, S.M.; Bentley, R.C.; Robboy, S.J. Natural history of biologically malignant struma ovarii: Analysis of 27 cases with extraovarian spread. Int. J. Gynecol. Pathol. 2010, 29, 212–227. [Google Scholar] [CrossRef]
- Li, S.; Kong, S.; Wang, X.; Zhang, X.; Yin, M.; Yang, J. Survival Outcomes and Prognostic Predictors in Patients with Malignant Struma Ovarii. Front. Med. 2021, 8, 774691. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.; Stefanova, D.; Thiesmeyer, J.W.; Lee, Y.J.; Greenberg, J.; Beninato, T.; Zarnegar, R.; Christos, P.J.; Klein, I.L.; Fahey, T.J., 3rd; et al. Proposed Risk Stratification and Patterns of Radioactive Iodine Therapy in Malignant Struma Ovarii. Thyroid 2022, 32, 1101–1108. [Google Scholar] [CrossRef]
- Neyrand, S.; Trecourt, A.; Lopez, J.; Just, P.A.; Descotes, F.; Borson-Chazot, F.; Ray-Coquard, I.; Decaussin-Petrucci, M.; Devouassoux-Shisheboran, M. Role of gene sequencing in classifying struma ovarii: BRAF p.G469A mutation and TERT promoter alterations favour malignant struma ovarii. Histopathology 2024, 84, 291–300. [Google Scholar] [CrossRef]
- Schmidt, J.; Derr, V.; Heinrich, M.C.; Crum, C.P.; Fletcher, J.A.; Corless, C.L.; Nosé, V. BRAF in papillary thyroid carcinoma of ovary (struma ovarii). Am. J. Surg. Pathol. 2007, 31, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.; Abu-Sini, H.; Billan, S. Tyrosine kinase inhibitor treatment and long-term follow-up for metastatic malignant struma ovarii. Pediatr. Hematol. Oncol. 2022, 39, 769–775. [Google Scholar] [CrossRef]
- Seban, R.D.; Bozec, L.; Nascimento-Leite, C.; Champion, L. Metabolic Response by 18F-FDG PET/CT in Metastatic Malignant Struma Ovarii Treated with Targeted Therapies. Clin. Nucl. Med. 2021, 46, 52–54. [Google Scholar] [CrossRef]
- Smith, L.P.; Brubaker, L.W.; Wolsky, R.J. It Does Exist! Diagnosis and Management of Thyroid Carcinomas Originating in Struma Ovarii. Surg. Pathol. Clin. 2023, 16, 75–86. [Google Scholar] [CrossRef] [PubMed]

| First Author | Ref. No. | Year | Country | N. Pts. | Age (Median) [Range] | DTC in SO:Other SO |
|---|---|---|---|---|---|---|
| Wei S | [3] | 2015 | USA | 11 | 59 years [35, 72] | 11:85 |
| Goffredo P | [6] | 2015 | USA | 68 | 43 years [16, 79] | 68:0 |
| Garg K | [7] | 2009 | USA | 10 | 41.5 years [32, 64] | 10:0 |
| Ryu HJ | [23] | 2024 | South Korea | 15 | 48 years [30, 70] | 15:155 |
| Gadducci | [24] | 2019 | Italy | 5 | na ^ | 5:0 |
| Li S | [25] | 2023 | China | 13 | na ^ | 13:262 |
| Addley S | [26] | 2021 | USA | 6 | na ^ | na |
| Devaney K | [27] | 1993 | USA | 13 | 50 years [30, 77] | 13:41 |
| Roth LM | [29] | 2008 | USA | 4 | 51 years [26, 70] | 4:0 |
| Marti JL | [30] | 2012 | USA | 4 | 44 years [43, 57] | 4:0 |
| Poli R | [31] | 2020 | Italy | 6 | 64.5 years [52, 66] | 6:0 |
| First Author | Ref. No. | PTC:FTC of HDFC:Other | FU Time (Mean) | Pelvic/Abdominal Surgery:TT:TT + RAI | Intraovarian:Extraovarian at Diagnosis | M0:M1 at Diagnosis | NED After First Line Therapy |
|---|---|---|---|---|---|---|---|
| Wei S | [3] | 10:1:0 | 79 months [1, 204] | 9:0:2 | 9:2 | 10:1 | 11:0 |
| Goffredo P | [6] | na | 96 months [2, 408] | na:na:6 | 52:13 * | 56:9 * | na |
| Garg K | [7] | 8:0:2 | 54 months [12, 168] | 10:0:0 | 10:0 | 10:0 | 8 |
| Ryu HJ | [23] | 7:4:4 | 33 months [4, 156] ** | 10:4:1 *** | 12:3 | 12:3 | 11:2 ** |
| Gadducci | [24] | 3:2:0 | 60 months [38, 203] | 4:0:1 *** | 5:0 | 5:0 | 5:0 |
| Li S | [25] | 8:5:0 | 74.4 months [9.6, 348] | 12:0:1 | 10:3 | 10:3 | 11:2 |
| Addley S | [26] | 6:0:0 | na | 3:0:3 | 6:0 | 6:0 | 6:0 |
| Devaney K | [27] | 11:2:0 | 87.6 months [24, 216] | 13:0:0 | 12:1 | 13:0 ^^ | 13:0 |
| Roth LM | [29] | 3:1:0 | na | 3:01 | 3:1 | na | na |
| Marti JL | [30] | 3:1:0 | 108 months [9.6, 156] | 3:0:1 | 4:0 | 4:0 | 4:0 |
| Poli R | [31] | 5:0:1 | 104 months [24, 240] | 5:0:1 | 6:0 | 6:0 | 6:0 |
| First Author | Ref. No. | Disease Relapse:No Disease Relapse During FU | RAI After Relapse |
|---|---|---|---|
| Wei S | [3] | 1:10 | na |
| Goffredo P | [6] | na | na |
| Garg K | [7] | 2:8 | 2 |
| Ryu HJ | [23] | 0:11 **** | na |
| Gadducci | [24] | 0:5 | na |
| Li S | [25] | 3:8 ***** | 2 |
| Addley S | [26] | 0:6 | na |
| Devaney K | [27] | 0:13 | na |
| Roth LM | [29] | na | na |
| Marti JL | [30] | 0:4 | na |
| Poli R | [31] | 0:6 | na |
| First Author | Ref. No. | Setting | Main Findings | TT + RAI |
|---|---|---|---|---|
| Wei S | [3] | Describe the pathology of SO in a single center experience. 11 of 96 of them were characterized as DTC in SO | PTC was the most frequent variant of DTC in SO (90.9%). TT and further RAI were performed only in 2 patients. The only 1 PTC patients with metastases discovered during follow up was also the only 1 with extraovarian spread at diagnosis. | RAI was performed in 2 patients: one presented a PTC fullicular variant (FVPTC) with round ligament involvement at diagnosis, the other one a FVPTC of 2.5 cm at diagnosis |
| Goffredo P | [6] | Describe the pathology, treatment and prognosis of MSO in the large database of Surveillance, Epidemiology, and End Results (SEER). 68 Patient with MSO defined as histologically identical to DTC were selected. | Radiation was not commonly administered as part of the treatment algorithm: only 6 patient received RAI (5 alone, 1 with EBRT). Overall survival rates (OSR) at 5, 10, and 20 years were 96.7%, 94.3%, and 84.9% respectively. | RAI was performed in 6 patients, particularly in 3 patients with concomitant or subsequent thyroid cancer. Histology was not reported. |
| Garg K | [7] | Describe the histopathology of MSO in a single center experience. 10 Patients with DTC developed in SO were described. | TT and further RAI were performed in the only 2 patients with metastases and/or disease relapse. Three patients with risk factor were treated conservatively. No cases of concomitant thyroid cancer were detected. | RAI was performed in 2 patients with a FVPTC in SO who developed metastases. In one case whole body scan (WBS) after RAI reveal a large liver metastasis. Both were alive with disease at the end of follow up. |
| Ryu HJ | [23] | Describe the pathology of SO in a single center experience. 15 of 170 of them were characterized as DTC in SO | PTC variant was the most frequent variant of DTC in SO (46.7%). Four patients presented also thyroid cancer. TT was performed in 4 patients, 3 for the thyroid cancer and one for MSO with metastases. Further RAI was performed in 3 of these patients. | RAI was performed in 2 patients with thyroid cancer and only in one case of MSO with metastases. No patients died for MSO. |
| Gadducci | [24] | Describe the pathology of malignant mature cystic teratoma in a multicentre experience. 5 of 23 of them were characterized as DTC. | PTC was the prevalent histotype (60%), all patients had a stage I FIGO. One patient had a previous thyroid cancer and performed RAI for them. No patients performed RAI after diagnosis of DTC in SO. | No patients presented metastases and/or died for MSO. No RAI was performed |
| Li S | [25] | Analyzing the prevalence and pathology of ovarian strumal disease in a single center experience. 275 patients with ovarian stumal disease, particularly 13 with MSO, were selected. | PTC was the predominant histotype (46.2%), 3 patients had metastases at diagnosis. Six patients received adjuvant therapy, but only 1 patient received TT + RAI at diagnosis. Three patients had disease relapse and one of them who not performed RAI died. | RAI was performed in 1 patient with metastatic MSO at diagnosis and 2 with disease relapse: two of them had a partial response and one a complete response. |
| Addley S | [26] | Describe the pathology of MSO in a single center experience. 11 patients were analyzed, particularly 6 patients with DTC in SO. | Patient with high risk, defined by aggressive histopathological features of PTC, a significant (>15 mm) disease deposit, close surgical margins and/or lympho-vascular invasion could benefit by TT and further RAI. None of the patients had metastases at diagnosis and none relapsed and/or died during FU. | TT + RAI was performed in 3 patients classified as “high risk”. |
| Devaney K | [27] | Describe the pathology of MSO in a single center experience. 41 patients were analyzed, particularly 11 patients with DTC in SO. | PTC was the prevalent histotype (84.6%). All of the patients performed only surgical therapy. One patient presented peritoneal involvement, but no adjuvant therapy was performed. | No RAI was performed. No patients died for MSO. |
| Roth LM | [29] | Describe 4 cases of DTC in MSO occurred in two centers experience. | Three PTC and one poorly differentiated FTC in SO were reported. This last patient presented metastases at diagnosis and performed chemotherapy and RAI but died anyway after 3 years. | TT + RAI was performed in one patient with poorly differentiated FTC. |
| Marti JL | [30] | Describe 4 cases of DTC in MSO occurred in a single center experiences. | Three PTC and one FTC in SO were reported. One patient performed TT + RAI with the discovery of a pT3N1a thyroid cancer. All patient achieved NED after therapy, and none died. | TT + RAI was performed in one patient with complete response. |
| Poli R | [31] | Analyzed the genetic characteristics of 6 DTC arised in SO in a multicentric experience | PTC was the main histotype (80%). BRAF, JAK3 and NRAS mutations were found. All of the patients achieved NED after therapy and no disease recrudescence and/or death occurred during FU. | TT + RAI was performed in one patient with PDC in SO with vascular invasion with a complete response. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, P.; Dondi, F.; Zilioli, V.; Gatta, E.; Cavadini, M.; Cappelli, C.; Viganò, G.L.; Bertagna, F. The Role of Radioiodine Therapy in Differentiated Thyroid Cancer Arising from Struma Ovarii: A Systematic Review. J. Clin. Med. 2024, 13, 7729. https://doi.org/10.3390/jcm13247729
Bellini P, Dondi F, Zilioli V, Gatta E, Cavadini M, Cappelli C, Viganò GL, Bertagna F. The Role of Radioiodine Therapy in Differentiated Thyroid Cancer Arising from Struma Ovarii: A Systematic Review. Journal of Clinical Medicine. 2024; 13(24):7729. https://doi.org/10.3390/jcm13247729
Chicago/Turabian StyleBellini, Pietro, Francesco Dondi, Valentina Zilioli, Elisa Gatta, Maria Cavadini, Carlo Cappelli, Gian Luca Viganò, and Francesco Bertagna. 2024. "The Role of Radioiodine Therapy in Differentiated Thyroid Cancer Arising from Struma Ovarii: A Systematic Review" Journal of Clinical Medicine 13, no. 24: 7729. https://doi.org/10.3390/jcm13247729
APA StyleBellini, P., Dondi, F., Zilioli, V., Gatta, E., Cavadini, M., Cappelli, C., Viganò, G. L., & Bertagna, F. (2024). The Role of Radioiodine Therapy in Differentiated Thyroid Cancer Arising from Struma Ovarii: A Systematic Review. Journal of Clinical Medicine, 13(24), 7729. https://doi.org/10.3390/jcm13247729





