A Randomized Controlled Trial on the Efficacy of 20% Human Albumin in Reducing Pleural Effusion After Cardiopulmonary Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Patients
2.3. Interventions
2.4. Outcomes
2.5. Statistical Data Analysis
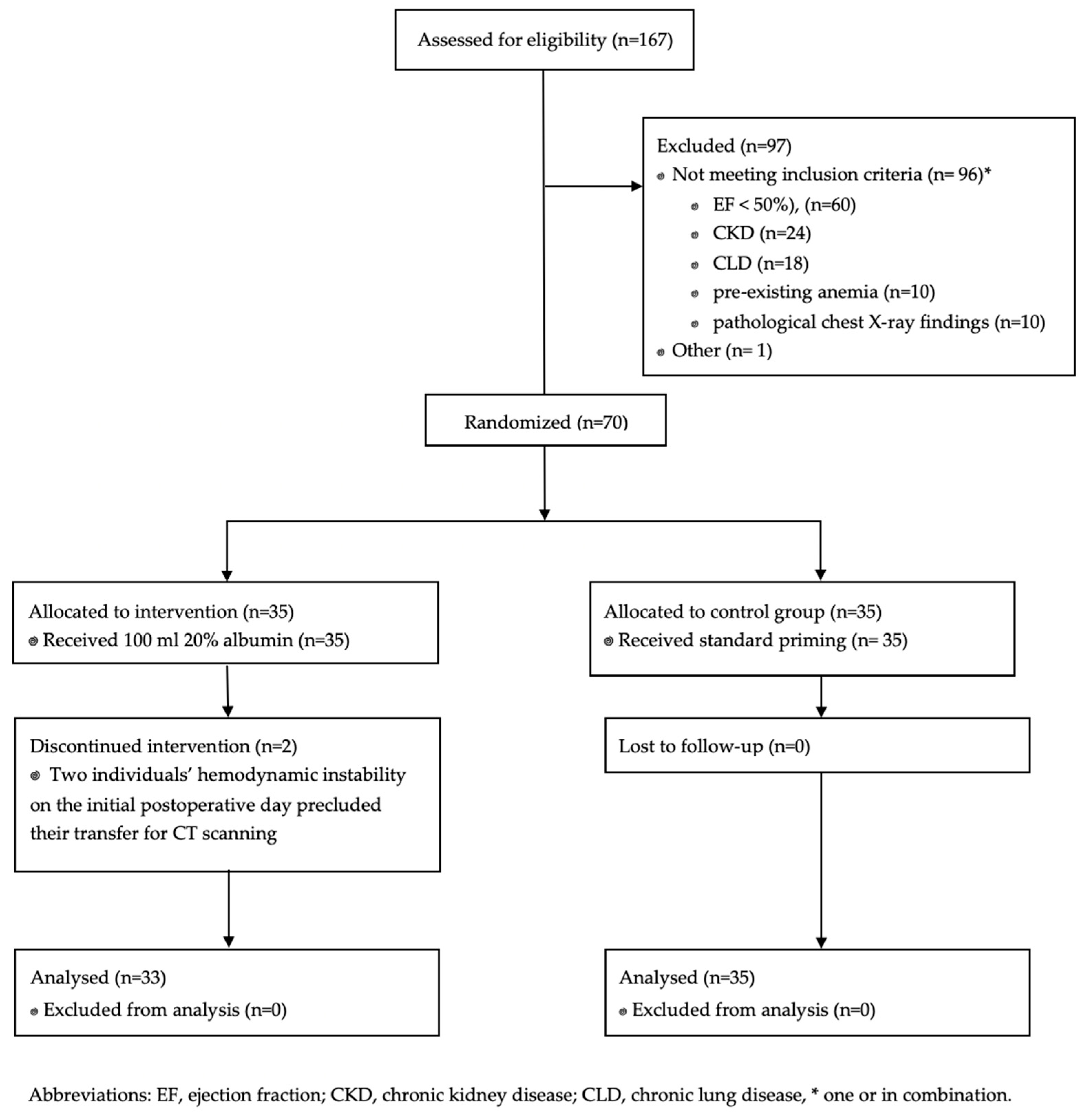
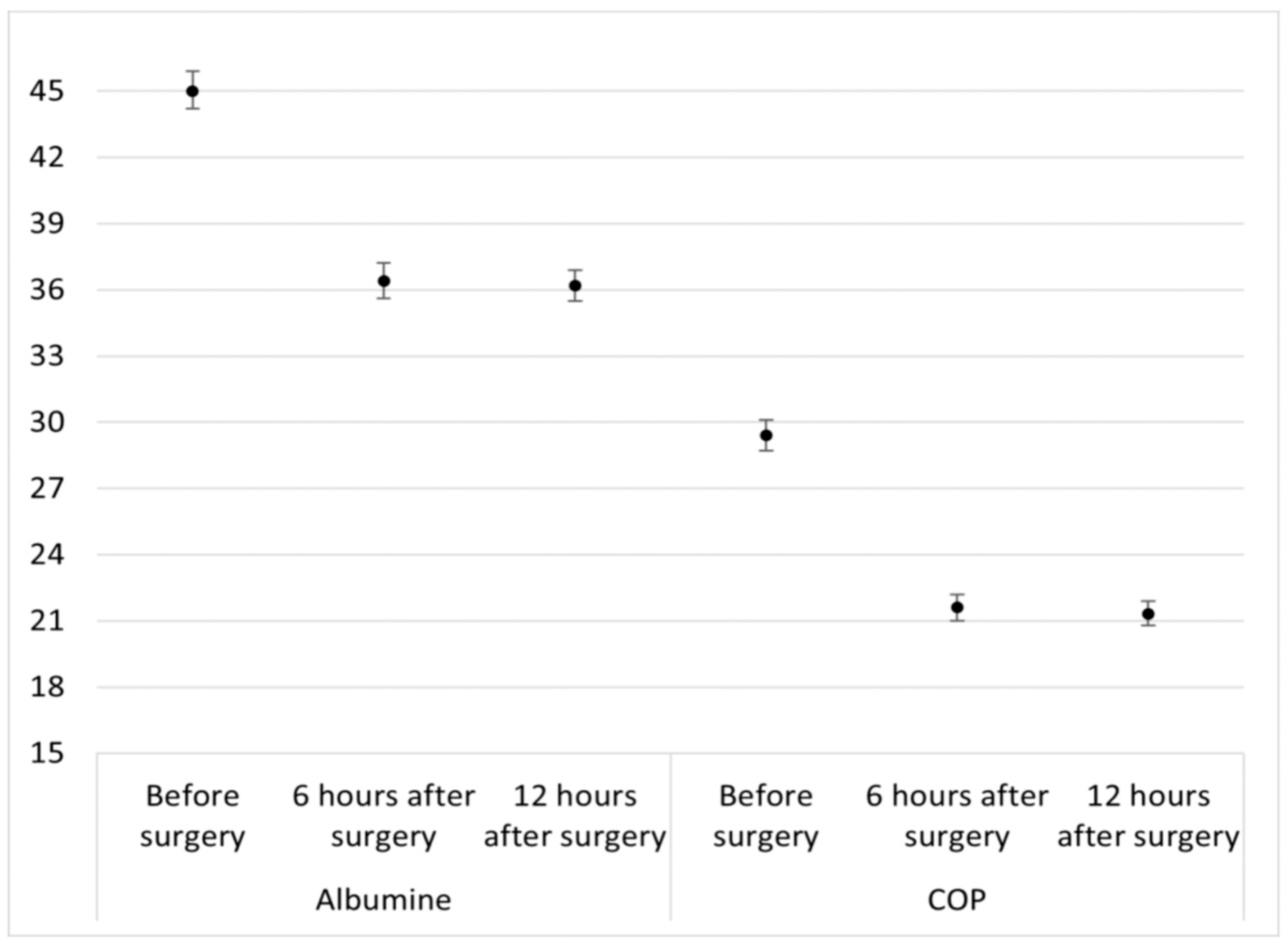
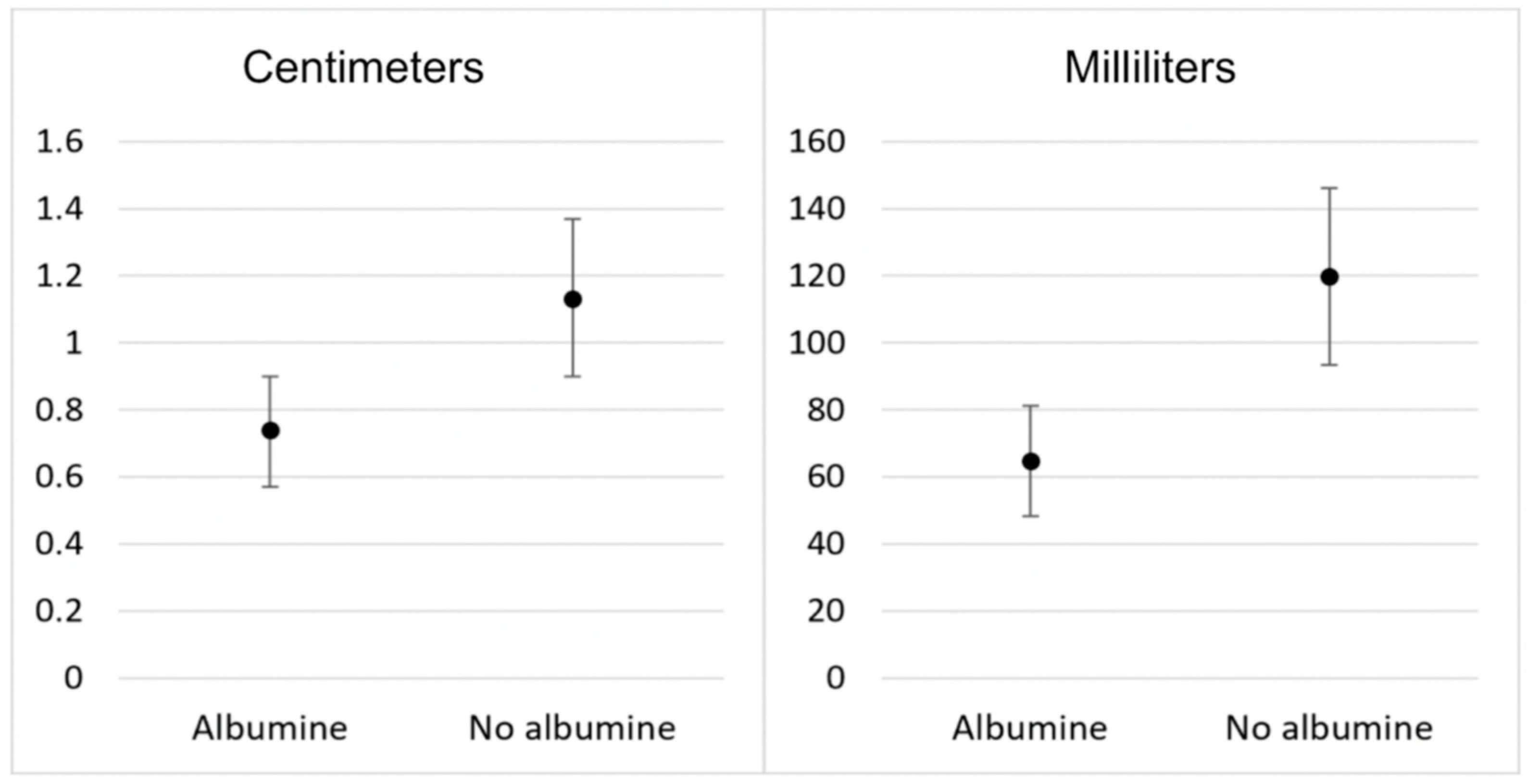
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huffmyer, J.L.; Groves, D.S. Pulmonary complications of cardiopulmonary bypass. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, A.; Korb, H.; Mehlhorn, U.; Stephan, H.; Sonntag, H. Priming of cardiopulmonary bypass with human albumin or Ringer lactate: Effect on colloid osmotic pressure and extravascular lung water. Br. J. Anaesth. 1991, 66, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Haghaninejad, H. Pulmonary complications following cardiac surgery. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, e280–e285. [Google Scholar] [CrossRef] [PubMed]
- den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef]
- Doguet, F.; Tamion, F.; Le Guillou, V.; Bubenheim, M.; Thuillez, C.; Richard, V.; Bessou, J.P. Albumin limits mesenteric endothelial dysfunction and inflammatory response in cardiopulmonary bypass. Artif. Organs 2012, 36, 962–971. [Google Scholar] [CrossRef]
- Vlasov, H.; Juvonen, T.; Hiippala, S.; Suojaranta, R.; Peltonen, M.; Schramko, A.; Arvonen, K.; Salminen, U.S.; Kleine Budde, I.; Eränen, T.; et al. Effect and safety of 4% albumin in the treatment of cardiac surgery patients: Study protocol for the randomized, double-blind, clinical ALBICS (ALBumin In Cardiac Surgery) trial. Trials 2020, 21, 235. [Google Scholar] [CrossRef]
- Beukers, A.M.; Hugo, J.V.; Haumann, R.G.; Boltje, J.W.T.; Ie, E.L.K.; Loer, S.A.; Bulte, C.S.E.; Vonk, A. Changes in colloid oncotic pressure during cardiac surgery with different prime fluid strategies. Perfusion 2024, 39, 1371–1379. [Google Scholar] [CrossRef]
- Berbel-Franco, D.; Lopez-Delgado, J.C.; Putzu, A.; Esteve, F.; Torrado, H.; Farrero, E.; Rodríguez-Castro, D.; Carrio, M.L.; Landoni, G. The influence of postoperative albumin levels on the outcome of cardiac surgery. J. Cardiothorac. Surg. 2020, 15, 78. [Google Scholar] [CrossRef]
- Moeller, C.; Fleischmann, C.; Thomas-Rueddel, D.; Vlasakov, V.; Rochwerg, B.; Theurer, P.; Gattinoni, L.; Reinhart, K.; Hartog, C.S. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J. Crit. Care 2016, 35, 75–83. [Google Scholar] [CrossRef]
- Maffezzoni, M.; Bellini, V. Con: Mechanical Ventilation During Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2024, 38, 1045–1048. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Xie, W.; Wang, Y.; Xu, Z.; Bai, Y.X.; Zhou, Q.; Wu, Q. Risk Factors and Short-Term Outcomes of Postoperative Pulmonary Complications in Elderly Patients After Cardiopulmonary Bypass. Clin. Interv. Aging 2024, 19, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.G.; Colvin, M.O. Pulmonary Complications of Cardiac Surgery. Lung 2020, 198, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Nematbakhsh, M.; Moradi, A. A new equation for calculation of colloid osmotic pressure based on serum total protein concentration and UV-light absorption. Curr. Anaesth. Crit. Care 2008, 19, 8–11. [Google Scholar] [CrossRef]
- Hoefs, J.C. Globulin correction of the albumin gradient: Correlation with measured serum to ascites colloid osmotic pressure gradients. Hepatology 1992, 16, 396–403. [Google Scholar] [CrossRef]
- Hazlinger, M.; Ctvrtlik, F.; Langova, K.; Herman, M. Quantification of pleural effusion on CT by simple measurement. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2014, 158, 107–111. [Google Scholar] [CrossRef]
- Khademi, S.; Heirany, F.; Jouybar, R.; Dehghanpisheh, L.; Ghazinoor, M.; Mehr, L.S. Effect of albumin usage during cardiopulmonary bypass on postoperative acute kidney injury in cardiac surgery patients: A historical cohort study. Ann. Card. Anaesth. 2023, 26, 288–294. [Google Scholar] [CrossRef]
- Skubas, N.J.; Callum, J.; Bathla, A.; Keshavarz, H.; Fergusson, D.; Wu, B.; Stanworth, S.; Shehata, N. Intravenous albumin in cardiac and vascular surgery: A systematic review and meta-analysis. Br. J. Anaesth. 2024, 132, 237–250. [Google Scholar] [CrossRef]
- Pesonen, E.; Vlasov, H.; Suojaranta, R.; Hiippala, S.; Schramko, A.; Wilkman, E.; Eränen, T.; Arvonen, K.; Mazanikov, M.; Salminen, U.S.; et al. Effect of 4% Albumin Solution vs Ringer Acetate on Major Adverse Events in Patients Undergoing Cardiac Surgery With Cardiopulmonary Bypass: A Randomized Clinical Trial. JAMA 2022, 328, 251–258. [Google Scholar] [CrossRef]
- Weissman, C. Pulmonary complications after cardiac surgery. Semin. Cardiothorac. Vasc. Anesth. 2004, 8, 185–211. [Google Scholar] [CrossRef]
- Setlers, K.; Jurcenko, A.; Arklina, B.; Zvaigzne, L.; Sabelnikovs, O.; Stradins, P.; Strike, E. Identifying Early Risk Factors for Postoperative Pulmonary Complications in Cardiac Surgery Patients. Medicina 2024, 60, 1398. [Google Scholar] [CrossRef]
- Klanderman, R.B.; Bosboom, J.J.; Korsten, H.; Zeiler, T.; Musson, R.E.A.; Veelo, D.P.; Geerts, B.F.; van Bruggen, R.; de Korte, D.; Vlaar, A.P.J. Colloid osmotic pressure of contemporary and novel transfusion products. Vox Sang. 2020, 115, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Verheij, J.; van Lingen, A.; Raijmakers, P.G.; Rijnsburger, E.R.; Veerman, D.P.; Wisselink, W.; Girbes, A.R.; Groeneveld, A.B. Effect of fluid loading with saline or colloids on pulmonary permeability, oedema and lung injury score after cardiac and major vascular surgery. Br. J. Anaesth. 2006, 96, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, W.J.; Driedger, A.A.; Wells, G.A.; Myers, M.L.; Lefcoe, M. The short-term effects of increasing plasma colloid osmotic pressure in patients with noncardiac pulmonary edema. Surgery 1983, 93, 620–633. [Google Scholar] [PubMed]
- Staton, G.W.; Williams, W.H.; Mahoney, E.M.; Hu, J.; Chu, H.; Duke, P.G.; Puskas, J.D. Pulmonary outcomes of off-pump vs on-pump coronary artery bypass surgery in a randomized trial. Chest 2005, 127, 892–901. [Google Scholar] [CrossRef]
- Jain, A.; Devarajan, A.; Assallum, H.; Malekan, R.; Lanier, G.M.; Epelbaum, O. Characteristics of early pleural effusions after orthotopic heart transplantation: Comparison with coronary artery bypass graft surgery. Pleura Peritoneum 2021, 6, 161–165. [Google Scholar] [CrossRef]
- Labidi, M.; Baillot, R.; Dionne, B.; Lacasse, Y.; Maltais, F.; Boulet, L.P. Pleural effusions following cardiac surgery: Prevalence, risk factors, and clinical features. Chest 2009, 136, 1604–1611. [Google Scholar] [CrossRef]
- Peng, M.J.; Vargas, F.S.; Cukier, A.; Terra-Filho, M.; Teixeira, L.R.; Light, R.W. Postoperative pleural changes after coronary revascularization. Comparison between saphenous vein and internal mammary artery grafting. Chest 1992, 101, 327–330. [Google Scholar] [CrossRef]
- Hurlbut, D.; Myers, M.L.; Lefcoe, M.; Goldbach, M. Pleuropulmonary morbidity: Internal thoracic artery versus saphenous vein graft. Ann. Thorac. Surg. 1990, 50, 959–964. [Google Scholar] [CrossRef]
- Landymore, R.W.; Howell, F. Pulmonary complications following myocardial revascularization with the internal mammary artery graft. Eur. J. Cardiothorac. Surg. 1990, 4, 156–161; discussion 61–62. [Google Scholar] [CrossRef]
- Light, R.W.; Rogers, J.T.; Moyers, J.P.; Lee, Y.C.; Rodriguez, R.M.; Alford, W.C., Jr.; Ball, S.K.; Burrus, G.R.; Coltharp, W.H.; Glassford, D.M., Jr.; et al. Prevalence and clinical course of pleural effusions at 30 days after coronary artery and cardiac surgery. Am. J. Respir. Crit. Care Med. 2002, 166, 1567–1571. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wu, T.; Zhang, A.; Li, Y. Association between obesity and systemic immune inflammation index, systemic inflammation response index among US adults: A population-based analysis. Lipids Health Dis. 2024, 23, 245. [Google Scholar] [CrossRef] [PubMed]
- Henriques, V.T.; Martinez, E.Z.; Divino-Filho, J.C.; Pecoits-Filho, R.; Cardeal da Costa, J.A. Increase in BMI Over Time Is Associated With Fluid Overload and Signs of Wasting in Incident Peritoneal Dialysis Patients. J. Ren. Nutr. 2013, 23, e51–e57. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Park, H.J.; Baik, J.E.; Li, C.; Shin, J.; Mehrara, B.J. Regulation of Lymphatic Function in Obesity. Front. Physiol. 2020, 11, 459. [Google Scholar] [CrossRef] [PubMed]
| Albumin (+) (35 Patients; 50%) | Albumin (−) (35 Patients; 50%) | p-Value | |
|---|---|---|---|
| Age (mean; SD) | 66.7 (12) | 69.3 (8) | 0.119 |
| Men (No. of patients; No. %) | 23 (66) | 27 (77) | 0.184 |
| Women (No. of patients; No. %) | 12 (34) | 8 (23) | 0.281 |
| BMI (mean; SD) | 28.6 (5) | 31.5 (3) | 0.027 |
| Type of surgery (No. %) | |||
| Coronary artery bypass graft (only) | 15 (43) | 20 (57) | 0.480 |
| CABG + valve replacement surgery | 2 (6) | 3 (9) | 0.648 |
| 1 valve replacement surgery (only) | 20 (57) | 16 (46) | 0.346 |
| 2 valve replacement surgery (only) | 0 (0) | 1 (3) | 0.321 |
| Preoperative, (mean; SD) | |||
| Hb (g/L) | 138.2 (16) | 138.8 (14) | 0.839 |
| Hct (%) | 41 (5) | 40 (4) | 0.953 |
| Albumin (g/L) | 45.2 (3) | 45.6 (3) | 0.895 |
| Total protein (g/L) | 70.1 (4) | 71.2 (6) | 0.887 |
| Creatinine (µmol/L) | 83.3 (18) | 93.8 (17) | 0.030 |
| Intraoperative (mean, SD) | |||
| Time of cardiopulmonary bypass (min) | 93 (30) | 93 (31) | 0.731 |
| Volume of cardioplegia (mL) | 1914 (565) | 1758 (492) | 0.314 |
| Diuresis (mL) | 1089 (482) | 1334 (761) | 0.120 |
| Postoperative fluid balance (mL) | +870 (546) | +782 (541) | 0.724 |
| Postoperative Hct (%) | 27 (3) | 29 (3) | 0.041 |
| Pleural Effusion | Albumin Level 6 h After Surgery | p | Albumin Level 12 h After Surgery | p | ||
|---|---|---|---|---|---|---|
| ≥35 g/L Median (Q1–Q3) | 30–34 g/L Median (Q1–Q3) | ≥35 g/L Median (Q1–Q3) | 30–34 g/L Median (Q1–Q3) | |||
| Right side (cm) | 0.8 (0–1.0) | 1.0 (0.9–2.0) | 0.001 | 0.8 (0–1.0) | 1.5 (1.0–2.0) | <0.001 |
| Right side (mL) | 36.7 (24.2–67.0) | 67.0 (51.9–215.0) | 0.002 | 36.7 (23.4–67.0) | 142.0 (67.0–215.0) | <0.001 |
| Left side (cm) | 0.8 (0–1.0) | 1.0 (0.9–1.0) | 0.002 | 0.8 (0–1.0) | 2.0 (1.0–2.0) | <0.001 |
| Left side (mL) | 36.7 (24.4–67.0) | 67.0 (51.9–215.0) | 0.004 | 36.7 (23.4–67.0) | 215.0 (67.0–215.0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setlers, K.; Aispure, K.; Zolovs, M.; Zvaigzne, L.; Sabelnikovs, O.; Stradins, P.; Strike, E. A Randomized Controlled Trial on the Efficacy of 20% Human Albumin in Reducing Pleural Effusion After Cardiopulmonary Bypass. J. Clin. Med. 2024, 13, 7693. https://doi.org/10.3390/jcm13247693
Setlers K, Aispure K, Zolovs M, Zvaigzne L, Sabelnikovs O, Stradins P, Strike E. A Randomized Controlled Trial on the Efficacy of 20% Human Albumin in Reducing Pleural Effusion After Cardiopulmonary Bypass. Journal of Clinical Medicine. 2024; 13(24):7693. https://doi.org/10.3390/jcm13247693
Chicago/Turabian StyleSetlers, Kaspars, Klaudija Aispure, Maksims Zolovs, Ligita Zvaigzne, Olegs Sabelnikovs, Peteris Stradins, and Eva Strike. 2024. "A Randomized Controlled Trial on the Efficacy of 20% Human Albumin in Reducing Pleural Effusion After Cardiopulmonary Bypass" Journal of Clinical Medicine 13, no. 24: 7693. https://doi.org/10.3390/jcm13247693
APA StyleSetlers, K., Aispure, K., Zolovs, M., Zvaigzne, L., Sabelnikovs, O., Stradins, P., & Strike, E. (2024). A Randomized Controlled Trial on the Efficacy of 20% Human Albumin in Reducing Pleural Effusion After Cardiopulmonary Bypass. Journal of Clinical Medicine, 13(24), 7693. https://doi.org/10.3390/jcm13247693





