Risk of Surgical Site Infection in Posterior Spine Surgery Using Different Closing Techniques: A Retrospective Study of Two Neurosurgical Centers
Abstract
1. Introduction
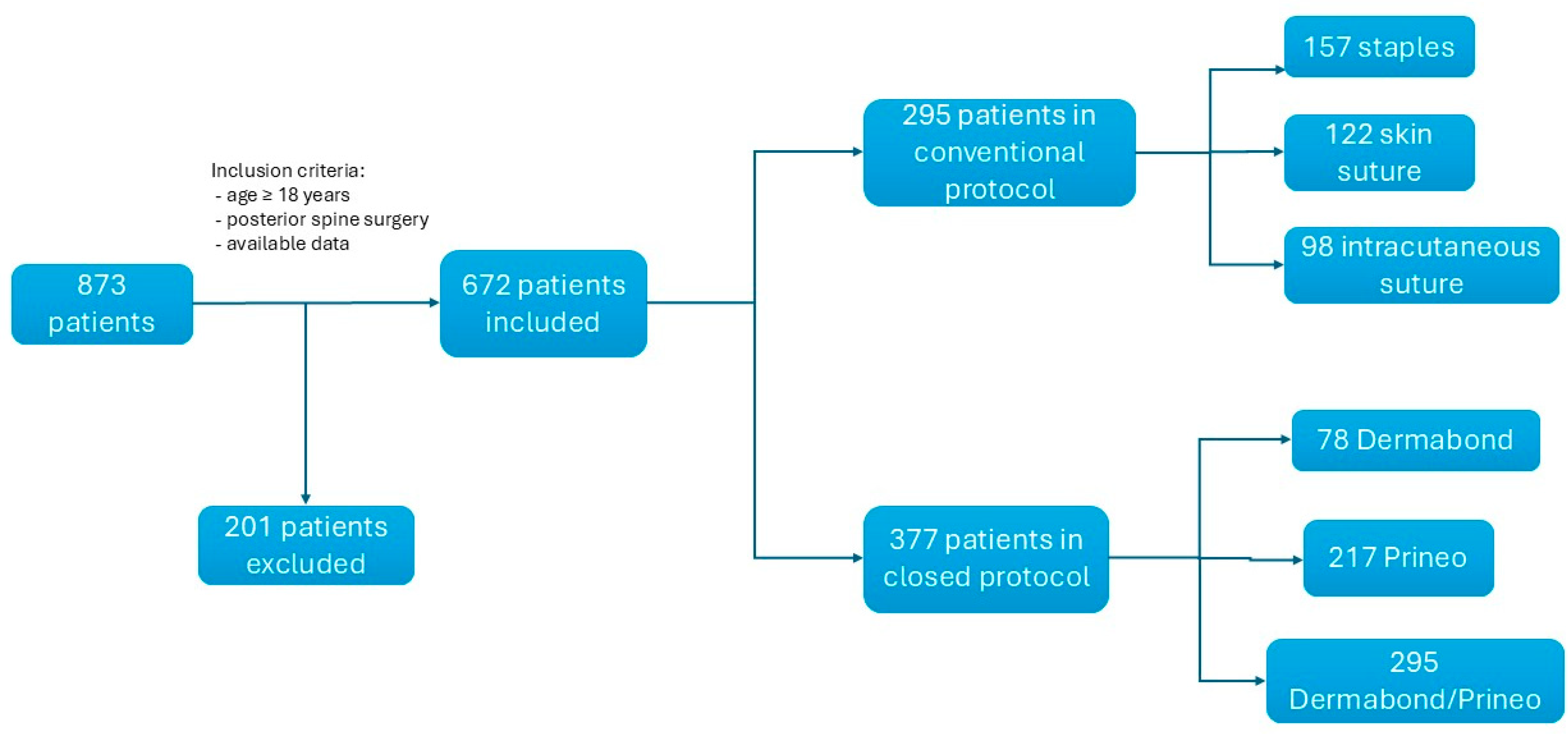
2. Methods
2.1. Institutional Antiseptic Procedure
2.2. Surgical Closure Techniques
2.3. Wound Monitoring
2.4. Statistics
3. Results
3.1. Infection Cohort Characteristics
3.2. Comparison Between the Conventional Protocol and Closed Protocol
3.3. Infection Rate According to the Wound Closure Technique
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| DBP | Dermabond® plus Prineo® |
| DB | Dermabond® |
| NNSI | National Nosocomial Infections Surveillance |
| SSI | Surgical Site Infection |
| NS | Not Significant |
References
- Zhou, J.; Wang, R.; Huo, X.; Xiong, W.; Kang, L.; Xue, Y. Incidence of Surgical Site Infection After Spine Surgery: A Systematic Review and Meta-analysis. Spine 2020, 45, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Owolabi, B.S.; Oseni, A.W.; Kanu, O.O.; Bankole, O.B. Surgical site infection in posterior spine surgery. Niger. J. Clin. Pract. 2016, 19, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, D.K.; Kim, J.W.; Kim, G.W. Particular Features of Surgical Site Infection in Posterior Lumbar Interbody Fusion. Clin. Orthop. Surg. 2015, 7, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.F.; Tomlinson, S.B.; Santangelo, G.; Van Galen, J.; De Andrea-Lazarus, I.; Towner, J.; Kimmell, K.T.; Silberstein, H.; Vates, G.E. Risk factors for wound complications following spine surgery. Surg. Neurol. Int. 2017, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.M.; Rechtine, G.; Norvell, D.C.; Dettori, J.R. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: A systematic review. Spine 2010, 35 (Suppl. S9), S125–S137. [Google Scholar] [CrossRef]
- Barker, F.G. Efficacy of prophylactic antibiotic therapy in spinal surgery: A meta-analysis. Neurosurgery 2002, 51, 391–400; discussion 400–401. [Google Scholar] [CrossRef]
- Yilmaz, E.; Blecher, R.; Moisi, M.; Ankush, C.; O’Lynnger, T.M.; Abdul-Jabbar, A.; Dettori, J.R.; Oskouian, R.J. Is There an Optimal Wound Closure Technique for Major Posterior Spine Surgery? A Systematic Review. Glob. Spine J. 2018, 8, 535–544. [Google Scholar] [CrossRef]
- Andrew Glennie, R.; Dea, N.; Street, J.T. Dressings and drains in posterior spine surgery and their effect on wound complications. J. Clin. Neurosci. 2015, 22, 1081–1087. [Google Scholar] [CrossRef]
- Ando, M.; Tamaki, T.; Yoshida, M.; Sasaki, S.; Toge, Y.; Matsumoto, T.; Maio, K.; Sakata, R.; Fukui, D.; Kanno, S.; et al. Surgical site infection in spinal surgery: A comparative study between 2-octyl-cyanoacrylate and staples for wound closure. Eur. Spine J. 2014, 23, 854–862. [Google Scholar] [CrossRef]
- Johnston, S.S.; Fortin, S.P.; Pracyk, J.B.; Tommaselli, G.A.; Elangovanraaj, N.; Chen, B.P. Economic and clinical outcomes of spinal fusion surgeries with skin closure through skin staples plus waterproof wound dressings versus 2-octyl cyanoacrylate plus polymer mesh tape. Spine J. 2021, 21, 45–54. [Google Scholar] [CrossRef]
- Howard, B.M.; Eshraghi, S.R.; Holland, C.M.; Refai, D. Octyl-cyanoacrylate skin adhesive is effective for wound closure in posterior spinal surgery without increased risk of wound complications. Clin. Neurol. Neurosurg. 2014, 125, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Bhende, S.; Rothenburger, S.; Spangler, D.J.; Dito, M. In Vitro Assessment of Microbial Barrier Properties of Dermabond® Topical Skin Adhesive. Surg. Infect. 2002, 3, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Adogwa, O.; Elsamadicy, A.A.; Han, J.L.; Karikari, I.O.; Cheng, J.; Bagley, C.A. 30-Day Readmission After Spine Surgery: An Analysis of 1400 Consecutive Spine Surgery Patients. Spine 2017, 42, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Rutges, J.; Marion, T.; Hunn, M.; Tee, J. Cyanoacrylate Dermal Closure in Spine Surgery: Systematic Review and Pooled Analysis. Glob. Spine J. 2020, 10, 493–498. [Google Scholar] [CrossRef]
- Mostofi, K.; Peyravi, M.; Shirbacheh, A.; Shirbache, K. A comparison between different suture techniques in lumbar spine surgery. Int. Wound J. 2023, 20, 296–301. [Google Scholar] [CrossRef]
- Yao, R.; Zhou, H.; Choma, T.J.; Kwon, B.K.; Street, J. Surgical Site Infection in Spine Surgery: Who Is at Risk? Glob. Spine J. 2018, 8 (Suppl. S4), 5S–30S. [Google Scholar] [CrossRef]
- Epstein, N.E. Preoperative measures to prevent/minimize risk of surgical site infection in spinal surgery. Surg. Neurol. Int. 2018, 9, 251. [Google Scholar] [CrossRef]
- Butler, J.S.; Wagner, S.C.; Morrissey, P.B.; Kaye, I.D.; Sebastian, A.S.; Schroeder, G.D.; Morrissey, P.B.; Kaye, I.D.; Sebastian, A.S.D.; Schroeder, G.D.; et al. Strategies for the Prevention and Treatment of Surgical Site Infection in the Lumbar Spine. Clin. Spine Surg. 2018, 31, 323–330. [Google Scholar] [CrossRef]
- Davidoff, C.L.; Rogers, J.M.; Simons, M.; Davidson, A.S. A systematic review and meta-analysis of wound drains in non-instrumented lumbar decompression surgery. J. Clin. Neurosci. 2018, 53, 55–61. [Google Scholar] [CrossRef]
- Takenaka, S.; Makino, T.; Sakai, Y.; Kashii, M.; Iwasaki, M.; Yoshikawa, H.; Kaito, T. Dural tear is associated with an increased rate of other perioperative complications in primary lumbar spine surgery for degenerative diseases. Medicine 2019, 98, e13970. [Google Scholar] [CrossRef]
- Wachter, D.; Brückel, A.; Stein, M.; Oertel, M.F.; Christophis, P.; Böker, D.K. 2-Octyl-cyanoacrylate for wound closure in cervical and lumbar spinal surgery. Neurosurg. Rev. 2010, 33, 483–489. [Google Scholar] [CrossRef]
| Patient Demographic | Total Cohort (n = 672) | Infection Cohort (n = 15) | p-Value | Closed Protocol (n = 295) | Conventional Protocol (n = 377) | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 61.32 ± 15.33 | 63.2 ± 11.3 | NS | 59.76 ± 16.01 | 62.47 ± 14.55 | <0.05 |
| Sex (F, %) | 44.64% (n = 300) | 46.67% (n = 7) | NS | 45.42% (n = 134) | 44.03% (n = 166) | NS |
| BMI (kg/m2) | 26.96 ± 4.87 | 29.46 ± 6.96 | <0.05 | 26.42 ± 4.26 | 27.38 ± 5.26 | 0.01 |
| Active Smoking (Y, %) | 28.57% (n = 192) | 26.67% (n = 4) | NS | 28.14% (n = 83) | 28.91% (n = 109) | NS |
| Comorbidities (n, %) | ||||||
| HIV | 0.45% (n = 3) | 6.67% (n = 1) | NS | 1.00% (n = 3) | 0.00% (n = 0) | NS |
| Cancer | 3.57% (n = 24) | 6.67% (n = 1) | NS | 1.35% (n = 4) | 5.31% (n = 20) | <0.01 |
| Diabetes | 15.03% (n = 101) | 6.67% (n = 1) | NS | 15.25% (n = 45) | 14.85% (n = 56) | NS |
| Immunsuppresion | 2.68% (n = 18) | 13.33% (n = 2) | NS | 2.37% (n = 7) | 2.92% (n = 11) | NS |
| Steroid treatment | 2.53% (n = 17) | 13.33% (n = 2) | NS | 3.05% (n = 9) | 2.12% (n = 8) | NS |
| MRSA status | 0.30% (n = 2) | 0.00% (n = 0) | NS | 0.00% (n = 0) | 0.68% (n = 2) | NS |
| Other site infection | 2.38% (n = 16) | 20.00% (n = 3) | <0.005 | 1.70% (n = 5) | 2.92% (n = 11) | NS |
| Risk Factors | ||||||
| ASA Score | ||||||
| 1 | 11.01% (n = 74) | 0.00% (n = 0) | NS | 15.25% (n = 45) | 7.69% (n = 29) | <0.001 |
| 2 | 65.63% (n = 441) | 60.00% (n = 9) | 66.78% (n = 197) | 64.72% (n = 244) | ||
| 3 | 22.92% (n = 154) | 40.00% (n = 6) | 17.29% (n = 51) | 27.32% (n = 103) | ||
| 4 | 0.45% (n = 3) | 0.00% (n = 0) | 0.68% (n = 2) | 0.26% (n = 1) | ||
| NNIS | ||||||
| 0 | 68.16% (n = 458) | 46.67% (n = 7) | <0.005 | 69.49% (n = 205) | 67.11% (n = 253) | NS |
| 1 | 28.42% (n = 191) | 33.33% (n = 2) | 26.44% (n = 78) | 29.97% (n = 113) | ||
| 2 | 3.27% (n = 22) | 20.00% (n = 3) | 4.07% (n = 12) | 2.65% (n = 10) | ||
| 3 | 0.15% (n = 1) | 0.00% (n = 0) | 0.00% (n = 0) | 0.26% (n = 1) | ||
| SSIRS | 2.02% ± 2.85% | 3.41% ± 3.20% | NS | 1.43% ± 0.87 | 2.49% ± 3.67% | <0.001 |
| Surgical Factors | ||||||
| Emergency Surgery (Y) | 9.67% (n = 65) | 20.00% (n = 3) | NS | 6.78% (n = 20) | 11.94% (n = 45) | <0.05 |
| Length of Surgery (min) | 141.00 ± 86.36 | 197.87 ± 118.33 | NS | 132.30 ± 90.05 | 147.75 ± 82.86 | <0.05 |
| Dural Tear (Y) | 7.14% (n = 48) | 6.67% (n = 1) | NS | 5.08% (n = 15) | 8.75% (n = 33) | NS |
| Blood Loss (mL) | 138.93 ± 241.12 | 313.33 ± 384.27 | NS | 121.90 ± 257.63 | 152.29 ± 226.81 | NS |
| Placement of drain (Y) | 42.56% (n = 286) | 66.67% (n = 10) | NS | 36.61% (n = 108) | 47.21% (n = 178) | <0.01 |
| Number of Surgical Levels | 1.37 ± 1.07 | 2.40 ± 2.67 | NS | 1.31 ± 1.08 | 1.42 ± 1.06 | NS |
| Revision Surgery (Y) | 2.38% (n = 16) | 0.00% (n = 0) | NS | 0.68% (n = 2) | 6.37% (n = 24) | <0.001 |
| Staple Closure | Skin Suture Closure | Intracutaneous Closure | Dermabond | Prineo | Dermabond/Prineo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional Protocol | Closed Protocol | |||||||||||
| Closure technique | Staples 23.36% (n = 157) | Other 76.64% (n = 515) | Skin suture 18.15% (n = 122) | Other 81.85% (n = 550) | Intracutaneous suture 14.58% (n = 98) | Other 85.42% (n = 574) | DB 11.61% (n = 78) | Other 88.39% (n = 594) | Prineo alone 32.29% (n = 217) | Other 67.71% (n = 455) | DBP 43.90% (n = 295) | Other 56.10% (n = 377) |
| SSI rate | 2.55% (n = 4/257) | 2.14% (n = 11/515) | 4.10% (n = 5/122) | 1.82% (n = 10/550) | 3.06% (n = 3/98) | 2.09% (n = 12/574) | 1.28% (n = 1/78) | 2.36% (n = 14/594) | 1.38% (n = 3/217) | 2.64% (n = 12/455) | 1.36% (n = 4/295) | 2.92% (n = 11/377) |
| p-value | NS | NS | NS | NS | NS | NS | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molliqaj, G.; Lener, S.; Da Broi, M.; Nouri, A.; Silva Baticam, N.; Schaller, K.; Thomé, C.; Girod, P.-P.; Tessitore, E. Risk of Surgical Site Infection in Posterior Spine Surgery Using Different Closing Techniques: A Retrospective Study of Two Neurosurgical Centers. J. Clin. Med. 2024, 13, 7675. https://doi.org/10.3390/jcm13247675
Molliqaj G, Lener S, Da Broi M, Nouri A, Silva Baticam N, Schaller K, Thomé C, Girod P-P, Tessitore E. Risk of Surgical Site Infection in Posterior Spine Surgery Using Different Closing Techniques: A Retrospective Study of Two Neurosurgical Centers. Journal of Clinical Medicine. 2024; 13(24):7675. https://doi.org/10.3390/jcm13247675
Chicago/Turabian StyleMolliqaj, Granit, Sara Lener, Michele Da Broi, Aria Nouri, Nalla Silva Baticam, Karl Schaller, Claudius Thomé, Pierre-Pascal Girod, and Enrico Tessitore. 2024. "Risk of Surgical Site Infection in Posterior Spine Surgery Using Different Closing Techniques: A Retrospective Study of Two Neurosurgical Centers" Journal of Clinical Medicine 13, no. 24: 7675. https://doi.org/10.3390/jcm13247675
APA StyleMolliqaj, G., Lener, S., Da Broi, M., Nouri, A., Silva Baticam, N., Schaller, K., Thomé, C., Girod, P.-P., & Tessitore, E. (2024). Risk of Surgical Site Infection in Posterior Spine Surgery Using Different Closing Techniques: A Retrospective Study of Two Neurosurgical Centers. Journal of Clinical Medicine, 13(24), 7675. https://doi.org/10.3390/jcm13247675






