Continuous Epidural Versus Non-Epidural Pain Management After Minimally Invasive Esophagectomy: A Real-Life, High-Case-Load Center Experience
Abstract
1. Introduction
2. Patients and Methods
2.1. Primary Outcome
2.2. Secondary Outcome
2.3. Operative Procedure
2.4. Catheter Insertion and Perioperative Pain Management
- For TEA (Group: TEA): The thoracic epidural catheter was placed in the interspace T5-7 prior to induction of anesthesia. The insertion site was determined using the classic landmark method, identifying the spinous process of T7 at the line crossing the inferior tip of the scapulae in the sitting position. An 18-gauge epidural needle was inserted through a paramedian or median approach, and the epidural space was identified using the loss of resistance technique. A test dose of 1.5 mL lidocaine 20 mg/mL with 0.005 mg/mL epinephrine was administered to rule out subarachnoid or intravascular placement. The TEA was then started with bupivacaine 2.5 mg/mL (bupivacaine 0.25% Bioren; Sintetica, Bioren, Switzerland) at a rate of 6-to-10 mL/h. No opioids were administered epidurally during the procedure. At the end of surgery, continuous epidural analgesia was maintained with an epidural mixture of bupivacaine 1 mg/mL, fentanyl 2 ug/mL, and epinephrine 2 ug/mL, using a CADD Legacy ambulatory infusion pump (model 6300; Deltec Inc., St Paul, MN, USA). The initial infusion rate was 6–8 mL/h, with additional bolus volumes of 5 mL (lockout time: 1 h).
- For the TAP catheter and PCA or single PCA (Group: TAP + PCA or PCA): In the period after TEA, but before the implementation of the current standard described below, patients received postoperatively either a tunneled, left-sided TAP catheter combined with an intravenous PCA using a CADD Legacy ambulatory infusion pump (model 6300; Deltec Inc., St Paul, MN, USA) or just the PCA. A 0.2% ropivacaine solution (Ropivacaine Fresenius Kabi 2 mg/mL, Fresenius Kabi, Kriens, Switzerland) was administered through the TAP catheter at a rate of 8-to-12 mL/h immediately after completion of surgery. With the intravenous PCA patients received 0.2 mg Hydromorphone (Hydromorphone Sintetica 20 mg/100 mL, Sintetica Switzerland) per dose. A lockout time of 7 min was programmed.
- For single-shot TAP and the PVB catheter and PCA (Group: PVB + PCA): The current pain management at our institution for McKeown MIE consists of an ultrasound-guided bilateral transversus abdominis plane block with 20 mL of Ropivacain 0.375% (Ropivacaine Fresenius Kabil 7.5 mg/mL, Fresenius Kabi Switzerland) for the laparoscopic portion of the MIE. This is performed immediately after induction of anesthesia. After completion of the thoracosopic part of the surgery, the surgeon places a paravertebral catheter (PVB) under direct thoracosopic vision. A 0.25% ropivacaine solution (Ropivacaine Fresenius Kabi 2.5 mg/mL, Fresenius Kabi Switzerland) is then administered through the catheter at a rate of 8-to-12 mL/h. Postoperatively, patients are supplied with an intravenous PCA pump at the same rate as described above.
- Standard intraoperative pain management: Further intraoperative pain management was at the discretion of the anesthesiologist in charge. In the postoperative period, all patients without contraindications received 1 g of metamizole (MINALGIN Inj Lös 1 g/2 mL i.v., Streuli Pharma Uznach, Switzerland) every 8 h. Rescue analgesics in the postoperative period were administered as needed, and the infusion rates of local anesthetics (TEA, TAP, and PVB) were adjusted within the above ranges to pain and sensory (distribution) levels by a member of the pain staff as needed. All catheters were usually left in place for 3 days. Before removal of the catheter, the dose of local anesthetic was reduced to ensure sufficient overlap with the systemic pain medication.
- Rescue analgesia protocols: There were no defined rescue analgesia protocols. Rescue analgesia was administered in the most suitable form determined by our pain staff: In the TEA group, in the first step, the infusion rate was increased (up to 15 mL/h); in the second step the composition of the TEA solution was changed (rotation to a “forte-mixture” of bupivacaine 2.5 mg/mL, fentanyl 2 ug/mL, and epinephrine 2 ug/mL) to achieve enhanced sensory blockade; and finally, if still judged insufficient, an additional PCA was used. In groups containing a PCA as part of the regimen an increase in the bolus volume was considered. Additionally, ketamine infusions were used to provide sufficient analgesia.
2.5. Statistical Methods
3. Results
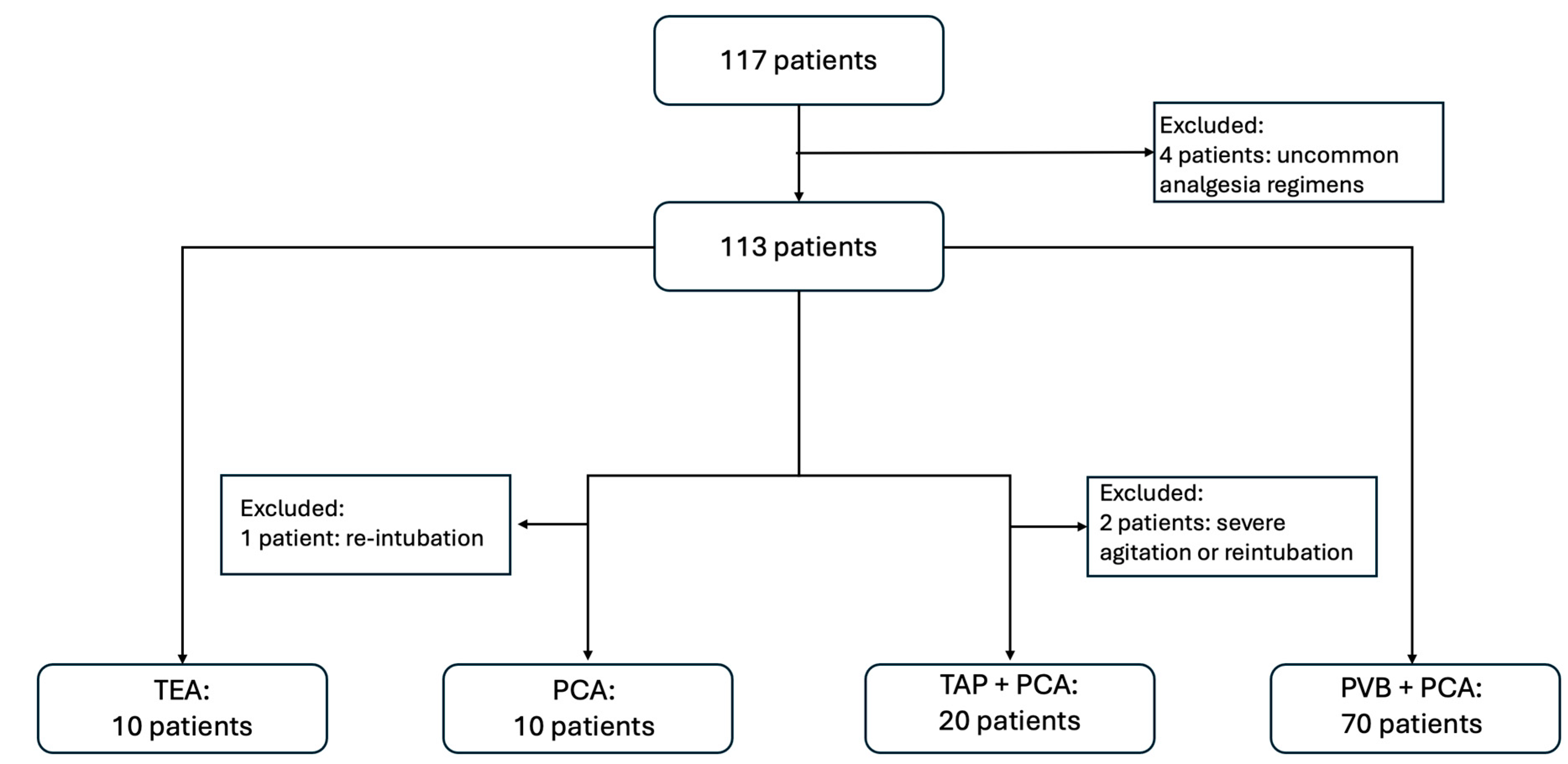
3.1. Participants
3.2. Descriptive and Outcome Data
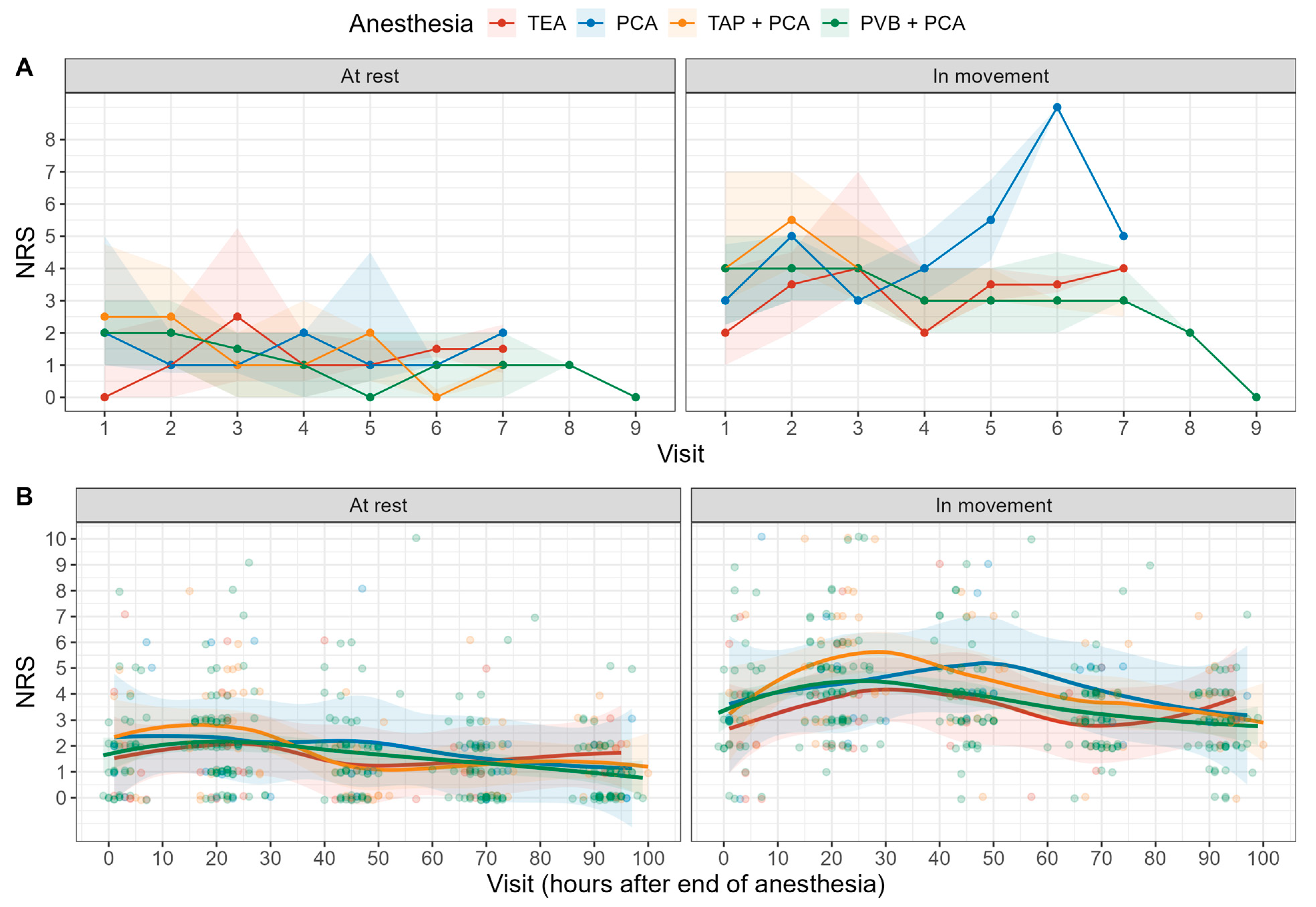
3.3. Primary Outcomes
3.4. Secondary Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Alsop, B.R.; Sharma, P. Esophageal Cancer. Gastroenterol. Clin. N. Am. 2016, 45, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Savage, C.; Mcquitty, C.; Wang, D.; Zwischenberger, J.B. Postthoracotomy pain management. Chest Surg. Clin. N. Am. 2002, 12, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Carli, F.; Kehlet, H.; Baldini, G.; Steel, A.; McRae, K.; Slinger, P.; Hemmerling, T.; Salinas, F.; Neal, J.M. Evidence Basis for Regional Anesthesia in Multidisciplinary Fast-Track Surgical Care Pathways. Reg. Anesth. Pain Med. 2011, 36, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Shea, R.A.; Brooks, J.A.; Dayhoff, N.E.; Keck, J. Pain Intensity and Postoperative Pulmonary Complications among the Elderly after Abdominal Surgery. Heart Lung J. Acute Crit. Care 2002, 31, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.; Marsman, M.; van Rossum, P.S.N.; Cheong, E.; Al-Naimi, K.; van Klei, W.A.; Ruurda, J.P.; van Hillegersberg, R. Postoperative Pain Management after Esophagectomy: A Systematic Review and Meta-Analysis. Dis. Esophagus 2017, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feray, S.; Lubach, J.; Joshi, G.P.; Bonnet, F.; Van de Velde, M.; PROSPECT Working Group *of the European Society of Regional Anaesthesia and Pain Therapy. PROSPECT guidelines for video-assisted thoracoscopic surgery: A systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2022, 77, 311–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coelho, F.d.S.; Barros, D.E.; Santos, F.A.; Meireles, F.C.; Maia, F.C.; Trovisco, R.A.; Machado, T.M.; Barbosa, J.A. Minimally Invasive Esophagectomy versus Open Esophagectomy: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021, 47, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Luketich, J.D.; Pennathur, A.; Awais, O.; Levy, R.M.; Keeley, S.; Shende, M.; Christie, N.A.; Weksler, B.; Landreneau, R.J.; Abbas, G.; et al. Outcomes after Minimally Invasive Esophagectomy: Review of over 1000 Patients. Ann. Surg. 2012, 256, 95–103. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamamoto, H.; Baba, H.; Miyata, H.; Watanabe, M.; Toh, Y.; Matsubara, H.; Kakeji, Y.; Seto, Y. Can Minimally Invasive Esophagectomy Replace Open Esophagectomy for Esophageal Cancer? Latest Analysis of 24,233 Esophagectomies from the Japanese National Clinical Database. Ann. Surg. 2020, 272, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kingma, B.F.; Visser, E.; Marsman, M.; Ruurda, J.P.; van Hillegersberg, R. Epidural analgesia after minimally invasive esophagectomy: Efficacy and complication profile. Dis. Esophagus 2019, 32, doy116. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, M.L.; van Berge Henegouwen, M.I.; Hollmann, M.W.; Hermanides, J.; Eshuis, W.J. Analgesia in Esophagectomy: A Narrative Review. J. Thorac. Dis. 2023, 15, 5099–5111. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.W.; Tabrett, K.; Cheong, E. Paravertebral Catheter Analgesia for Minimally Invasive Ivor Lewis Oesophagectomy. J. Thorac. Dis. 2019, 11, S786–S793. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, M.L.; Ten Hoope, W.; Hermanides, J.; Gisbertz, S.S.; Hollmann, M.W.; Van Berge Henegouwen, M.I.; Eshuis, W.J.; Hermanides, J.; Surg, A. Optimal Perioperative Pain Management in Esophageal Surgery: An Evaluation of Paravertebral Analgesia. Ann. Surg. Oncol. 2021, 28, 6321–6328. [Google Scholar] [CrossRef]
- Feenstra, M.L.; Kooij, C.D.; Eshuis, W.J.; de Groot, E.M.; Hermanides, J.; Kingma, B.F.; Gisbertz, S.S.; Ruurda, J.P.; Daams, F.; Marsman, M.; et al. Paravertebral versus EPidural Analgesia in Minimally Invasive Esophageal ResectioN (PEPMEN). Ann. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lamperti, M.; Romero, C.S.; Guarracino, F.; Cammarota, G.; Vetrugno, L.; Tufegdzic, B.; Lozsan, F.; Macias Frias, J.J.; Duma, A.; Bock, M.; et al. Preoperative Assessment of Adults Undergoing Elective Noncardiac Surgery: Updated Guide-lines from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2024, 41, 1–35. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.W.; Gao, S.G.; Xue, Q.; Mao, Y.S.; Wang, D.; Zhao, J.; Gao, Y.S.; Huang, J.F.; He, J. Updated Experiences with Minimally Invasive McKeown Esophagectomy for Esophageal Cancer. World J. Gastroenterol. 2015, 21, 12873–12881. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 1 August 2024).
- Hermanides, J.; Hollmann, M.W.; Stevens, M.F.; Lirk, P. Failed Epidural: Causes and Management. Br. J. Anaesth. 2012, 109, 144–154. [Google Scholar] [CrossRef] [PubMed]
| ALL | TEA | PCA | TAP + PCA | PVB + PCA | p | |
|---|---|---|---|---|---|---|
| n = 110 | n = 10 | n = 10 | n = 20 | n = 70 | ||
| Sex (female) | 22 (20%) | 4 (40%) | 3 (30%) | 4 (20%) | 11 (16%) | 0.258 |
| Age (yrs, [IQR]) | 64 [58;69] | 67 [63;71] | 66 [64;71] | 63 [56;72] | 64 [57;68] | 0.215 |
| BMI (kg · m−2 [IQR]) | 26 [22;28] | 24 [21;28] | 25 [24;28] | 25 [22;29] | 26 [22;28] | 0.902 |
| ASA status | 0.630 | |||||
| 2 | 7 (6%) | 1 (10%) | 1 (10%) | 0 (0%) | 5 (7%) | |
| 3 | 94 (85%) | 7 (70%) | 8 (80%) | 19 (95%) | 60 (86%) | |
| 4 | 9 (8%) | 2 (20%) | 1 (10%) | 1 (5%) | 5 (7%) | |
| CHD (Yes) | 16 (15%) | 0 (0%) | 4 (40%) | 5 (25%) | 7 (10%) | 0.021 |
| Hypertension (Yes) | 54 (49%) | 5 (50%) | 7 (70%) | 11 (55%) | 31 (44%) | 0.476 |
| COPD | 0.338 | |||||
| GOLD 2 | 1 (1%) | 1 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| GOLD 3 | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | |
| None | 108 (98%) | 9 (90%) | 10 (100%) | 20 (100%) | 69 (99%) | |
| OSAS (Yes) | 8 (7%) | 1 (10%) | 1 (10%) | 2 (10%) | 4 (6%) | 0.941 |
| Metabolism | 0.090 | |||||
| None | 90 (82%) | 10 (100%) | 6 (60%) | 18 (90%) | 56 (80%) | |
| IDDM/NIDDM | 20 (18%) | 0 (0%) | 4 (40%) | 2 (10%) | 14 (20%) | |
| CKD * | 16 (15%) | 1 (10%) | 2 (20%) | 3 (15%) | 10 (14%) | >0.99 |
| Regimen | Risk of NRS > 3 (in Movement) | Risk of NRS > 3 (at Rest) |
|---|---|---|
| TEA | 47.1% (95%-CI: 22.9–71.2%) | 8.3% (95%-CI: −3.2–19.8%) |
| PCA | 51.0% (95%-CI: 24.1–77.8%) | 4.3% (95%-CI: −4.6–13.1%) |
| TAP + PCA | 60.1% (95%-CI: 43.4–76.7%) | 11.2% (95%-CI: 0.5–21.8%) |
| PVB + PCA | 48.3% (95%-CI: 39.4–57.3%) | 5.0% (95%-CI: 1.1–9.0%) |
| Contrasts | Differences in risk of NRS > 3 (in movement) | Differences in risk of NRS > 3 (at rest) |
| TEA vs. PCA | −3.9% (95%-CI: −51.1–43.3%, p > 0.99) | 4.1% (95%-CI: −14.2–22.4%, p = 0.941) |
| TEA vs. TAP + PCA | −13.0% (95%-CI: −51.5–25.6%, p = 0.823) | −2.8% (95%-CI: −22.5–16.9%, p = 0.983) |
| TEA vs. PVB + PCA | −1.3% (95%-CI: −35.0–32.4%, p > 0.99) | 3.3% (95%-CI: −11.7–18.3%, p = 0.943) |
| PCA vs. TAP + PCA | −9.1% (95%-CI: −50.5–32.3%, p = 0.943) | −6.9% (95%-CI: −24.2–10.4%, p = 0.734) |
| PCA vs. PVB + PCA | 2.6% (95%-CI: −34.4–39.7%, p > 0.99) | −0.8% (95%-CI: −12.4–10.8%, p > 0.99) |
| TAP + PCA vs. PVB + PCA | 11.7% (95%-CI: −13.1–36.5%, p = 0.619) | 6.1% (95%-CI: −7.6–19.9%, p = 0.661) |
| ALL | TEA | PCA | TAP + PCA | PVB + PCA | p | |
|---|---|---|---|---|---|---|
| n = 110 | n = 10 | n = 10 | n = 20 | n = 70 | ||
| Induction (min [IQR])) | 32 [26;46] | 37 [24;47] | 40 [32;60] | 32 [26;42] | 32 [26;44] | 0.458 |
| Emergence (min [IQR])) | 35 [24;53] | 31 [20;37] | 26 [20;45] | 52 [32;60] | 34 [24;54] | 0.037 |
| Fentanyl (mcg/kg [IQR]) | 8 [6;11] | 6 [5;8] | 9 [6;11] | 9 [7;11] | 8 [7;11] | 0.079 |
| Hydromorphon (Yes) | 16 (15%) | 1 (10%) | 2 (20%) | 3 (15%) | 10 (14%) | >0.99 |
| Methadon (Yes) | 6 (5%) | 0 (0%) | 1 (10%) | 0 (0%) | 5 (7%) | 0.447 |
| Remifentanil (Yes) | 15 (14%) | 1 (10%) | 2 (20%) | 3 (15%) | 9 (13%) | 0.962 |
| Ringer’s lactate (mL) | 2125 [1900;3000] | 2225 [1700;2925] | 2150 [2000;2875] | 1950 [1300;3000] | 2200 [1925;3000] | 0.357 |
| RCC (Yes): | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | >0.99 |
| FFP (Yes): | 9 (8%) | 2 (20%) | 1 (10%) | 0 (0%) | 6 (9%) | 0.282 |
| TC (No) | 110 (100%) | 10 (100%) | 10 (100%) | 20 (100%) | 70 (100%) | |
| Norepinephrine (mcg/kg [IQR]) | 18 [13;26] | 30 [16;46] | 16 [13;18] | 15 [9;24] | 19 [14;26] | 0.086 |
| Clavien–Dindo | 0.669 | |||||
| 0 | 40 (36%) | 5 (50%) | 2 (20%) | 6 (30%) | 27 (39%) | |
| 1 | 6 (5%) | 0 (0%) | 1 (10%) | 3 (15%) | 2 (3%) | |
| 2 | 23 (21%) | 1 (10%) | 1 (10%) | 7 (35%) | 14 (20%) | |
| 3a | 13 (12%) | 0 (0%) | 3 (30%) | 1 (5%) | 9 (13%) | |
| 3b | 9 (8%) | 1 (10%) | 1 (10%) | 1 (5%) | 6 (9%) | |
| 4 | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | |
| 4a | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | |
| 4b | 8 (7%) | 1 (10%) | 1 (10%) | 0 (0%) | 6 (9%) | |
| 5 | 3 (3%) | 1 (10%) | 0 (0%) | 0 (0%) | 2 (3%) |
| All | TEA | PCA | TAP + PCA | PVB + PCA | p | |
|---|---|---|---|---|---|---|
| n = 110 | n = 10 | n = 10 | n = 20 | n = 70 | ||
| Satisfaction: | 0.806 | |||||
| Not assessed | 11 (10%) | 1 (10%) | 2 (20%) | 3 (15%) | 5 (7%) | |
| Not satisfied | 4 (4%) | 1 (10%) | 0 (0%) | 0 (0%) | 3 (4%) | |
| Satisfied | 25 (23%) | 1 (10%) | 2 (20%) | 4 (20%) | 18 (26%) | |
| Very satisfied | 70 (64%) | 7 (70%) | 6 (60%) | 13 (65%) | 44 (63%) | |
| Number of visits | ||||||
| At rest | 4 [3;5] | 4 [2;5] | 2 [2;4] | 4 [3;5] | 4 [3;5] | 0.222 |
| In movement | 4 [3;5] | 4 [2;5] | 2 [2;3] | 4 [3;5] | 4 [3;5] | 0.067 |
| ALL | TEA | PCA | TAP + PCA | PVB + PCA | p † | N | |
|---|---|---|---|---|---|---|---|
| n = 110 | n = 10 | n = 10 | n = 20 | n = 70 | |||
| Time to first flatus (days) | 5 [4;6] | 6 [4;6] | 4 [3;6] | 4 [4;5] | 5 [4;7] | 0.994 | 73 |
| Time to first BM (days) | 5 [4;6] | 6 [3;6] | 5 [4;6] | 5 [4;6] | 6 [4;7] | 0.606 | 94 |
| Time to first micturition (days) | 5 [3;7] | 6 [2;6] | 6 [4;8] | 6 [4;7] | 5 [3;7] | 0.478 | 81 |
| LOS ICU (days) | 2 [1;4] | 2 [1;3] | 3 [1;5] | 2 [1;3] | 2 [1;4] | 0.688 | 94 |
| LOS Hospital (days) | 15 [12;20] | 15 [9;18] | 14 [13;26] | 14 [11;15] | 15 [12;20] | 0.518 | 95 |
| All | TEA | PCA | TAP + PCA | PVB + PCA | p | |
|---|---|---|---|---|---|---|
| n = 110 | n = 10 | n = 10 | n = 20 | n = 70 | ||
| Undesired effects | 0.256 | |||||
| Not present | 68 (61.8%) | 5 (50.0%) | 7 (70.0%) | 9 (45.0%) | 47 (67.1%) | |
| Present | 42 (38.2%) | 5 (50.0%) | 3 (30.0%) | 11 (55.0%) | 23 (32.9%) | |
| Type (multiple possible): | N = 54 | N = 10 | N = 3 | N = 14 | N = 27 | 0.051 |
| impaired breathing | 28 (51.9%) | 5 (50.0%) | 2 (66.7%) | 5 (35.7%) | 16 (59.3%) | |
| hypotension | 4 (7.4%) | 3 (30.0%) | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) | |
| nausea | 3 (5.6%) | 0 (0.0%) | 1 (33.3%) | 1 (7.1%) | 1 (3.7%) | |
| catheter or pump dysfunction | 19 (35.2%) | 2 (20.0%) | 0 (0.0%) | 7 (50.0%) | 10 (37.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boehler, S.; Huber, M.; Wuethrich, P.Y.; Beilstein, C.M.; Arigoni, S.M.; Furrer, M.A.; Borbély, Y.; Engel, D. Continuous Epidural Versus Non-Epidural Pain Management After Minimally Invasive Esophagectomy: A Real-Life, High-Case-Load Center Experience. J. Clin. Med. 2024, 13, 7669. https://doi.org/10.3390/jcm13247669
Boehler S, Huber M, Wuethrich PY, Beilstein CM, Arigoni SM, Furrer MA, Borbély Y, Engel D. Continuous Epidural Versus Non-Epidural Pain Management After Minimally Invasive Esophagectomy: A Real-Life, High-Case-Load Center Experience. Journal of Clinical Medicine. 2024; 13(24):7669. https://doi.org/10.3390/jcm13247669
Chicago/Turabian StyleBoehler, Sebastian, Markus Huber, Patrick Y. Wuethrich, Christian M. Beilstein, Stefano M. Arigoni, Marc A. Furrer, Yves Borbély, and Dominique Engel. 2024. "Continuous Epidural Versus Non-Epidural Pain Management After Minimally Invasive Esophagectomy: A Real-Life, High-Case-Load Center Experience" Journal of Clinical Medicine 13, no. 24: 7669. https://doi.org/10.3390/jcm13247669
APA StyleBoehler, S., Huber, M., Wuethrich, P. Y., Beilstein, C. M., Arigoni, S. M., Furrer, M. A., Borbély, Y., & Engel, D. (2024). Continuous Epidural Versus Non-Epidural Pain Management After Minimally Invasive Esophagectomy: A Real-Life, High-Case-Load Center Experience. Journal of Clinical Medicine, 13(24), 7669. https://doi.org/10.3390/jcm13247669





