Gout and Hyperuricemia: A Narrative Review of Their Comorbidities and Clinical Implications
Abstract
1. Introduction
2. Uric Acid and Formation of Monosodium Urate Crystals
3. Risk Factors of Hyperuricemia and Gout
3.1. Sex
3.2. Age
3.3. Body Composition
3.4. Genetic Factors and Ethnicity
3.5. Dietary Factors
3.6. Medication
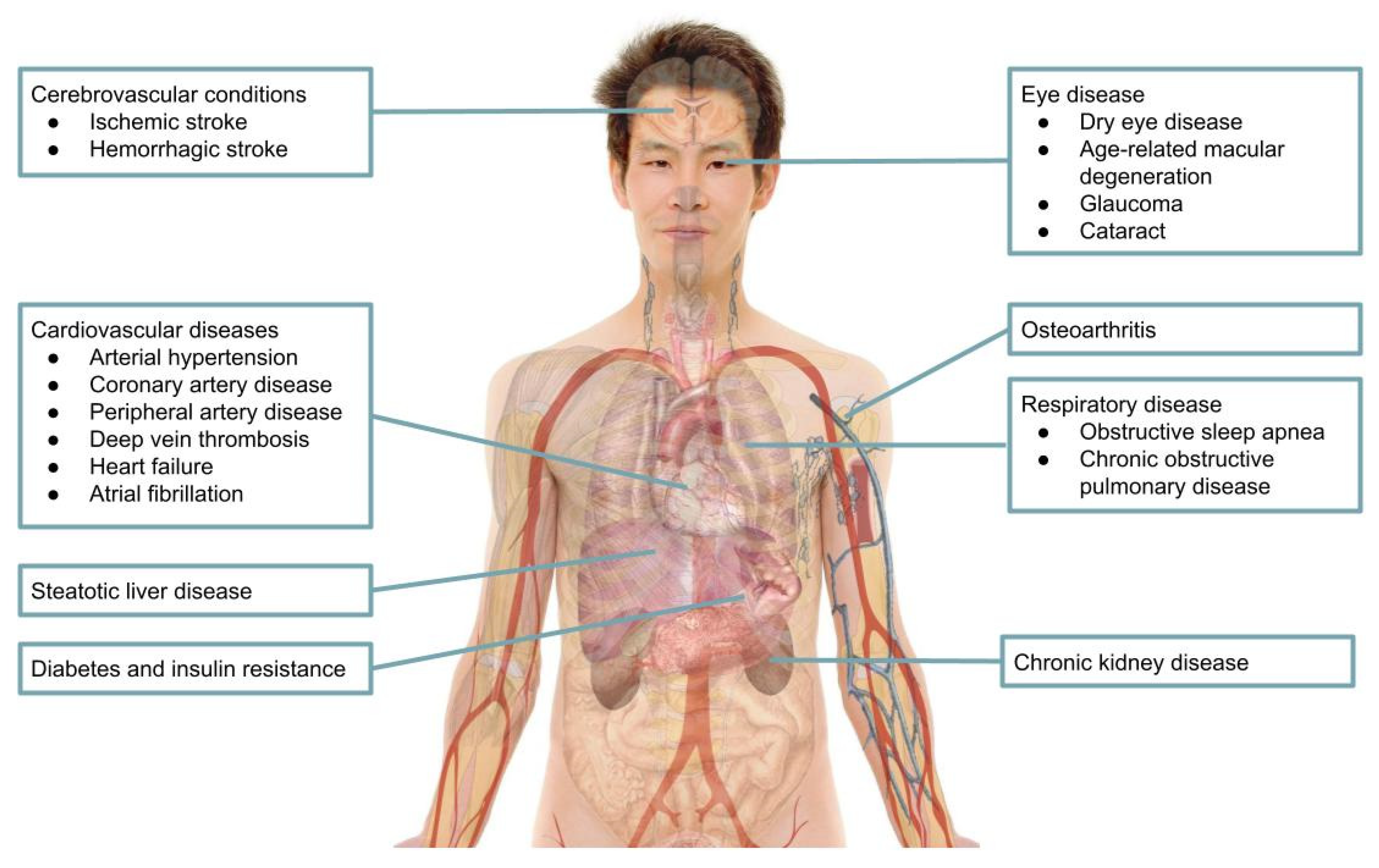
4. Comorbidities Associated with Gout and Hyperuricemia
4.1. Cardiovascular Diseases
4.1.1. Arterial Hypertension
4.1.2. Arterial Diseases
4.1.3. Heart Failure
4.1.4. Atrial Fibrillation
4.1.5. Cerebrovascular Conditions
4.2. Chronic Kidney Disease
4.3. Diabetes and Insulin Resistance
4.4. Steatotic Liver Disease
4.5. Osteoarthritis
4.6. Respiratory Disease
4.7. Eye Disease
5. Clinical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, J.A.; Gaffo, A. Gout epidemiology and comorbidities. Semin. Arthritis Rheum. 2020, 50 (Suppl. S3), S11–S16. [Google Scholar] [CrossRef]
- Smith, G.E.; Jones, F.W. The Archeological Survey of Nubia, Report for 1907–1989; National Printing Department: Cairo, Egypt, 1910; Volume 2, pp. 44–269.
- McCarty, D.J. A historical note: Leeuwenhoek’s description of crystals from a gouty tophus. Arthritis Rheum. 1970, 13, 414–418. [Google Scholar] [CrossRef]
- Rai, S.K.; Choi, H.K.; Choi, S.H.J.; Townsend, A.F.; Shojania, K.; De Vera, M.A. Key barriers to gout care: A systematic review and thematic synthesis of qualitative studies. Rheumatology 2018, 57, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Elfishawi, M.M.; Zleik, N.; Kvrgic, Z.; Michet, C.J., Jr.; Crowson, C.S.; Matteson, E.L.; Bongartz, T. The rising incidence of gout and the increasing burden of comorbidities: A population-based study over 20 years. J. Rheumatol. 2018, 45, 574–579. [Google Scholar] [CrossRef]
- Adams, F. (Ed.) The Genuine Works of Hippocrates; Wood: New York, NY, USA, 1886; Volume I–II. [Google Scholar]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Keenan, R.T. The biology of urate. Semin. Arthritis Rheum. 2020, 50 (Suppl. S3), S2–S10. [Google Scholar] [CrossRef]
- Wilcox, W.R.; Khalaf, A.A. Nucleation of monosodium urate crystals. Ann. Rheum. Dis. 1975, 34, 332–339. [Google Scholar] [CrossRef]
- Loeb, J.N. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972, 15, 189–192. [Google Scholar] [CrossRef]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Lee, G.; Lee, G.S. Lower temperatures exacerbate NLRP3 inflammasome activation by promoting monosodium urate crystallization, causing gout. Cells 2021, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Chatchawan, U.; Narkto, P.; Damri, T.; Yamauchi, J. An exploration of the relationship between foot skin temperature and blood flow in type 2 diabetes mellitus patients: A cross-sectional study. J. Phys. Ther. Sci. 2018, 30, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Desideri, G.; Castaldo, G.; Lombardi, A.; Mussap, M.; Testa, A.; Pontremoli, R.; Punzi, L.; Borghi, C. Is it time to revise the normal range of serum uric acid levels? Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1295–1306. [Google Scholar] [PubMed]
- Bardin, T. Hyperuricemia starts at 360 micromoles (6 mg/dL). Jt. Bone Spine 2015, 82, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Hisatome, I.; Ichida, K.; Mineo, I.; Ohtahara, A.; Ogino, K.; Kuwabara, M.; Ishizaka, N.; Uchida, S.; Kurajoh, M.; Kohagura, K.; et al. Japanese Society of Gout and Uric & Nucleic Acids 2019 guidelines for management of hyperuricemia and gout. Gout Uric Nucleic Acids 2020, 44, sp40. [Google Scholar]
- Guo, Y.; Huang, H.; Chen, Y.; Shen, C.; Xu, C. Association between circulating cystatin C and hyperuricemia: A cross-sectional study. Clin. Rheumatol. 2022, 41, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Akashi, N.; Kuwabara, M.; Matoba, T.; Kohro, T.; Oba, Y.; Kabutoya, T.; Imai, Y.; Kario, K.; Kiyosue, A.; Mizuno, Y.; et al. Hyperuricemia predicts increased cardiovascular events in patients with chronic coronary syndrome after percutaneous coronary intervention: A nationwide cohort study from Japan. Front. Cardiovasc. Med. 2023, 9, 1062894. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Leslie, S.W.; Minter, D.A. Hyperuricemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459218/ (accessed on 12 October 2024).
- Yang, Y.; Zhou, W.; Wang, Y.; Zhou, R. Gender-specific association between uric acid level and chronic kidney disease in the elderly health checkup population in China. Ren. Fail. 2019, 41, 197–203. [Google Scholar] [CrossRef]
- Zhang, W.Z. Why does hyperuricemia not necessarily induce gout? Biomolecules 2021, 11, 280. [Google Scholar] [CrossRef]
- Bhole, V.; de Vera, M.; Rahman, M.M.; Krishnan, E.; Choi, H. Epidemiology of gout in women: Fifty-two-year follow-up of a prospective cohort. Arthritis Rheum. 2010, 62, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Campion, E.W.; Glynn, R.J.; DeLabry, L.O. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am. J. Med. 1987, 82, 421–426. [Google Scholar] [CrossRef]
- Zhang, W.Z. Uric acid en route to gout. Adv. Clin. Chem. 2023, 116, 209–275. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Gout Collaborators. Global, regional, and national burden of gout, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2024, 6, e507–e517. [Google Scholar] [CrossRef]
- Wallace, K.L.; Riedel, A.A.; Joseph-Ridge, N.; Wortmann, R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J. Rheumatol. 2004, 31, 1582–1587. [Google Scholar] [PubMed]
- Hak, A.E.; Choi, H.K. Menopause, postmenopausal hormone use and serum uric acid levels in US women—The Third National Health and Nutrition Examination Survey. Arthritis Res. Ther. 2008, 10, R116. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, T.; Shan, L.; Cao, L.; Zhu, X.; Xue, Y. Estradiol regulates intestinal ABCG2 to promote urate excretion via the PI3K/Akt pathway. Nutr. Metab. 2021, 18, 63. [Google Scholar] [CrossRef]
- Kuzuya, M.; Ando, F.; Iguchi, A.; Shimokata, H. Effect of aging on serum uric acid levels: Longitudinal changes in a large Japanese population group. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M660–M664. [Google Scholar] [CrossRef]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality: The NHANES I epidemiologic follow-up study, 1971–1992. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.; Owczarek, A.J.; Mossakowska, M.; Broczek, K.; Grodzicki, T.; Wierucki, Ł.; Chudek, J. Prevalence of hyperuricemia and the use of allopurinol in older Poles—Results from a population-based PolSenior Study. Int. J. Environ. Res. Public Health 2021, 18, 387. [Google Scholar] [CrossRef] [PubMed]
- Timsans, J.; Kauppi, J.E.; Kerola, A.M.; Lehto, T.M.; Kautiainen, H.; Kauppi, M.J. Hyperuricaemia: Prevalence and association with mortality in an elderly Finnish population. BMJ Open 2023, 13, e072110. [Google Scholar] [CrossRef] [PubMed]
- Franchi, C.; Salerno, F.; Conca, A.; Djade, C.D.; Tettamanti, M.; Pasina, L.; Corrao, S.; Marengoni, A.; Marcucci, M.; Mannucci, P.M.; et al. Gout, allopurinol intake and clinical outcomes in the hospitalized multimorbid elderly. Eur. J. Intern. Med. 2014, 25, 847–852. [Google Scholar] [CrossRef]
- Roubenoff, R.; Klag, M.J.; Mead, L.A.; Liang, K.Y.; Seidler, A.J.; Hochberg, M.C. Incidence and risk factors for gout in white men. JAMA 1991, 266, 3004–3007. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Curhan, G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: The health professionals follow-up study. Arch. Intern. Med. 2005, 165, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Xie, R.; Dai, W.; Gao, C.; Shen, P.; Huang, X.; Zhang, F.; Yang, X.; Ji, G. Association of serum uric acid with body mass index: A cross-sectional study from Jiangsu Province, China. Iran J. Public Health 2014, 43, 1503–1509. [Google Scholar]
- Wang, H.; Yao, J.; Ding, N.; He, Y. Correlation of uric acid with body mass index based on NHANES 2013–2018 data: A cross-sectional study. Medicine 2022, 101, e30646. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Vatten, L.J. Body mass index and the risk of gout: A systematic review and dose-response meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.W.; McAdams DeMarco, M.A.; Baer, A.N.; Köttgen, A.; Folsom, A.R.; Coresh, J.; Gelber, A.C. Incident gout in women and association with obesity in the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Med. 2012, 125, 717.e9–717.e17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.H.; Pan, W.H.; Hsu, C.C.; Yeh, W.T.; Chuang, S.Y.; Chen, P.Y.; Chen, H.C.; Chang, C.T.; Huang, W.L. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: A prospective study. Arthritis Care Res. 2013, 65, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Bartels, E.M.; Henriksen, M.; Wæhrens, E.E.; Gudbergsen, H.; Bliddal, H.; Astrup, A.; Knop, F.K.; Carmona, L.; Taylor, W.J.; et al. Weight loss for overweight and obese individuals with gout: A systematic review of longitudinal studies. Ann. Rheum. Dis. 2017, 76, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.; Kaushal, S.; Lim, B.; Syn, N.; Oo, A.M.; Rao, J.; Koura, A.; Yeo, D. Impact of bariatric surgery on serum uric acid levels and the incidence of gout—A meta-analysis. Obes. Rev. 2019, 20, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, Y.; Nishizawa, H.; Tochino, Y.; Nakatsuji, H.; Sekimoto, R.; Nagao, H.; Shirakura, T.; Kato, K.; Imaizumi, K.; Takahashi, H.; et al. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 2013, 288, 27138–27149. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Jha, R.K.; Keerti, A. Chronic kidney disease: Its relationship with obesity. Cureus 2022, 14, e30535. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grade inflammation and its markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, K.; Li, C. Dietary factors and risk of gout and hyperuricemia: A meta-analysis and systematic review. Asia Pac. J. Clin. Nutr. 2018, 27, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.W.; McAdams-DeMarco, M.A.; Law, A.; Kao, L.; Gelber, A.C.; Coresh, J.; Baer, A.N. Racial differences in gout incidence in a population-based cohort: Atherosclerosis risk in communities study. Am. J. Epidemiol. 2014, 179, 576–583. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Thomas, J.; Thomas, D.J.; Mead, L.; Levine, D.M.; Klag, M.J. Racial differences in the incidence of gout: The role of hypertension. Arthritis Rheum. 1995, 38, 628–632. [Google Scholar] [CrossRef]
- McCormick, N.; Lu, N.; Yokose, C.; Joshi, A.D.; Sheehy, S.; Rosenberg, L.; Warner, E.T.; Dalbeth, N.; Merriman, T.R.; Saag, K.G.; et al. Racial and sex disparities in gout prevalence among US adults. JAMA Netw. Open 2022, 5, e2226804. [Google Scholar] [CrossRef]
- Howard, A.G.; Attard, S.M.; Herring, A.H.; Wang, H.; Du, S.; Gordon-Larsen, P. Socioeconomic gradients in the Westernization of diet in China over 20 years. SSM Popul. Health 2021, 16, 100943. [Google Scholar] [CrossRef] [PubMed]
- Colozza, D.; Avendano, M. Urbanisation, dietary change and traditional food practices in Indonesia: A longitudinal analysis. Soc. Sci. Med. 2019, 233, 103–112. [Google Scholar] [CrossRef]
- Krishnan, E.; Lienesch, D.; Kwoh, C.K. Gout in ambulatory care settings in the United States. J. Rheumatol. 2008, 35, 498–501. [Google Scholar] [PubMed]
- Yokose, C.; McCormick, N.; Lu, N.; Tanikella, S.; Lin, K.; Joshi, A.D.; Raffield, L.M.; Warner, E.; Merriman, T.; Hsu, J.; et al. Trends in prevalence of gout among US Asian adults, 2011–2018. JAMA Netw. Open 2023, 6, e239501. [Google Scholar] [CrossRef] [PubMed]
- Winnard, D.; Wright, C.; Taylor, W.J.; Jackson, G.; Te Karu, L.; Gow, P.J.; Arroll, B.; Thornley, S.; Gribben, B.; Dalbeth, N. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology 2012, 51, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Taylor, R.; Faaiuso, S.; Ainuu, S.P.; Whitehouse, S.; Zimmet, P. Hyperuricaemia and gout in Western Samoans. J. Chronic Dis. 1981, 34, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Pascart, T.; Wasik, K.A.; Preda, C.; Chune, V.; Torterat, J.; Prud’homme, N.; Nassih, M.; Martin, A.; Le Masson, J.; Rodière, V.; et al. The gout epidemic in French Polynesia: A modelling study of data from the Ma’i u’u epidemiological survey. Lancet Glob. Health 2024, 12, e685–e696. [Google Scholar] [CrossRef] [PubMed]
- Te Karu, L.; Dalbeth, N.; Stamp, L.K. Inequities in people with gout: A focus on Māori (Indigenous People) of Aotearoa New Zealand. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211028007. [Google Scholar] [CrossRef] [PubMed]
- Nian, Y.L.; You, C.G. Susceptibility genes of hyperuricemia and gout. Hereditas 2022, 159, 30. [Google Scholar] [CrossRef] [PubMed]
- Danve, A.; Sehra, S.T.; Neogi, T. Role of diet in hyperuricemia and gout. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101723. [Google Scholar] [CrossRef] [PubMed]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The association between purine-rich food intake and hyperuricemia: A cross-sectional study in Chinese adult residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour-Koujan, S.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Consumption of sugar-sweetened beverages and serum uric acid concentrations: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2021, 34, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Meneses-León, J.; León-Maldonado, L.; Macías, N.; Torres-Ibarra, L.; Hernández-López, R.; Rivera-Paredez, B.; Flores, M.; Flores, Y.N.; Barrientos-Gutiérrez, T.; Quezada-Sánchez, A.D.; et al. Sugar-sweetened beverage consumption and risk of hyperuricemia: A longitudinal analysis of the Health Workers Cohort Study participants in Mexico. Am. J. Clin. Nutr. 2020, 112, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, H.; Cheng, X.; Li, Y.; Zhao, Y.; Mei, W.; Wei, X.; Zhou, H.; Du, Y.; Zeng, C. Dietary intake of fructose increases purine de novo synthesis: A crucial mechanism for hyperuricemia. Front. Nutr. 2022, 9, 1045805. [Google Scholar] [CrossRef]
- Gaffo, A.L.; Roseman, J.M.; Jacobs, D.R., Jr.; Lewis, C.E.; Shikany, J.M.; Mikuls, T.R.; Jolly, P.E.; Saag, K.G. Serum urate and its relationship with alcoholic beverage intake in men and women: Findings from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Ann. Rheum. Dis. 2010, 69, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sakurai, M.; Miura, K.; Morikawa, Y.; Yoshita, K.; Ishizaki, M.; Kido, T.; Naruse, Y.; Suwazono, Y.; Nakagawa, H. Alcohol intake and the risk of hyperuricemia: A 6-year prospective study in Japanese men. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shin, D. Association between alcoholic beverage intake and hyperuricemia in Chinese adults: Findings from the China Health and Nutrition Survey. Medicine 2023, 102, e33861. [Google Scholar] [CrossRef]
- Lyu, J.Q.; Miao, M.Y.; Wang, J.M.; Qian, Y.W.; Han, W.W.; Peng, X.Z.; Tao, H.W.; Yang, J.; Chen, J.S.; Qin, L.Q.; et al. Consumption of total and specific alcoholic beverages and long-term risk of gout among men and women. JAMA Netw. Open 2024, 7, e2430700. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Okada, M.; Rahman, M.; Matsui, H.; Shiraishi, A.; Nakai, T.; Tamaki, H.; Kishimoto, M.; Hasegawa, H.; Matsuda, T.; et al. Differences in the association between alcoholic beverage type and serum urate levels using standardized ethanol content. JAMA Netw. Open 2023, 6, e233398. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Curhan, G. Beer, liquor, and wine consumption and serum uric acid level: The third national health and nutrition examination survey. Arthritis Rheum. 2004, 51, 1023–1029. [Google Scholar] [CrossRef]
- Neogi, T.; Chen, C.; Niu, J.; Chaisson, C.; Hunter, D.J.; Zhang, Y. Alcohol quantity and type on risk of recurrent gout attacks: An internet-based case-crossover study. Am. J. Med. 2014, 127, 311–318. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B. The role of alcohol consumption in pathogenesis of gout. Crit. Rev. Food Sci. Nutr. 2022, 62, 7129–7137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Shan, L.; You, Z.; Xia, Y.; Zhao, Y.; Zhang, H.; Zhao, Z. Dietary patterns, uric acid levels, and hyperuricemia: A systematic review and meta-analysis. Food Funct. 2023, 14, 7853–7868. [Google Scholar] [CrossRef]
- Ou, G.; Wu, J.; Wang, S.; Jiang, Y.; Chen, Y.; Kong, J.; Xu, H.; Deng, L.; Zhao, H.; Chen, X.; et al. Dietary factors and risk of gout: A two-sample Mendelian randomization study. Foods 2024, 13, 1269. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Kim, H.J.; Ahn, H.S.; Kim, S.H.; Park, E.J.; Yim, S.Y.; Jun, J.B. Effects of coffee consumption on serum uric acid: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2016, 45, 580–586. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, T.; Zeng, C.; Wei, J.; Li, H.; Xiong, Y.L.; Yang, Y.; Ding, X.; Lei, G. Is coffee consumption associated with a lower risk of hyperuricaemia or gout? A systematic review and meta-analysis. BMJ Open 2016, 6, e009809. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Nakayama, A.; Kawamura, Y.; Toyoda, Y.; Nakatochi, M.; Shimizu, S.; Shinomiya, N.; Okada, Y.; Matsuo, H. Coffee consumption reduces gout risk independently of serum uric acid levels: Mendelian randomization analyses across ancestry populations. ACR Open Rheumatol. 2022, 4, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, S.; Peng, H.; Wang, M.; Li, L.; Huang, J.; Wu, T. Dose-response relationships of tea and coffee consumption with gout: A prospective cohort study in the UK Biobank. Rheumatology 2023, 62, 3043–3050. [Google Scholar] [CrossRef]
- Song, J.; Li, H.; Fang, X. Inverted U-shaped relationship between coffee consumption and serum uric acid in American chronic kidney disease population. Front. Nutr. 2023, 10, 1286430. [Google Scholar] [CrossRef]
- Ben Salem, C.; Slim, R.; Fathallah, N.; Hmouda, H. Drug-induced hyperuricaemia and gout. Rheumatology 2017, 56, 679–688. [Google Scholar] [CrossRef]
- Howard, S.C.; Avagyan, A.; Workeneh, B.; Pui, C.H. Tumour lysis syndrome. Nat. Rev. Dis. Primers 2024, 10, 58. [Google Scholar] [CrossRef]
- Wilson, F.P.; Berns, J.S. Tumor lysis syndrome: New challenges and recent advances. Adv. Chronic Kidney Dis. 2014, 21, 18–26. [Google Scholar] [CrossRef]
- Leung, N.; Yip, K.; Pillinger, M.H.; Toprover, M. Lowering and raising serum urate levels: Off-label effects of commonly used medications. Mayo Clin. Proc. 2022, 97, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, A.; Takkunen, H.; Knekt, P.; Aromaa, A. Hyperuricemia as a risk factor for cardiovascular mortality. Acta Med. Scand. Suppl. 1982, 668, 49–59. [Google Scholar] [CrossRef]
- Bengtsson, C.; Lapidus, L.; Stendahl, C.; Waldenström, J. Hyperuricaemia and risk of cardiovascular disease and overall death. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Acta Med. Scand. 1988, 224, 549–555. [Google Scholar] [CrossRef]
- Niskanen, L.K.; Laaksonen, D.E.; Nyyssönen, K.; Alfthan, G.; Lakka, H.M.; Lakka, T.A.; Salonen, J.T. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: A prospective cohort study. Arch. Intern. Med. 2004, 164, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Tunstall-Pedoe, H.; Woodward, M. Serum uric acid and the risk of mortality during 23 years follow-up in the Scottish Heart Health Extended Cohort Study. Atherosclerosis 2014, 233, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.C.; Chen, Y.T.; Ou, S.M.; Shih, C.J.; Tarng, D.C.; Taiwan Geriatric Kidney Disease (TGKD) Research Group. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: The role of malnourishment. J. Am. Heart Assoc. 2018, 7, e007523. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Chang, Y.; Kim, I.; Ryu, S. U-shaped association between serum uric acid level and risk of mortality: A cohort study. Arthritis Rheumatol. 2018, 70, 1122–1132. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hu, G.; Xu, B.P.; Zhu, L.; Zhou, W.; Wang, T.; Bao, H.; Cheng, X. U-shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: A cohort study. J. Clin. Endocrinol. Metab. 2020, 105, dgz068. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Go, S.; Son, H.E.; Ryu, J.Y.; Lee, H.; Heo, N.J.; Chin, H.J.; Park, J.H. Association between serum uric acid level and ESRD or death in a Korean population. J. Korean Med. Sci. 2020, 35, e254. [Google Scholar] [CrossRef] [PubMed]
- Cang, Y.; Xu, S.; Zhang, J.; Ju, J.; Chen, Z.; Wang, K.; Li, J.; Xu, Y. Serum uric acid revealed a U-shaped relationship with all-cause mortality and cardiovascular mortality in high atherosclerosis risk patients: The ASSURE study. Front. Cardiovasc. Med. 2021, 8, 641513. [Google Scholar] [CrossRef]
- Ungar, A.; Rivasi, G.; Di Bari, M.; Virdis, A.; Casiglia, E.; Masi, S.; Mengozzi, A.; Barbagallo, C.M.; Bombelli, M.; Bruno, B.; et al. The association of uric acid with mortality modifies at old age: Data from the uric acid right for heart health (URRAH) study. J. Hypertens. 2022, 40, 704–711. [Google Scholar] [CrossRef]
- Masulli, M.; D’Elia, L.; Angeli, E.; Esposito, D.; Vaccaro, O.; Giugliano, D.; Rivellese, A.A.; Barbagallo, C.M.; Barrea, A.; Battista, F.; et al. Uric acid as a risk factor for cardiovascular disease and mortality in individuals with type 2 diabetes. Nutrients 2023, 15, 394. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kawamoto, R.; Ninomiya, D.; Kumagi, T. Hyperuricemia is associated with all-cause mortality among males and females: Findings from a study on Japanese community-dwelling individuals. Metabol. Open 2022, 14, 100186. [Google Scholar] [CrossRef]
- Timsans, J.; Kauppi, J.E.; Kerola, A.M.; Lehto, T.M.; Kautiainen, H.J.; Kauppi, M.J. Hyperuricaemia-associated all-cause mortality risk effect is increased by non-impaired kidney function—Is renal hyperuricaemia less dangerous? Eur. J. Intern. Med. 2024, 121, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Timsans, J.; Kerola, A.M.; Rantalaiho, V.M.; Hakkarainen, K.N.; Kautiainen, H.J.; Kauppi, M.J. “Metabolic” type of hyperuricemia increases mortality mainly by leading to premature death from cardiovascular disease. Mayo Clin. Proc. 2024, 99, 1835–1837. [Google Scholar] [CrossRef]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Grassi, G.; Angeli, F.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid/serum creatinine ratio as a predictor of cardiovascular events: Detection of prognostic cardiovascular cut-off values. J. Hypertens. 2023, 41, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Wang, H.; Chen, M.; Wen, C.; Huang, L.; Zhou, M. All-cause and specific mortality in patients with gout: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2023, 63, 152273. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xie, Z.; Pan, B.; Zhang, J. Impact of gout on cardiovascular disease mortality: A meta-analysis. Z. Rheumatol. 2024, 63, 1–9. [Google Scholar] [CrossRef]
- Ubhadiya, T.J.; Dubey, N.; Sojitra, M.H.; Shah, K.; Joshi, S.; Gandhi, S.K.; Patel, P. Exploring the effects of elevated serum uric acid levels on hypertension: A scoping review of hyperuricemia. Cureus 2023, 15, e43361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am. J. Med. 2012, 125, 679–687.e1. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Yu, H.P.; Chang, Y.J.; Wang, L.C.; Chen, C.K.; Zhang, W.; Doherty, M.; Chang, S.H.; Hsu, J.T.; Yu, K.H.; et al. Assessing the causal relationships between gout and hypertension: A bidirectional Mendelian randomization study with coarsened exposures. Arthritis Res. Ther. 2022, 24, 243. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Pazoki, R.; Uitterlinden, A.G.; Hofman, A.; Stricker, B.H.; Ikram, M.A.; Franco, O.H.; Dehghan, A. Association of uric acid genetic risk score with blood pressure: The Rotterdam study. Hypertension 2014, 64, 1061–1066. [Google Scholar] [CrossRef]
- Li, K.; Li, K.; Yao, Q.; Shui, X.; Zheng, J.; He, Y.; Lei, W. The potential relationship of coronary artery disease and hyperuricemia: A cardiometabolic risk factor. Heliyon 2023, 9, e16097. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the risk for coronary heart disease morbidity and mortality: A systematic review and dose-response meta-analysis. Sci. Rep. 2016, 6, 19520. [Google Scholar] [CrossRef]
- Cox, P.; Gupta, S.; Zhao, S.S.; Hughes, D.M. The incidence and prevalence of cardiovascular diseases in gout: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 1209–1219. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Y.; Zhang, H.; Qu, Y.; Ying, Z.; Sun, Y.; Hu, Y.; Chen, W.; Yang, H.; Yang, J.; et al. The association of hyperuricemia and gout with the risk of cardiovascular diseases: A cohort and Mendelian randomization study in UK Biobank. Front. Med. 2022, 8, 817150. [Google Scholar] [CrossRef]
- Cipolletta, E.; Tata, L.J.; Nakafero, G.; Avery, A.J.; Mamas, M.A.; Abhishek, A. Association between gout flare and subsequent cardiovascular events among patients with gout. JAMA 2022, 328, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, E.; Tata, L.J.; Nakafero, G.; Avery, A.J.; Mamas, M.A.; Abhishek, A. Risk of venous thromboembolism with gout flares. Arthritis Rheumatol. 2023, 75, 1638–1647. [Google Scholar] [CrossRef]
- Cipolletta, E.; Nakafero, G.; Richette, P.; Avery, A.J.; Mamas, M.A.; Tata, L.J.; Abhishek, A. Short-term risk of cardiovascular events in people newly diagnosed with gout. Arthritis Rheumatol. 2024. [Google Scholar] [CrossRef]
- Andrés, M. Gout flares as vascular red flags. Arthritis Rheumatol. 2024. [Google Scholar] [CrossRef]
- Gupta, M.K.; Singh, J.A. Cardiovascular disease in gout and the protective effect of treatments including urate-lowering therapy. Drugs 2019, 79, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.; Fang, C.; Pendse, J.; Toprover, M.; Pillinger, M.H. Narrative review: Peripheral arterial disease in patients with hyperuricemia and gout. Curr. Rheumatol. Rep. 2023, 25, 83–97. [Google Scholar] [CrossRef]
- Wang, J.C.; Tsai, S.H.; Chien, W.C.; Chung, C.H.; Lin, S.J.; Chen, Y.T.; Huang, P.H. Association between gout and abdominal aortic aneurysm. J. Cardiol. 2023, 82, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.P.; He, H.Q.; Aierken, Y.; Wu, Y.; Liu, Y. Effect of serum uric acid on the risk of aortic aneurysm and dissection: A mendelian randomization analysis. Biochem. Biophys. Rep. 2024, 38, 101743. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Lu, Y.; Guo, L.; Ma, L. Preoperative uric acid predicts in-hospital death in patients with acute type a aortic dissection. J. Cardiothorac. Surg. 2020, 15, 21. [Google Scholar] [CrossRef]
- Klauser, A.S.; Halpern, E.J.; Strobl, S.; Gruber, J.; Feuchtner, G.; Bellmann-Weiler, R.; Weiss, G.; Stofferin, H.; Jaschke, W. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol. 2019, 4, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Pascart, T.; Carpentier, P.; Choi, H.K.; Norberciak, L.; Ducoulombier, V.; Luraschi, H.; Houvenagel, E.; Legrand, J.; Verclytte, S.; Becce, F.; et al. Identification and characterization of peripheral vascular color-coded DECT lesions in gout and non-gout patients: The VASCURATE study. Semin. Arthritis Rheum. 2021, 51, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Barazani, S.H.; Chi, W.W.; Pyzik, R.; Chang, H.; Jacobi, A.; O’Donnell, T.; Fayad, Z.A.; Ali, Y.; Mani, V. Quantification of uric acid in vasculature of patients with gout using dual-energy computed tomography. World J. Radiol. 2020, 12, 184–194. [Google Scholar] [CrossRef]
- Ren, H.; Qu, H.; Zhang, Y.; Gu, Y.; Zhao, Y.; Xu, W.; Zhou, M.; Wang, W. Detection of monosodium urate depositions and atherosclerotic plaques in the cardiovascular system by dual-energy computed tomography. Heliyon 2024, 10, e24548. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, C.; Yao, L.; Liu, Y.; Hao, P. HDL-C mediates the causal relationship between serum urate and aortic aneurysm: A Mendelian randomization study. Cardiol. Plus 2024, 9, 180–186. [Google Scholar] [CrossRef]
- Si, K.; Wei, C.; Xu, L.; Zhou, Y.; Lv, W.; Dong, B.; Wang, Z.; Huang, Y.; Wang, Y.; Chen, Y. Hyperuricemia and the risk of heart failure: Pathophysiology and therapeutic implications. Front. Endocrinol. 2021, 12, 770815. [Google Scholar] [CrossRef]
- Huang, H.; Huang, B.; Li, Y.; Huang, Y.; Li, J.; Yao, H.; Jing, X.; Chen, J.; Wang, J. Uric acid and risk of heart failure: A systematic review and meta-analysis. Eur. J. Heart Fail. 2014, 16, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Nyrnes, A.; Toft, I.; Njølstad, I.; Mathiesen, E.B.; Wilsgaard, T.; Hansen, J.B.; Løchen, M.L. Uric acid is associated with future atrial fibrillation: An 11-year follow-up of 6308 men and women—The Tromso Study. Europace 2014, 16, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.J.; Jiao, H.C.; Liu, X.J.; Hao, H.; Liu, Y.; Xue, Y.T.; Li, Y. Association of serum uric acid with non-valvular atrial fibrillation: A retrospective study in China. Int. J. Gen. Med. 2024, 17, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Viet, N.N.; Gigante, B.; Lind, V.; Hammar, N.; Modig, K. Elevated uric acid is associated with new-onset atrial fibrillation: Results from the Swedish AMORIS cohort. J. Am. Heart Assoc. 2023, 12, e027089. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.J.; Tsai, T.H.; Chang, H.P.; Chua, S.; Chung, S.Y.; Yang, C.H.; Lin, C.J.; Wu, C.J.; Hang, C.L. The risk of atrial fibrillation in patients with gout: A nationwide population-based study. Sci. Rep. 2016, 6, 32220. [Google Scholar] [CrossRef][Green Version]
- Quesada, A.; Quesada-Ocete, J.; Quesada-Ocete, B.; González-Ritonnale, A.; Marcaida-Benito, G.; Moral-Ronda, V.D.; Jiménez-Bello, J.; Sahuquillo-Frias, L.; Rubini-Costa, R.; Lavie, C.J.; et al. Long-term hyperuricemia impact on atrial fibrillation outcomes. Curr. Probl. Cardiol. 2024, 49, 102608. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the diagnosis and management of atrial fibrillation: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Yang, X.; Xu, Z.; Feng, Y. Incorporating uric acid into the CHA2DS2-VASc score improves the prediction of new-onset atrial fibrillation in patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2023, 23, 522. [Google Scholar] [CrossRef]
- Hong, M.; Park, J.W.; Yang, P.S.; Hwang, I.; Kim, T.H.; Yu, H.T.; Uhm, J.S.; Joung, B.; Lee, M.H.; Jee, S.H.; et al. A Mendelian randomization analysis: The causal association between serum uric acid and atrial fibrillation. Eur. J. Clin. Investig. 2020, 50, e13300. [Google Scholar] [CrossRef]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Rheum. 2009, 61, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, W.; Zhang, X.; Hu, L.; Tang, Z. Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclerosis 2014, 232, 265–270. [Google Scholar] [CrossRef]
- Zhong, C.; Zhong, X.; Xu, T.; Zhang, Y. Sex-specific relationship between serum uric acid and risk of stroke: A dose-response meta-analysis of prospective studies. J. Am. Heart Assoc. 2017, 6, e005042. [Google Scholar] [CrossRef]
- Tu, W.; Wu, J.; Jian, G.; Lori, J.; Tang, Y.; Cheng, H.; Wu, X.; Wang, N. Asymptomatic hyperuricemia and incident stroke in elderly Chinese patients without comorbidities. Eur. J. Clin. Nutr. 2019, 73, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Wu, H.; Peng, W. The relationship between elevated serum uric acid and risk of stroke in adults: An updated and dose-response meta-analysis. Front. Neurol. 2021, 12, 674398. [Google Scholar] [CrossRef]
- Tsai, P.H.; Kuo, C.F.; See, L.C.; Li, P.R.; Chen, J.S.; Tseng, W.Y. Stroke risk in patients with gout: A nationwide retrospective cohort study in Taiwan. J. Clin. Med. 2022, 11, 3779. [Google Scholar] [CrossRef] [PubMed]
- Padda, J.; Khalid, K.; Padda, S.; Boddeti, N.L.; Malhi, B.S.; Nepal, R.; Cooper, A.C.; Jean-Charles, G. Hyperuricemia and its association with ischemic stroke. Cureus 2021, 13, e18172. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez Lozada, L.G.; Lanaspa, M.A.; Piani, F.; Borghi, C. Uric acid and chronic kidney disease: Still more to do. Kidney Int. Rep. 2022, 8, 229–239. [Google Scholar] [CrossRef]
- Yin, H.; Liu, N.; Chen, J. The role of the intestine in the development of hyperuricemia. Front. Immunol. 2022, 13, 845684. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, T.; Liu, Y.; Chang, Q.; Zhao, Y.; Guo, C.; Xia, Y. Prevalence of diabetes in patients with hyperuricemia and gout: A systematic review and meta-analysis. Curr. Diab. Rep. 2023, 23, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Ogbera, A.O.; Azenabor, A.O. Hyperuricaemia and the metabolic syndrome in type 2 DM. Diabetol. Metab. Syndr. 2010, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, R.P.; Lei, L.; Song, Q.Q.; Zhang, R.Y.; Li, Y.B.; Yang, C.; Lin, S.D.; Chen, L.S.; Wang, Y.L.; et al. Prevalence and determinants of hyperuricemia in type 2 diabetes mellitus patients with central obesity in Guangdong Province in China. Asia Pac. J. Clin. Nutr. 2013, 22, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Mundhe, S.; Mhasde, D. The study of prevalence of hyperuricemia and metabolic syndrome in type 2 diabetes mellitus. Int. J. Adv. Med. 2016, 3, 241–249. [Google Scholar] [CrossRef]
- Woyesa, S.B.; Hirigo, A.T.; Wube, T.B. Hyperuricemia and metabolic syndrome in type 2 diabetes mellitus patients at Hawassa university comprehensive specialized hospital, South West Ethiopia. BMC Endocr. Disord. 2017, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Billa, G.; Dargad, R.; Mehta, A. Prevalence of hyperuricemia in Indian subjects attending hyperuricemia screening programs—A retrospective study. J. Assoc. Physicians India 2018, 66, 43–46. [Google Scholar]
- Abujbara, M.; Al Hourani, H.M.; Al-Raoush, R.I.; Khader, Y.S.; Ajlouni, K. Prevalence of hyperuricemia and associated factors among type 2 diabetic patients in Jordan. Int. J. Gen. Med. 2022, 15, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- Pfister, R.; Barnes, D.; Luben, R.; Forouhi, N.G.; Bochud, M.; Khaw, K.T.; Wareham, N.J.; Langenberg, C. No evidence for a causal link between uric acid and type 2 diabetes: A Mendelian randomisation approach. Diabetologia 2011, 54, 2561–2569. [Google Scholar] [CrossRef]
- Sluijs, I.; Holmes, M.V.; van der Schouw, Y.T.; Beulens, J.W.; Asselbergs, F.W.; Huerta, J.M.; Palmer, T.M.; Arriola, L.; Balkau, B.; Barricarte, A.; et al. A Mendelian randomization study of circulating uric acid and type 2 diabetes. Diabetes 2015, 64, 3028–3036. [Google Scholar] [CrossRef]
- Keerman, M.; Yang, F.; Hu, H.; Wang, J.; Wang, F.; Li, Z.; Yuan, J.; Yao, P.; Zhang, X.; Guo, H.; et al. Mendelian randomization study of serum uric acid levels and diabetes risk: Evidence from the Dongfeng-Tongji cohort. BMJ Open Diabetes Res. Care 2020, 8, e000834. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, J.; Jiang, F.; Zhang, R.; Wang, T.; Wang, S.; Peng, D.; He, Z.; Chen, H.; Bao, Y.; et al. A causal relationship between uric acid and diabetic macrovascular disease in Chinese type 2 diabetes patients: A Mendelian randomization analysis. Int. J. Cardiol. 2016, 214, 194–199. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, J.; Xu, Y. Effects of uric acid on diabetes mellitus and its chronic complications. Int. J. Endocrinol. 2019, 2019, 9691345. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Perreault, L.; Ji, L.; Dagogo-Jack, S. Diagnosis and management of prediabetes: A review. JAMA 2023, 329, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 2023, 51, 3000605231164548. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Insulin resistance and type 2 diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, E.; Pandya, B.J.; Chung, L.; Hariri, A.; Dabbous, O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: A 15-year follow-up study. Am. J. Epidemiol. 2012, 176, 108–116. [Google Scholar] [CrossRef]
- Timsans, J.; Kauppi, J.; Kerola, A.; Rantalaiho, V.; Kautiainen, H.; Kauppi, M. Hyperuricemia is associated with higher levels of fasting plasma glucose and insulin resistance in non-diabetic subjects. Arthritis Rheumatol. 2024, 76 (Suppl. S9). Available online: https://acrabstracts.org/abstract/hyperuricemia-is-associated-with-higher-levels-of-fasting-plasma-glucose-and-insulin-resistance-in-non-diabetic-subjects/ (accessed on 1 October 2024).
- Han, T.; Lan, L.; Qu, R.; Xu, Q.; Jiang, R.; Na, L.; Sun, C. Temporal relationship between hyperuricemia and insulin resistance and its impact on future risk of hypertension. Hypertension 2017, 70, 703–711. [Google Scholar] [CrossRef]
- McCormick, N.; O’Connor, M.J.; Yokose, C.; Merriman, T.R.; Mount, D.B.; Leong, A.; Choi, H.K. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional Mendelian randomization. Arthritis Rheumatol. 2021, 73, 2096–2104. [Google Scholar] [CrossRef]
- Mandal, A.K.; Leask, M.P.; Estiverne, C.; Choi, H.K.; Merriman, T.R.; Mount, D.B. Genetic and physiological effects of insulin on human urate homeostasis. Front. Physiol. 2021, 12, 713710. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, L.; Yang, J.; Fan, J.; Tse, L.A.; Li, Y. Genetic Predisposition to Type 2 Diabetes and Insulin Levels Is Positively Associated with Serum Urate Levels. J. Clin. Endocrinol. Metab. 2021, 106, e2547–e2556. [Google Scholar] [CrossRef]
- Hu, X.; Rong, S.; Wang, Q.; Sun, T.; Bao, W.; Chen, L.; Liu, L. Association between Plasma Uric Acid and Insulin Resistance in Type 2 Diabetes: A Mendelian Randomization Analysis. Diabetes Res. Clin. Pract. 2021, 171, 108542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Yu, C.; Xu, L.; Miao, M. Association of Serum Uric Acid Level with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. J. Hepatol. 2009, 50, 1029–1034. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, X.; Wang, T.; Zhu, L.; Zhou, W.; Bao, H.; Cheng, X. Positive Correlation Between Fatty Liver Index and Hyperuricemia in Hypertensive Chinese Adults: A H-Type Hypertension Registry Study. Front. Endocrinol. 2023, 14, 1183666. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, T.; Manji, L.; Liu, Y.; Chang, Q.; Zhao, Y.; Ding, Y.; Xia, Y. Association Between Serum Uric Acid and Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Meta-Analysis. Clin. Epidemiol. 2023, 15, 683–693. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Timsans, J.; Kauppi, J.; Kautiainen, H.; Kauppi, M. Hyperuricemia—Especially “Metabolic Hyperuricemia”—Is Independently Associated with Higher Risk of Fatty Liver. Arthritis Rheumatol. 2023, 75 (Suppl. S9). Available online: https://acrabstracts.org/abstract/hyperuricemia-especially-metabolic-hyperuricemia-is-independently-associated-with-higher-risk-of-fatty-liver/ (accessed on 1 October 2024).
- Brennan, P.; Clare, K.; George, J.; Dillon, J.F. Determining the Role for Uric Acid in Non-Alcoholic Steatohepatitis Development and the Utility of Urate Metabolites in Diagnosis: An Opinion Review. World J. Gastroenterol. 2020, 26, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, Y.; Liu, P.; Liu, C.; Zhong, R.; Yu, X.; Xiao, L.; Du, M.; Yang, L.; Yuan, J.; et al. No Evidence for a Causal Link Between Serum Uric Acid and Non-Alcoholic Fatty Liver Disease from the Dongfeng-Tongji Cohort Study. Oxid. Med. Cell. Longev. 2022, 2022, 6687626. [Google Scholar] [CrossRef]
- Li, S.; Fu, Y.; Liu, Y.; Zhang, X.; Li, H.; Tian, L.; Zhuo, L.; Liu, M.; Cui, J. Serum Uric Acid Levels and Non-Alcoholic Fatty Liver Disease: A 2-Sample Bidirectional Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e3497–e3503. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Comorbidities in Patients with Gout Prior to and Following Diagnosis: Case-Control Study. Ann. Rheum. Dis. 2016, 75, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.G.; Samuels, J.; Gyftopoulos, S.; Krasnokutsky, S.; Leung, J.; Swearingen, C.J.; Pillinger, M.H. Presence of Gout Is Associated with Increased Prevalence and Severity of Knee Osteoarthritis Among Older Men: Results of a Pilot Study. J. Clin. Rheumatol. 2015, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bevis, M.; Marshall, M.; Rathod, T.; Roddy, E. The Association Between Gout and Radiographic Hand, Knee, and Foot Osteoarthritis: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2016, 17, 169. [Google Scholar] [CrossRef]
- Ding, X.; Zeng, C.; Wei, J.; Li, H.; Yang, T.; Zhang, Y.; Xiong, Y.L.; Gao, S.G.; Li, Y.S.; Lei, G.H. The Associations of Serum Uric Acid Level and Hyperuricemia with Knee Osteoarthritis. Rheumatol. Int. 2016, 36, 567–573. [Google Scholar] [CrossRef]
- Dalbeth, N.; Aati, O.; Kalluru, R.; Gamble, G.D.; Horne, A.; Doyle, A.J.; McQueen, F.M. Relationship Between Structural Joint Damage and Urate Deposition in Gout: A Plain Radiography and Dual-Energy CT Study. Ann. Rheum. Dis. 2015, 74, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Comberg, H.U.; Schach, S. Hyperuricemia Is Associated with Musculoskeletal Pain—Results from a Cross-Sectional Study. Open Pain J. 2016, 9, 15–25. [Google Scholar] [CrossRef]
- Jonsson, H.; Aspelund, T.; Eiriksdottir, G.; Harris, T.B.; Launer, L.J.; Gudnason, V. Hyperuricemia Is Associated with Intermittent Hand Joint Pain in a Cross-Sectional Study of Elderly Females: The AGES-Reykjavik Study. PLoS ONE 2019, 14, e0221474. [Google Scholar] [CrossRef]
- Timsans, J.; Kerola, A.; Kauppi, J.; Rantalaiho, V.; Paldanius, M.; Kautiainen, H.; Kauppi, M. POS0561: The Effect of Hyperuricaemia on the Use of Non-Opioid and Opioid Analgesics. Ann. Rheum. Dis. 2024, 83, 564. [Google Scholar]
- Kuo, C.F.; Chou, I.J.; See, L.C.; Chen, J.S.; Yu, K.H.; Luo, S.F.; Hsieh, A.H.; Zhang, W.; Doherty, M. Urate-Lowering Treatment and Risk of Total Joint Replacement in Patients with Gout. Rheumatology 2018, 57, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Nishimura, M.; Shibuya, E.; Makita, H.; Tsujino, I.; Miyamoto, K.; Kawakami, Y. Tissue Hypoxia in Sleep Apnea Syndrome Assessed by Uric Acid and Adenosine. Chest 2002, 122, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Niu, J.; Neogi, T.; Chen, C.A.; Chaisson, C.; Hunter, D.; Zhang, Y. Nocturnal risk of gout attacks. Arthritis Rheumatol. 2015, 67, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peloquin, C.E.; Dubreuil, M.; Roddy, E.; Lu, N.; Neogi, T.; Choi, H.K. Sleep apnea and the risk of incident gout: A population-based, body mass index-matched cohort study. Arthritis Rheumatol. 2015, 67, 3298–3302. [Google Scholar] [CrossRef]
- Van Durme, C.; Spaetgens, B.; Driessen, J.; Nielen, J.; Sastry, M.; Boonen, A.; de Vries, F. Obstructive sleep apnea and the risk of gout: A population-based case-control study. Arthritis Res. Ther. 2020, 22, 92. [Google Scholar] [CrossRef] [PubMed]
- Khandwala, P.; Desai, D.; Sen, M. Obstructive sleep apnea: A contributing factor in gout. Cureus 2024, 16, e52115. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, C.; Tufik, S.; Guindalini, C.; Mazzotti, D.R.; Bittencourt, L.R.; Andersen, M.L. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS ONE 2013, 8, e66891. [Google Scholar] [CrossRef]
- Park, S.L.; Lim, J.; Lee, J.H. The association of serum uric acid with risk of obstructive sleep apnea: The Korean national health and nutrition examination survey 2019–2021. J. Pers. Med. 2024, 14, 532. [Google Scholar] [CrossRef]
- Zeng, Z.; Jin, T.; Ni, J.; Huang, L.; Xie, Y.; He, W.; Zhang, L.; Ding, C.; Cen, H. Assessing the causal associations of obstructive sleep apnea with serum uric acid levels and gout: A bidirectional two-sample mendelian randomization study. Semin. Arthritis Rheum. 2022, 57, 152095. [Google Scholar] [CrossRef] [PubMed]
- Abrams, B. Premature mortality with gout and hyperuricemia may be reduced by early resolution of comorbid obstructive sleep apnea. Explor. Musculoskelet. Dis. 2023, 1, 106–120. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Xiao, S.; Dai, C.; Wen, X.; Wu, F.; Peng, J.; Tian, H.; Zhou, Y.; Ran, P. Association between serum uric acid and lung function in people with and without chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Bartziokas, K.; Papaioannou, A.I.; Loukides, S.; Papadopoulos, A.; Haniotou, A.; Papiris, S.; Kostikas, K. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respir. J. 2014, 43, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Spiropoulos, K.; Trakada, G.; Nikolaou, E.; Prodromakis, E.; Efremidis, G.; Pouli, A.; Koniavitou, A. Endothelin-1 levels in the pathophysiology of chronic obstructive pulmonary disease and bronchial asthma. Respir. Med. 2003, 97, 983–989. [Google Scholar] [CrossRef]
- Chen, T.; Chen, J.; Zhao, C.; Li, X. Correlation between gout and dry eye disease. Int. Ophthalmol. 2024, 44, 102. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Cleveland, J.D. Gout and the risk of age-related macular degeneration in the elderly. PLoS ONE 2018, 13, e0199562. [Google Scholar] [CrossRef]
- Hsu, M.H.; Hsu, C.A.; Lai, S.C.; Yen, J.C. Gout as a risk factor for age-related macular degeneration in Taiwanese adults—A population-based study in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 10142. [Google Scholar] [CrossRef]
- Mohammadi, M.; Yarmohammadi, A.; Salehi-Abargouei, A.; Ghasemirad, H.; Shirvani, M.; Ghoshouni, H. Uric acid and glaucoma: A systematic review and meta-analysis. Front. Med. 2023, 10, 1159316. [Google Scholar] [CrossRef]
- Bhat, V.G.; Patra, R.; Raghuram, C.J.; Giri, A.; Rao, N.L. Association between serum uric acid levels and primary open-angle glaucoma: A cross-sectional study. J. Clin. Diagn. Res. 2023, 17, BC10–BC13. [Google Scholar] [CrossRef]
- Luo, C.; Chen, X.; Jin, H.; Yao, K. The association between gout and cataract risk: A meta-analysis. PLoS ONE 2017, 12, e0180188. [Google Scholar] [CrossRef]
- Qin, Y.J.; Chan, S.O.; Lin, H.L.; Zhang, Y.Q.; Chen, Y.L.; Niu, Y.Y.; Xie, W.J.; Chu, W.K.; Pang, C.P.; Zhang, H.Y. Elevated level of uric acid in aqueous humour is associated with posterior subcapsular cataract in human lens. Clin. Exp. Ophthalmol. 2020, 48, 1183–1191. [Google Scholar] [CrossRef]
- Lin, H.L.; Wang, S.; Sato, K.; Zhang, Y.Q.; He, B.T.; Xu, J.; Nakazawa, T.; Qin, Y.J.; Zhang, H.Y. Uric acid-driven NLRP3 inflammasome activation triggers lens epithelial cell senescence and cataract formation. Cell Death Discov. 2024, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castañeda-Sanabria, J.; Coyfish, M.; Guillo, S.; Jansen, T.L.; Janssens, H.; et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 2017, 76, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Dessein, P.H.; Shipton, E.A.; Stanwix, A.E.; Joffe, B.I.; Ramokgadi, J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: A pilot study. Ann. Rheum. Dis. 2000, 59, 539–543. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 744–760, Erratum in Arthritis Care Res. 2020, 72, 1187; Arthritis Care Res. 2021, 73, 458. [Google Scholar] [CrossRef]
- McCormick, N.; Yokose, C.; Wei, J.; Lu, N.; Wexler, D.J.; Aviña-Zubieta, J.A.; De Vera, M.A.; Zhang, Y.; Choi, H.K. Comparative Effectiveness of Sodium-Glucose Cotransporter-2 Inhibitors for Recurrent Gout Flares and Gout-Primary Emergency Department Visits and Hospitalizations: A General Population Cohort Study. Ann. Intern. Med. 2023, 176, 1067–1080. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Abhishek, A.; Dalbeth, N.; Pascart, T.; Lioté, F.; Gaffo, A.; FitzGerald, J.; Terkeltaub, R.; Sise, M.E.; et al. The Clinical Benefits of Sodium-Glucose Cotransporter Type 2 Inhibitors in People with Gout. Nat. Rev. Rheumatol. 2024, 20, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Hui, M.; Carr, A.; Cameron, S.; Davenport, G.; Doherty, M.; Forrester, H.; Jenkins, W.; Jordan, K.M.; Mallen, C.D.; McDonald, T.M.; et al. The British Society for Rheumatology Guideline for the Management of Gout. Rheumatology 2017, 56, 1056–1059, Erratum in Rheumatology 2017, 56, 1246. [Google Scholar] [CrossRef] [PubMed]
- Pascart, T.; Latourte, A.; Flipo, R.M.; Chalès, G.; Coblentz-Baumann, L.; Cohen-Solal, A.; Ea, H.K.; Grichy, J.; Letavernier, E.; Lioté, F.; et al. 2020 recommendations from the French Society of Rheumatology for the management of gout: Urate-lowering therapy. Jt. Bone Spine 2020, 87, 395–404. [Google Scholar] [CrossRef]
- Tykarski, A.; Filipiak, K.J.; Januszewicz, A.; Litwin, M.; Narkiewicz, K.; Prejbisz, A.; Ostalska-Nowicka, D.; Widecka, K.; Kostka-Jeziorny, K. Zasady Postępowania w Nadciśnieniu Tętniczym—2019 Rok. Nadciśnienie Tętnicze w Prakt. 2019, 5, 1–86. [Google Scholar]
| Non-Modifiable Risk Factors | Comments | Modifiable Risk Factors | Comments |
|---|---|---|---|
| Age | The prevalence of hyperuricemia and gout rises with age | Body composition | Obesity and abdominal adiposity increase the risk of hyperuricemia and gout; weight loss has a protective effect against gout |
| Sex | Hyperuricemia and gout are more prevalent in men; in postmenopausal women, however, the SUA levels are close to those of men of the same age | Dietary factors | Dietary factors that increase the risk of hyperuricemia and/or gout:
Dietary factors that decrease the risk of hyperuricemia and/or gout:
|
| Genetic factors and ethnicity | Over 20 susceptibility genes for hyperuricemia and gout have been identified Gout appears to be more prevalent in Black and Asian individuals than in White individuals | Medication | Medications that increase SUA levels
Medications that decrease SUA levels
|
| Factors That Need to Be Addressed | Comments |
|---|---|
| Cardiovascular risks |
|
| Other comorbidities |
|
| Weight |
|
| Physical activity |
|
| Dietary factors |
|
| Medications prescribed for indications other than treating hyperuricemia |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timsans, J.; Palomäki, A.; Kauppi, M. Gout and Hyperuricemia: A Narrative Review of Their Comorbidities and Clinical Implications. J. Clin. Med. 2024, 13, 7616. https://doi.org/10.3390/jcm13247616
Timsans J, Palomäki A, Kauppi M. Gout and Hyperuricemia: A Narrative Review of Their Comorbidities and Clinical Implications. Journal of Clinical Medicine. 2024; 13(24):7616. https://doi.org/10.3390/jcm13247616
Chicago/Turabian StyleTimsans, Janis, Antti Palomäki, and Markku Kauppi. 2024. "Gout and Hyperuricemia: A Narrative Review of Their Comorbidities and Clinical Implications" Journal of Clinical Medicine 13, no. 24: 7616. https://doi.org/10.3390/jcm13247616
APA StyleTimsans, J., Palomäki, A., & Kauppi, M. (2024). Gout and Hyperuricemia: A Narrative Review of Their Comorbidities and Clinical Implications. Journal of Clinical Medicine, 13(24), 7616. https://doi.org/10.3390/jcm13247616





