Interstitial High-Dose-Rate Brachytherapy Combined with External Beam Radiation Therapy for Dose Escalation in the Primary Treatment of Locally Advanced, Non-Resectable Superior Sulcus (Pancoast) Tumors: Results of a Monocentric Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Technique
2.3. Response and Toxicity Evaluation Criteria
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rusch, V.W.; Giroux, D.J.; Kraut, M.J.; Crowley, J.; Hazuka, M.; Winton, T.; Johnson, D.H.; Shulman, L.; Shepherd, F.; Deschamps, C.; et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: Long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J. Clin. Oncol. 2007, 25, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kunitoh, H.; Kato, H.; Tsuboi, M.; Shibata, T.; Asamura, H.; Ichinose, Y.; Katakami, N.; Nagai, K.; Mitsudomi, T.; Matsumura, A.; et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: Report of Japan Clinical Oncology Group trial 9806. J. Clin. Oncol. 2008, 26, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Jeannin, G.; Merle, P.; Janicot, H.; Thibonnier, L.; Kwiatkowski, F.; Naame, A.; Chadeyras, J.B.; Galvaing, G.; Belliere, A.; Filaire, M.; et al. Combined treatment modalities in Pancoast tumor: Results of a monocentric retrospective study. Chin. Clin. Oncol. 2015, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.; Mai, G.T.; Vinod, S.; Babington, S.; Ruben, J.; Kron, T.; Chesson, B.; Herschtal, A.; Vanevski, M.; Rezo, A.; et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019, 20, 494–503. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Wujanto, C.; Vellayappan, B.; Siva, S.; Louie, A.V.; Guckenberger, M.; Slotman, B.J.; Onishi, H.; Nagata, Y.; Liu, M.; Lo, S.S. Stereotactic Body Radiotherapy for Oligometastatic Disease in Non-small Cell Lung Cancer. Front. Oncol. 2019, 9, 1219. [Google Scholar] [CrossRef]
- Falcinelli, L.; Menichelli, C.; Casamassima, F.; Aristei, C.; Borghesi, S.; Ingrosso, G.; Draghini, L.; Tagliagambe, A.; Badellino, S.; di Monale, E.B.M.B. Stereotactic radiotherapy for lung oligometastases. Rep. Pract. Oncol. Radiother. 2022, 27, 23–31. [Google Scholar] [CrossRef]
- Chatzikonstantinou, G.; Zamboglou, N.; Baltas, D.; Ferentinos, K.; Bon, D.; Tselis, N. Image-guided interstitial high-dose-rate brachytherapy for dose escalation in the radiotherapy treatment of locally advanced lung cancer: A single-institute experience. Brachytherapy 2019, 18, 829–834. [Google Scholar] [CrossRef]
- Kolotas, C.; Baltas, D.; Zamboglou, N. CT-Based interstitial HDR brachytherapy. Strahlenther. Onkol. 1999, 175, 419–427. [Google Scholar] [CrossRef]
- Ricke, J.; Wust, P.; Wieners, G.; Hengst, S.; Pech, M.; Lopez Hänninen, E.; Felix, R. CT-guided interstitial single-fraction brachytherapy of lung tumors: Phase I results of a novel technique. Chest 2005, 127, 2237–2242. [Google Scholar] [CrossRef]
- Nath, R.; Anderson, L.L.; Luxton, G.; Weaver, K.A.; Williamson, J.F.; Meigooni, A.S. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med. Phys. 1995, 22, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Rivard, M.J.; Coursey, B.M.; DeWerd, L.A.; Hanson, W.F.; Huq, M.S.; Ibbott, G.S.; Mitch, M.G.; Nath, R.; Williamson, J.F. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med. Phys. 2004, 31, 633–674. [Google Scholar] [CrossRef] [PubMed]
- Senan, S.; De Ruysscher, D.; Giraud, P.; Mirimanoff, R.; Budach, V. Literature-based recommendations for treatment planning and execution in high-dose radiotherapy for lung cancer. Radiother. Oncol. 2004, 71, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Fornacon-Wood, I.; Chan, C.; Bayman, N.; Banfill, K.; Coote, J.; Garbett, A.; Harris, M.; Hudson, A.; Kennedy, J.; Pemberton, L.; et al. Impact of Introducing Intensity Modulated Radiotherapy on Curative Intent Radiotherapy and Survival for Lung Cancer. Front. Oncol. 2022, 12, 835844. [Google Scholar] [CrossRef] [PubMed]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- National Care Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017. [Google Scholar]
- Department of Radiotherapy and Radiobiology, Medical University of Vienna. HDR Radiobiologic Dose Equivalent Worksheet #1. Available online: https://www.americanbrachytherapy.org/for-physicists/physics-corner/ (accessed on 1 September 2024).
- Unal, S.; Feller, R.; Stadhouder, A.; Heineman, D.J.; Jiya, T.U.; van Dorp, M.; Bahce, I.; Braun, J.; Senan, S.; Dahele, M.; et al. Superior Sulcus Tumors Invading the Spine: Multimodal Treatment Outcomes From the Preimmunotherapy Era. JTO Clin. Res. Rep. 2023, 4, 100582. [Google Scholar] [CrossRef]
- Hutchings, H.E.; Cox, J.; Westra, J.; Kuo, Y.F.; Okereke, I.C. Treatment patterns and outcomes in patients with Pancoast tumors: A national cancer database analysis. J. Thorac. Dis. 2023, 15, 33–41. [Google Scholar] [CrossRef]
- Gundepalli, S.G.; Tadi, P. Lung Pancoast Tumor; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rzyman, W.; Łazar-Poniatowska, M.; Dziedzic, R.; Marjański, T.; Łapiński, M.; Dziadziuszko, R. Trimodality Treatment of Superior Sulcus Non-Small Cell Lung Cancer: An Institutional Series of 47 Consecutive Patients. Curr. Oncol. 2023, 30, 4551–4562. [Google Scholar] [CrossRef]
- Deutsche Krebsgesellschaft; Deutsche Krebshilfe; AWMF. S3-Leitlinie Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms, Kurzversion 2.1, 2022, AWMF-Registernummer: 020-007OL; Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften: Frankfurt, Germany, 2023. [Google Scholar]
- Vokes, E.E.; Herndon, J.E., 2nd; Kelley, M.J.; Cicchetti, M.G.; Ramnath, N.; Neill, H.; Atkins, J.N.; Watson, D.M.; Akerley, W.; Green, M.R.; et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J. Clin. Oncol. 2007, 25, 1698–1704. [Google Scholar] [CrossRef]
- Caglar, H.B.; Baldini, E.H.; Othus, M.; Rabin, M.S.; Bueno, R.; Sugarbaker, D.J.; Mentzer, S.J.; Janne, P.A.; Johnson, B.E.; Allen, A.M. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer 2009, 115, 4156–4166. [Google Scholar] [CrossRef]
- Fong, T.; Morgensztern, D.; Govindan, R. EGFR inhibitors as first-line therapy in advanced non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.Y.; Lee, J.M.; Lee, Y.C.; Han, Y.M.; Lim, Y.S. Primary and secondary lung malignancies treated with percutaneous radiofrequency ablation: Evaluation with follow-up helical CT. Am. J. Roentgenol. 2004, 183, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Fieguth, H.G.; Eichler, K.; Straub, R.; Lehnert, T.; Zangos, S.; Mack, M. Laser-induced thermotherapy of lung metastases and primary lung tumors. Radiologe 2004, 44, 693–699. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, G.; Liang, X.; Wu, J.; Zhang, P.; Wang, N.; Li, Z.; Cao, P.; Wang, G.; Cai, H.; et al. The safety and feasibility of three-dimensional visualization planning system for CT-guided microwave ablation of stage I NSCLC (diameter ≤2.5 cm): A pilot study. J. Cancer Res. Ther. 2023, 19, 64–70. [Google Scholar] [CrossRef]
- Imamura, F.; Ueno, K.; Kusunoki, Y.; Uchida, J.; Yoshimura, M.; Koizumi, M.; Yamasaki, H.; Nishiyama, K. High-dose-rate brachytherapy for small-sized peripherally located lung cancer. Strahlenther. Onkol. 2006, 182, 703–707. [Google Scholar] [CrossRef]
- Peters, N.; Wieners, G.; Pech, M.; Hengst, S.; Rühl, R.; Streitparth, F.; Lopez Hänninen, E.; Felix, R.; Wust, P.; Ricke, J. CT-guided interstitial brachytherapy of primary and secondary lung malignancies: Results of a prospective phase II trial. Strahlenther. Onkol. 2008, 184, 296–301. [Google Scholar] [CrossRef]
- Rashid, A.; Pinkawa, M.; Haddad, H.; Hermani, H.; Temming, S.; Schäfer, A.; Bischoff, P.; Kovács, A. Interstitial single fraction brachytherapy for malignant pulmonary tumours. Strahlenther. Onkol. 2021, 197, 416–422. [Google Scholar] [CrossRef]
- Yoon, S.M.; Suh, R.; Abtin, F.; Moghanaki, D.; Genshaft, S.; Kamrava, M.; Drakaki, A.; Liu, S.; Venkat, P.; Lee, A.; et al. Outcomes with multi-disciplinary management of central lung tumors with CT-guided percutaneous high dose rate brachyablation. Radiat. Oncol. 2021, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Kolotas, C.; Röddiger, S.; Strassmann, G.; Martin, T.; Tselis, N.; Aebersold, D.M.; Baltas, D.; Zamboglou, N. Palliative interstitial HDR brachytherapy for recurrent rectal cancer. Implantation techniques and results. Strahlenther. Onkol. 2003, 179, 458–463. [Google Scholar] [CrossRef]
- Martin, T.; Baltas, D.; Kurek, R.; Roddiger, S.; Kontova, M.; Anagnostopoulos, G.; Dannenberg, T.; Buhleier, T.; Skazikis, G.; Tunn, U.; et al. 3-D conformal HDR brachytherapy as monotherapy for localized prostate cancer. A pilot study. Strahlenther. Onkol. 2004, 180, 225–232. [Google Scholar] [CrossRef]
- Wieners, G.; Pech, M.; Rudzinska, M.; Lehmkuhl, L.; Wlodarczyk, W.; Miersch, A.; Hengst, S.; Felix, R.; Wust, P.; Ricke, J. CT-guided interstitial brachytherapy in the local treatment of extrahepatic, extrapulmonary secondary malignancies. Eur. Radiol. 2006, 16, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Chatzikonstantinou, G.; Ulrich, P.; Archavlis, E.; Zamboglou, N.; Strouthos, I.; Zoga, E.; Baltas, D.; Tselis, N. Interstitial high-dose-rate brachytherapy in the primary treatment of inoperable glioblastoma multiforme. J. Contemp. Brachytherapy 2019, 11, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Dammerer, D.; Neugebauer, J.; Braito, M.; Wagner, M.; Neubauer, M.; Moser, L.; Suss, M.; Liebensteiner, M.; Putzer, D. Midterm Results of High-Dose-Rate Intraoperative Brachytherapy in the Treatment of Soft Tissue Sarcomas. Cancers 2023, 15, 2854. [Google Scholar] [CrossRef]
- Laskar, S.; Manjali, J.J.; Chargari, C.; Chard, J. Brachytherapy for Organ and Function Preservation in Soft-Tissue Sarcomas in Adult and Paediatric Patients. Clin. Oncol. 2023, 35, 533–540. [Google Scholar] [CrossRef]
- Neugebauer, J.; Blum, P.; Keiler, A.; Suss, M.; Neubauer, M.; Moser, L.; Dammerer, D. Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities-A Current Concept and Systematic Review of the Literature. Cancers 2023, 15, 1133. [Google Scholar] [CrossRef]
- Ferenczi, Ö.; Major, T.; Fröhlich, G.; Béla, D.; Tódor, S.; Polgár, C.; Akiyama, H.; Bukovszky, B.; Takácsi-Nagy, Z. Dosimetric comparison of postoperative interstitial high-dose-rate brachytherapy and modern external beam radiotherapy modalities in tongue and floor of the mouth tumours in terms of doses to critical organs. Radiol. Oncol. 2023, 57, 516–523. [Google Scholar] [CrossRef]
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Gutierrez Miguelez, C.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238. [Google Scholar] [CrossRef]
- Herein, A.; Stelczer, G.; Pesznyák, C.; Fröhlich, G.; Smanykó, V.; Mészáros, N.; Polgár, C.; Major, T. Multicatheter interstitial brachytherapy versus stereotactic radiotherapy with CyberKnife for accelerated partial breast irradiation: A comparative treatment planning study with respect to dosimetry of organs at risk. Radiol. Oncol. 2021, 55, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef]
- Gupta, I.J.; Ghosh, A.; Yadav, J.; Tuteja, J.S.; Gupta, R.; Srivastava, K.; Verma, M.; Gupta, S.; Srivastava, S.; Bhatt, M.L.B. External Beam Radiotherapy Interdigitated with High Dose Rate(HDR) Intracavitary Brachytherapy versus External Beam Radiotherapy followed by Sequential HDR Intracavitary Brachytherapy for Locally Advanced Carcinoma Cervix-Randomized Control Study. Asian Pac. J. Cancer Prev. 2023, 24, 3441–3445. [Google Scholar] [CrossRef]
- Hsieh, K.; Bloom, J.R.; Dickstein, D.R.; Hsieh, C.; Marshall, D.; Ghiassi-Nejad, Z.; Raince, J.; Lymberis, S.; Chadha, M.; Gupta, V. Dose and fractionation regimen for brachytherapy boost in cervical cancer in the US. Gynecol. Oncol. 2023, 180, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Tharavichitkul, E.; Jia-Mahasap, B.; Muangwong, P.; Chakrabandhu, S.; Klunklin, P.; Onchan, W.; Tippanya, D.; Nobnop, W.; Watcharawipha, A.; Kittidachanan, K.; et al. Survival outcome of cervical cancer patients treated by image-guided brachytherapy: A ‘real world’ single center experience in Thailand from 2008 to 2018. J. Radiat. Res. 2022, 63, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Willner, J.; Baier, K.; Caragiani, E.; Tschammler, A.; Flentje, M. Dose, volume, and tumor control prediction in primary radiotherapy of non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 382–389. [Google Scholar] [CrossRef]
- Hagan, M.P.; Choi, N.C.; Mathisen, D.J.; Wain, J.C.; Wright, C.D.; Grillo, H.C. Superior sulcus lung tumors: Impact of local control on survival. J. Thorac. Cardiovasc. Surg. 1999, 117, 1086–1094. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Kataoka, M.; Yamashita, M.; Shinkai, T.; Kubo, Y.; Sugawara, Y.; Inoue, T.; Sakai, S.; Aono, S.; Takahashi, T.; et al. Local control of metastatic lung tumors treated with SBRT of 48 Gy in four fractions: In comparison with primary lung cancer. Jpn. J. Clin. Oncol. 2010, 40, 125–129. [Google Scholar] [CrossRef]
- Timmerman, R.D.; Park, C.; Kavanagh, B.D. The North American experience with stereotactic body radiation therapy in non-small cell lung cancer. J. Thorac. Oncol. 2007, 2, S101–S112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, B.; Wu, Z.; Xiao, H.; Yang, Y.; Fan, J.; Gu, Y.; Chen, C.; Wu, J. The occurrence and development of radiation-induced lung injury after interstitial brachytherapy and stereotactic radiotherapy in SD rats. J. Inflamm. 2023, 20, 23. [Google Scholar] [CrossRef]
- Walter, F.; Nierer, L.; Rottler, M.; Duque, A.S.; Weingandt, H.; Well, J.; Shpani, R.; Landry, G.; Seidensticker, M.; Streitparth, F.; et al. Comparison of liver exposure in CT-guided high-dose rate (HDR) interstitial brachytherapy versus SBRT in hepatocellular carcinoma. Radiat. Oncol. 2021, 16, 86. [Google Scholar] [CrossRef]
- Bilski, M.; Korab, K.; Stąpór-Fudzińska, M.; Ponikowska, J.; Brzozowska, A.; Sroka, Ł.; Wojtyna, E.; Sroka, S.; Szlag, M.; Cisek, P.; et al. HDR brachytherapy versus robotic-based and linac-based stereotactic ablative body radiotherapy in the treatment of liver metastases—A dosimetric comparison study of three radioablative techniques. Clin. Transl. Radiat. Oncol. 2024, 48, 100815. [Google Scholar] [CrossRef]
- Parisi, S.; Ferini, G.; Lillo, S.; Brogna, A.; Chillari, F.; Ferrantelli, G.; Settineri, N.; Santacaterina, A.; Platania, A.; Leotta, S.; et al. Stereotactic boost on residual disease after external-beam irradiation in clinical stage III non-small cell lung cancer: Mature results of stereotactic body radiation therapy post radiation therapy (SBRTpostRT) study. Radiol. Med. 2023, 128, 877–885. [Google Scholar] [CrossRef]
- Ferini, G.; Valenti, V.; Viola, A.; Umana, G.E.; Illari, S.I.; Parisi, S.; Pontoriero, A.; Pergolizzi, S. First-ever Clinical Experience With Magnetic Resonance-based Lattice Radiotherapy for Treating Bulky Gynecological Tumors. Anticancer Res. 2022, 42, 4641–4646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, Y.; Wang, F.; Badkul, R.; Chen, R.C.; Gao, H. Lattice position optimization for LATTICE therapy. Med. Phys. 2023, 50, 7359–7367. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.W.; Mazur, T.R.; Schmidt, M.C.; Hilliard, J.; Badiyan, S.; Spraker, M.B.; Kavanaugh, J.A. Script-based implementation of automatic grid placement for lattice stereotactic body radiation therapy. Phys. Imaging. Radiat. Oncol. 2024, 29, 100549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Griffin, R.J.; Galhardo, E.P.; Penagaricano, J. Feasibility Study of 3D-VMAT-Based GRID Therapy. Technol. Cancer Res. Treat. 2022, 21, 15330338221086420. [Google Scholar] [CrossRef]
- Tselis, N.; Ferentinos, K.; Kolotas, C.; Schirren, J.; Baltas, D.; Antonakakis, A.; Ackermann, H.; Zamboglou, N. Computed tomography-guided interstitial high-dose-rate brachytherapy in the local treatment of primary and secondary intrathoracic malignancies. J. Thorac. Oncol. 2011, 6, 545–552. [Google Scholar] [CrossRef]
- Ferentinos, K.; Karagiannis, E.; Strouthos, I.; Vrachimis, A.; Doolan, P.J.; Zamboglou, N. Computed tomography guided interstitial percutaneous high-dose-rate brachytherapy in the management of lung malignancies. A review of the literature. Brachytherapy 2021, 20, 892–899. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Mou, K.; Zheng, Y.; Li, H.; Ren, P.; Ye, H.; Lin, S.; Pang, H.; Wu, J.; et al. Risk Factors for Operation Complications of High Dose Rate 3-Dimensional Interstitial Brachytherapy for Lung Cancer. Clin. Lung Cancer 2023, 24, e187–e194. [Google Scholar] [CrossRef]
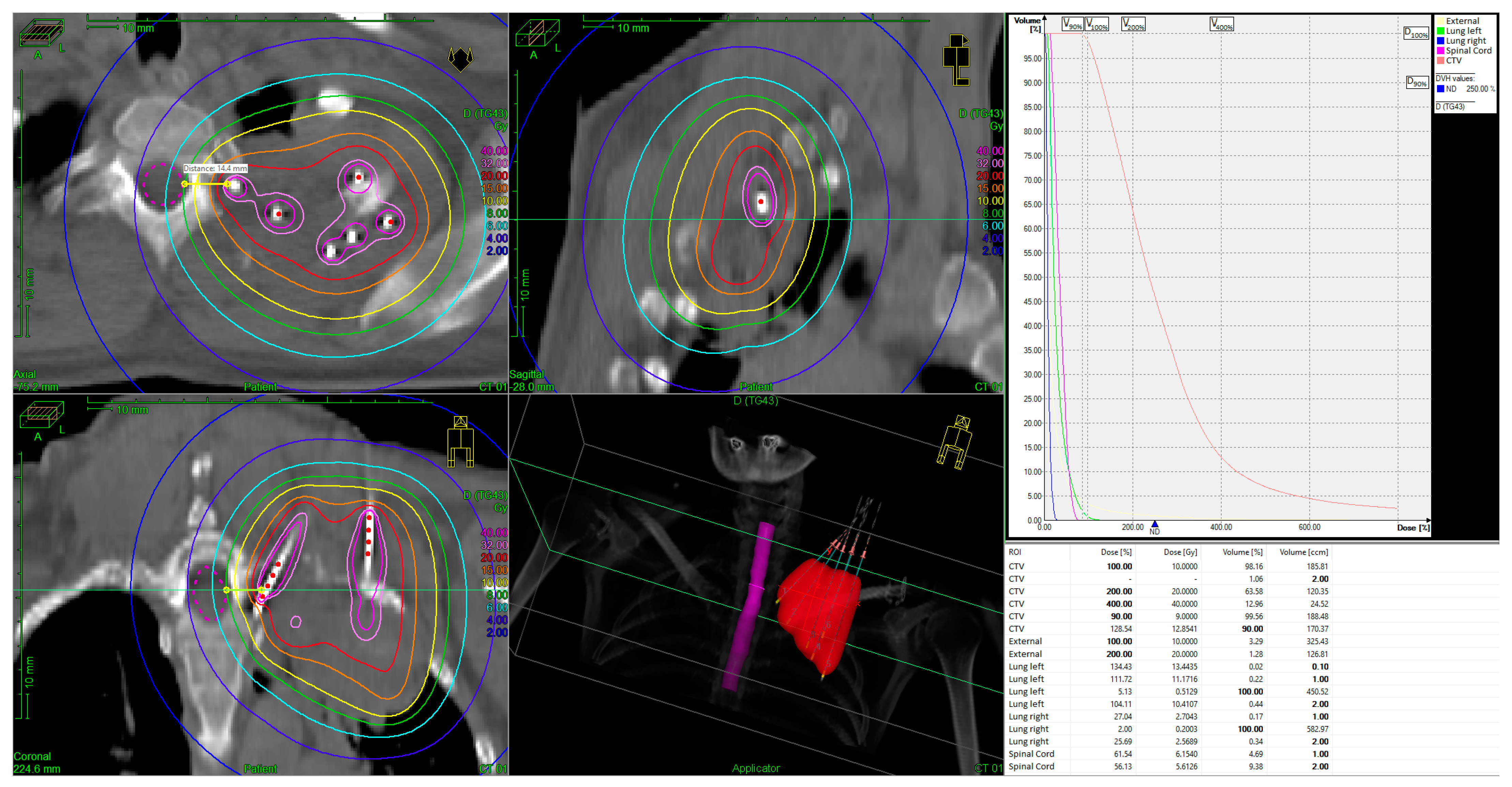
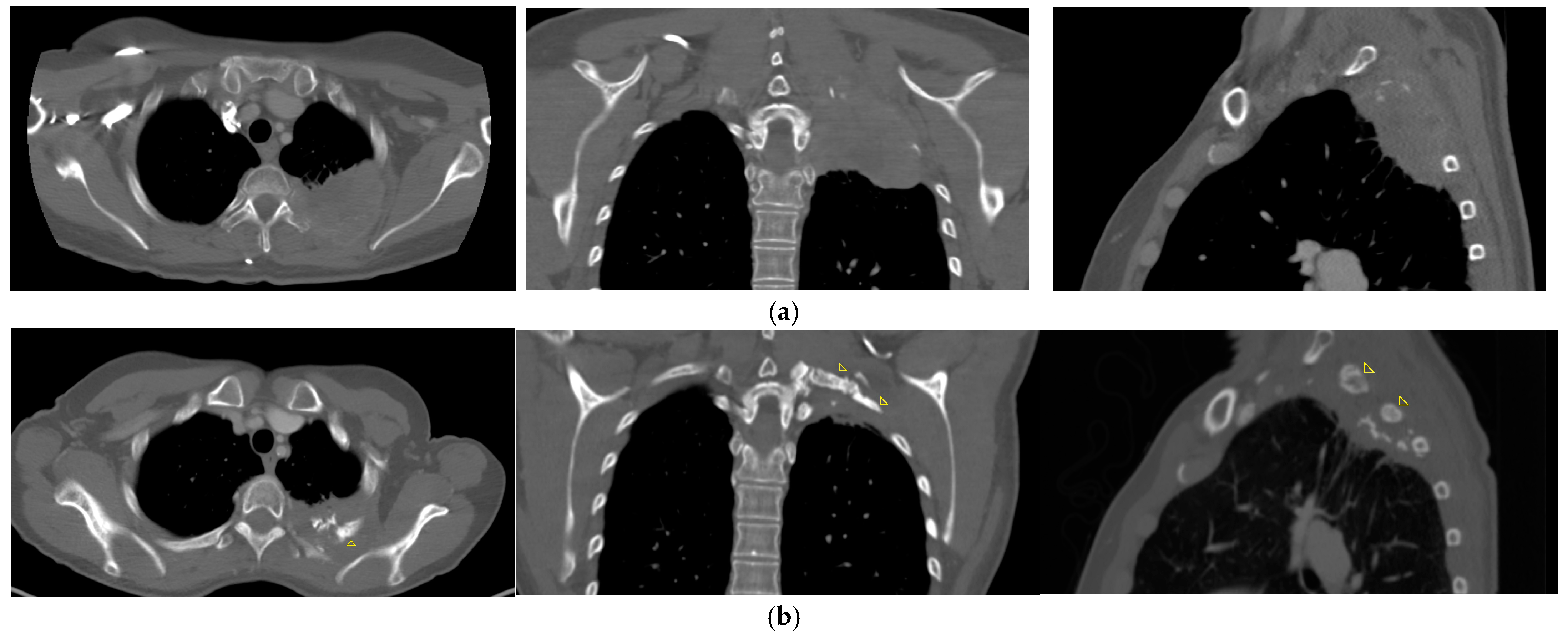
| Patient No. | Age (yrs), Sex | Histological Subtype | TNM | BT: Dose (Gy) | EBRT: Total Dose (Gy) | Tumor Volume at BT (cm3) | CHT Simultaneous to EBRT | CHT Upon Progression |
|---|---|---|---|---|---|---|---|---|
| 1 | 79, F | SCC | T2 N0 M0 | 10 | 54 | 29.3 | + | - |
| 2 | 74, M | SCC | T4 N2 M1 | 8 | 26 * | 146.8 | - | - |
| 3 | 50, F | SCC | T4 N0 M0 | 10 | 54 | 185.7 | + | - |
| 4 | 64, M | SCC | T4 N0 M0 | 5 | 59 | 130.4 | - | - |
| 5 | 49, F | ADC | T4 N3 M1 | 8 | 59 | 84.6 | + | - |
| 6 | 67, F | ADC | T4 N0 M0 | 8 | 59 | 149.7 | + | - |
| 7 | 63, F | ADC | T4 N2 M1 | 5 | 30 | 242.3 | - | + |
| mean | 63.7 | 7.7 | 48.7 | 138.4 | ||||
| median | 64 | 8 | 54 | 146.8 |
| Patient No. | BT: Dose (Gy) | EBRT: Total Dose (Gy) | EBRT: Dose per Fraction (Gy) | BED | EQD2 | Total BED | Total EQD2 |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 54 | 2 | 64.8 | 54 | 84.8 | 70.7 |
| 2 | 8 | 26 | 2 | 31.2 | 26 | 45.6 | 38 |
| 3 | 10 | 54 | 1.8 | 63.7 | 53,1 | 83.7 | 69.8 |
| 4 | 5 | 59 | 1.8 | 70.1 | 58,4 | 77.6 | 64.7 |
| 5 | 8 | 59 | 1.8 | 70.1 | 58,4 | 84.5 | 70.4 |
| 6 | 8 | 59 | 1.8 | 70.1 | 58,4 | 84.5 | 70.4 |
| 7 | 5 | 30 | 3 | 39 | 32,5 | 46.5 | 38.8 |
| mean | 7.7 | 48.7 | 2 | 58.4 | 48.7 | 72.5 | 60.4 |
| median | 8 | 54 | 1.8 | 64.8 | 54 | 83.7 | 69.8 |
| Patient No. | KPS Score * PreRT | KPS Score 1.5-Month | KPS Score 12-Month | OS ** | LC *** | Patient Alive | Cause of Death | Comments |
|---|---|---|---|---|---|---|---|---|
| 1 | 80 | 70 | 90 | 131 | 131 | yes | Painless after IRT BT. No evidence of local recurrence: complete metabolic response (CMR) | |
| 2 | 60 | 0 | 0 | 0 | 0 | no | Rapid progression of systemic disease | Died during EBRT at 26 Gy. (initial distant metastasis) |
| 3 | 70 | 60 | 90 | 95 | 95 | yes | Clinically significant improvement in pain symptoms and motor skills upon completion of IRT BT. Recalcification of the rib. No evidence of local recurrence (CMR), new singular pulmonary lesion at 54 months after initial therapy, treated with SBRT | |
| 4 | 60 | 60 | 0 | 8 | 7 | no | Progressive systemic disease | Died of local and systemic progression of the disease at 8 months after radiotherapy |
| 5 | 80 | 80 | 80 | 39 | 39 | yes | Progressive systemic disease (initial distant metastasis) with new cerebral metastasis, under treatment. Locally negative PET-CT scan (CMR) | |
| 6 | 70 | 60 | 90 | 38 | 38 | yes | Clinically significant improvement in pain symptoms upon completion of IRT BT. No evidence of local recurrence (CMR) | |
| 7 | 60 | 60 | 60 | 31 | 31 | yes | Progressive systemic disease (initial distant metastasis), under treatment. Locally negative PET-CT scan (CMR) | |
| mean (n) | 68.6 (7) | 65 (6) | 82 (5) | 48.9 | 48.7 | |||
| median (n) | 70 (7) | 60 (6) | 90 (5) | 38 | 38 |
| Patient No. | Fatigue | Dysesthesia | Dysphagia | Pneumonitis | Pulmonary Fibrosis | Hemorrhage | Pneumothorax |
|---|---|---|---|---|---|---|---|
| 1 | - | - | - | - | - | - | - |
| 2 | II° | II° | - | - | - | - | - |
| 3 | - | I° | - | I° | - | - | - |
| 4 | II° | I° | - | - | - | - | - |
| 5 | I° | - | - | - | - | - | - |
| 6 | - | I° | - | I° | - | - | - |
| 7 | I° | I° | - | I° | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neu, M.; Kahl, K.-H.; Körner, M.; Walter, R.; Raab, S.; Jehs, B.; Käsmann, L.; Strnad, V.; Stüben, G.; Balagiannis, N. Interstitial High-Dose-Rate Brachytherapy Combined with External Beam Radiation Therapy for Dose Escalation in the Primary Treatment of Locally Advanced, Non-Resectable Superior Sulcus (Pancoast) Tumors: Results of a Monocentric Retrospective Study. J. Clin. Med. 2024, 13, 7550. https://doi.org/10.3390/jcm13247550
Neu M, Kahl K-H, Körner M, Walter R, Raab S, Jehs B, Käsmann L, Strnad V, Stüben G, Balagiannis N. Interstitial High-Dose-Rate Brachytherapy Combined with External Beam Radiation Therapy for Dose Escalation in the Primary Treatment of Locally Advanced, Non-Resectable Superior Sulcus (Pancoast) Tumors: Results of a Monocentric Retrospective Study. Journal of Clinical Medicine. 2024; 13(24):7550. https://doi.org/10.3390/jcm13247550
Chicago/Turabian StyleNeu, Maria, Klaus-Henning Kahl, Melina Körner, Renate Walter, Stephan Raab, Bertram Jehs, Lukas Käsmann, Vratislav Strnad, Georg Stüben, and Nikolaos Balagiannis. 2024. "Interstitial High-Dose-Rate Brachytherapy Combined with External Beam Radiation Therapy for Dose Escalation in the Primary Treatment of Locally Advanced, Non-Resectable Superior Sulcus (Pancoast) Tumors: Results of a Monocentric Retrospective Study" Journal of Clinical Medicine 13, no. 24: 7550. https://doi.org/10.3390/jcm13247550
APA StyleNeu, M., Kahl, K.-H., Körner, M., Walter, R., Raab, S., Jehs, B., Käsmann, L., Strnad, V., Stüben, G., & Balagiannis, N. (2024). Interstitial High-Dose-Rate Brachytherapy Combined with External Beam Radiation Therapy for Dose Escalation in the Primary Treatment of Locally Advanced, Non-Resectable Superior Sulcus (Pancoast) Tumors: Results of a Monocentric Retrospective Study. Journal of Clinical Medicine, 13(24), 7550. https://doi.org/10.3390/jcm13247550






