Haemodynamic Assessment and Outcomes of Aortic Valvuloplasty for Aortic Regurgitation in Patients with Bicuspid Aortic Valve
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethical Approval
2.3. Operative Techniques
2.4. Echocardiographic Assessments
2.5. Clinical End Points
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Postoperative Echocardiographic Findings
3.3. Postoperative Echocardiographic Findings Using the IPTW Method
3.4. Risk Factors for Higher Peak PG After AVP in BAV Patients
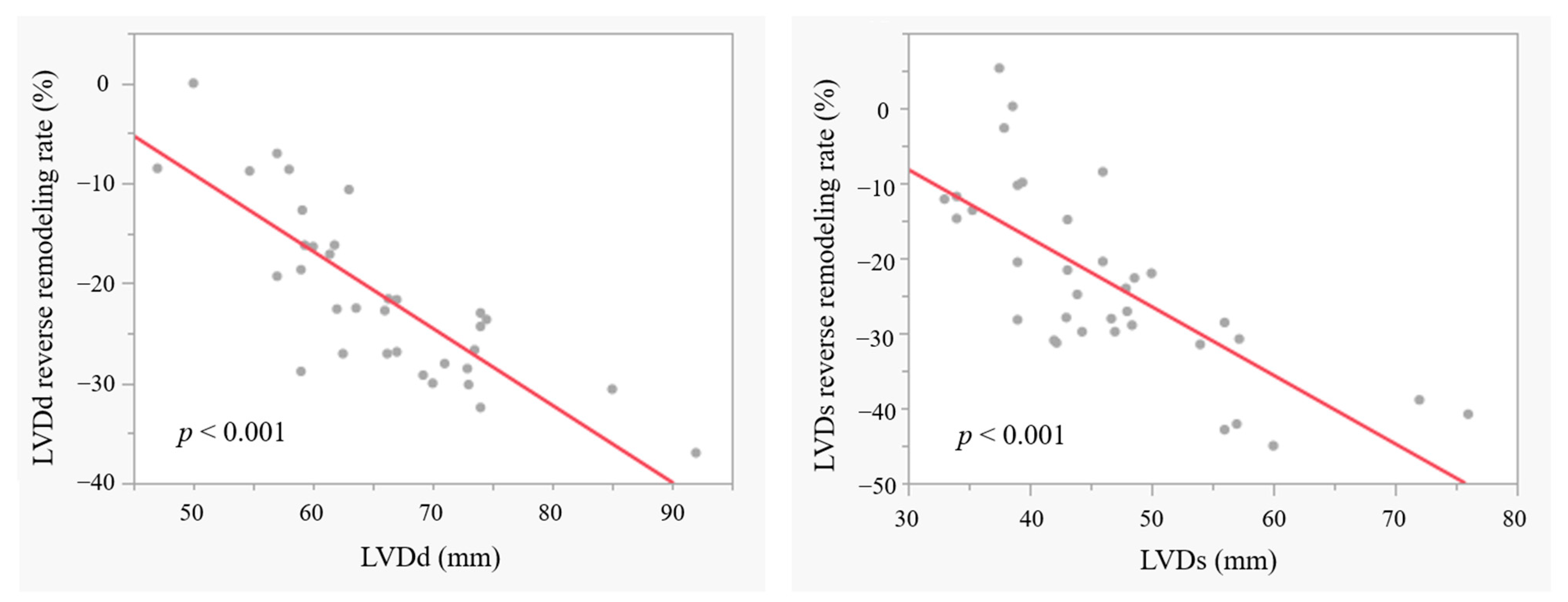
3.5. LV Reverse Remodelling in Patients with BAV
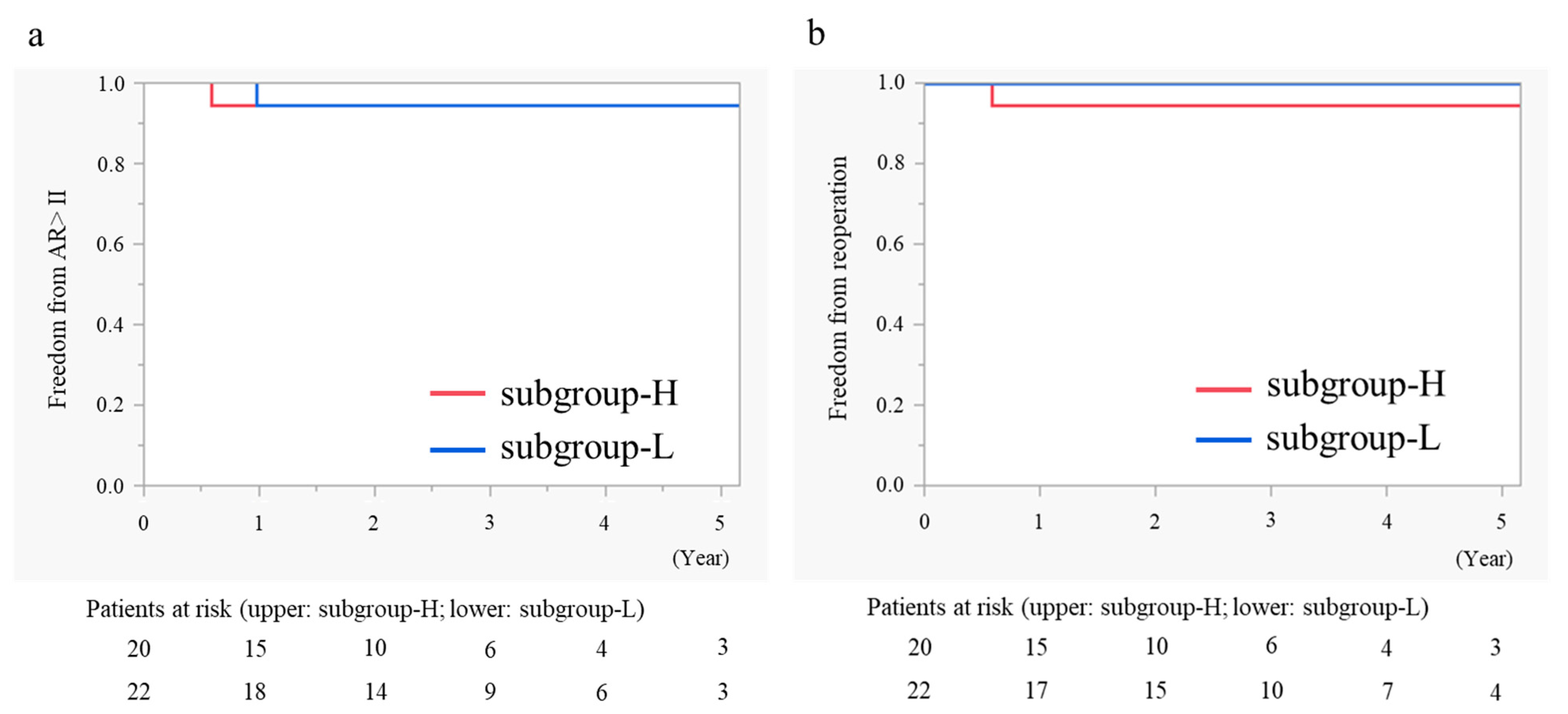
3.6. Mid-Term Outcomes in AVP for BAV
3.7. Subgroup Analysis in AVP for BAV According to Postoperative Peak PG
4. Discussion
4.1. Advantages of AVP with Regard to Valve Function
4.2. Higher PG After AVP in BAV Patients
4.3. Mid-Term Outcomes After AVP in BAV Patients
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Schäfers, H.J.; Raddatz, A.; Schmied, W.; Takahashi, H.; Miura, Y.; Kunihara, T.; Aicher, D. Reexamining remodeling. J. Thorac. Cardiovasc. Surg. 2015, 149, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Tamer, S.; Mastrobuoni, S.; Lemaire, G.; Jahanyar, J.; Navarra, E.; Poncelet, A.; Astarci, P.; El Khoury, G.; de Kerchove, L. Two decades of valve-sparing root reimplantation in tricuspid aortic valve: Impact of aortic regurgitation and cusp repair. Eur. J. Cardiothorac. Surg. 2021, 59, 1069–1076. [Google Scholar] [CrossRef]
- de Kerchove, L.; Mastrobuoni, S.; Froede, L.; Tamer, S.; Boodhwani, M.; van Dyck, M.; EL Khoury, G.; Schäfers, H.J. Variability of repairable bicuspid aortic valve phenotypes: Towards an anatomical and repair-oriented classification. Eur. J. Cardiothorac. Surg. 2019, 56, 351–359. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Vohra, H.A.; Whistance, R.N.; de Kerchove, L.; Glineur, D.; Noirhomme, P.; El Khoury, G. Influence of higher valve gradient on long-term outcome after aortic valve repair. Ann. Cardiothorac. Surg. 2013, 2, 30–39. [Google Scholar]
- Kunihara, T.; Arimura, S.; Sata, F.; Giebels, C.; Schneider, U.; Schäfers, H.J. Aortic annulus does not dilate over time after aortic root remodeling with or without annuloplasty. J. Thorac. Cardiovasc. Surg. 2018, 155, 885–894. [Google Scholar] [CrossRef]
- Kunihara, T. Toward standardization of valve-sparing root replacement and annuloplasty. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, H.J.; Bierbach, B.; Aicher, D. A new approach to the assessment of aortic cusp geometry. J. Thorac. Cardiovasc. Surg. 2006, 132, 436–438. [Google Scholar] [CrossRef]
- Klotz, S.; Stock, S.; Sievers, H.H.; Diwoky, M.; Petersen, M.; Stierle, U.; Richardt, D. Survival and reoperation pattern after 20 years of experience with aortic valve-sparing root replacement in patients with tricuspid and bicuspid valves. J. Thorac. Cardiovasc. Surg. 2018, 155, 1403–1411. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar]
- Gocoł, R.; Bis, J.; Malinowski, M.; Ciosek, J.; Hudziak, D.; Morkisz, Ł.; Jasiński, M.; Deja, M.A. Comparison of bicuspid and tricuspid aortic valve repair. Eur. J. Cardiothorac. Surg. 2021, 59, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Korteland, N.M.; Etnel, J.R.G.; Arabkhani, B.; Mokhles, M.M.; Mohamad, A.; Roos-Hesselink, J.W.; Bogers, A.J.J.C.; Takkenberg, J.J.M. Mechanical aortic valve replacement in non-elderly adults: Meta-analysis and microsimulation. Eur. Heart. J. 2017, 38, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Elbatarny, M.; Tam, D.Y.; Edelman, J.J.; Rocha, R.V.; Chu, M.W.A.; Peterson, M.D.; El-Hamamsy, I.; Appoo, J.J.; Friedrich, J.O.; Boodhwani, M.; et al. Valve-Sparing Root Replacement Versus Composite Valve Grafting in Aortic Root Dilation: A Meta-Analysis. Ann. Thorac. Surg. 2020, 110, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Fattouch, K.; Murana, G.; Castrovinci, S.; Nasso, G.; Mossuto, C.; Corrado, E.; Ruvolo, G.; Speziale, G. Outcomes of aortic valve repair according to valve morphology and surgical techniques. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 644–650. [Google Scholar] [CrossRef]
- Patlolla, S.H.; Schaff, H.V.; Stulak, J.M.; Michelena, H.I.; Saran, N.; King, K.S. Bicuspid Aortic Valve Repair: Causes of Valve Failure and Long-Term Outcomes. Ann. Thorac. Surg. 2021, 111, 1225–1233. [Google Scholar] [CrossRef]
- Pettersson, G.B.; Crucean, A.C.; Savage, R.; Halley, C.M.; Grimm, R.A.; Svensson, L.G.; Naficy, S.; Gillinov, A.M.; Feng, J.; Blackstone, E.H. Toward Predictable Repair of Regurgitant Aortic Valves. J. Am. Coll. Cardiol. 2008, 52, 40–49. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Desai, N.; Szeto, W.Y.; Komlo, C.; Rhode, T.; Wallen, T.; Vallabhajosyula, P. Valve-sparing root reimplantation and leaflet repair in a bicuspid aortic valve: Comparison with the 3-cusp David procedure. J. Thorac. Cardiovasc. Surg. 2015, 149, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Prinzing, A.; Boehm, J.; Erlebach, M.; Sideris, K.; Lange, R.; Krane, M. Comparison of outcomes following isolated repair of tricuspid versus bicuspid aortic valves. J. Thorac. Dis. 2020, 12, 3514–3533. [Google Scholar] [CrossRef]
- Schäfers, H.J.; Schmied, W.; Marom, G.; Aicher, D. Cusp height in aortic valves. J. Thorac. Cardiovasc. Surg. 2013, 146, 269–274. [Google Scholar] [CrossRef]
- Karliova, I.; Schneider, U.; Ehrlich, T.; Schäfers, H.J. Results of Pericardial Patches in Tricuspid and Bicuspid Aortic Cusp Repair. Ann. Thorac. Surg. 2020, 109, 728–735. [Google Scholar] [CrossRef]
- Izumi, C.; Kitai, T.; Kume, T.; Onishi, T.; Yuda, S.; Hirata, K.; Yamashita, E.; Kawata, T.; Nishimura, K.; Takeuchi, M.; et al. Effect of Left Ventricular Reverse Remodeling on Long-term Outcomes After Aortic Valve Replacement. Am. J. Cardiol. 2019, 124, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Izumi, C.; Imamura, S.; Onishi, N.; Tamaki, Y.; Enomoto, S.; Miyake, M.; Tamura, T.; Kondo, H.; Kaitani, K.; et al. Late recurrence of left ventricular dysfunction after aortic valve replacement for severe chronic aortic regurgitation. Int. J. Cardiol. 2016, 224, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, N.; Nikolaou, P.E.; Karakasis, P.; Stachteas, P.; Fragakis, N.; Andreadou, I. Endothelial Protection by Sodium-Glucose Cotransporter 2 Inhibitors: A Literature Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 7274. [Google Scholar] [CrossRef] [PubMed]
| AVP (n = 108) | AVR (n = 132) | p-Value | ||
|---|---|---|---|---|
| Group-PB (n = 41) | Group-PT (n = 67) | Group-R (n = 132) | ||
| Patient characteristics | ||||
| Age | 40.0 (26.0–49.0) | 57.0 (50.0–66.0) | 65.5 (55.0–73.0) | <0.001 |
| Body surface area (m2) | 1.81 (1.72–1.91) | 1.78 (1.62–1.90) | 1.71 (1.56–1.85) | 0.002 |
| Male sex (%) | 40 (97.6) | 58 (87.9) | 106 (80.3) | 0.019 |
| Preoperative echocardiography | ||||
| LVDd (mm) | 65.8 (59.1–72.9) | 59.4 (53.0–67.0) | 65.0 (60.0–69.0) | 0.002 |
| LVDs (mm) | 46.5 (39.4–52.5) | 40.0 (35.6–48.3) | 47.0 (41.0–52.0) | 0.001 |
| LVEF (%) | 57.6 (51.0–60.2) | 58.3 (52.2–62.1) | 53.0 (47.0–61.0) | 0.119 |
| Peak PG (mmHg) | 17.0 (11.8–23.2) | 8.6 (5.1–12.7) | <0.001 | |
| Mean PG (mmHg) | 9.1 (6.5–12.3) | 4.4 (2.8–7.1) | 0.001 | |
| Vmax (m/s) | 2.2 (1.8–2.4) | 1.6 (1.2–2.0) | <0.001 | |
| AVA (cm2) | 3.4 (2.9–4.2) | 3.5 (2.9–4.1) | 0.804 | |
| Operations | ||||
| VSRR (remodelling) (%) | 16 (39.0) | 29 (43.3) | 0.663 | |
| AVP (%) | 25 (61.0) | 38 (56.7) | ||
| AVP details | ||||
| Cusp plication (%) | 38 (92.7) | 46 (68.7) | 0.004 | |
| External suture annuloplasty (%) | 41 (100) | 59 (88.1) | 0.022 | |
| External suture annuloplasty size (mm) | 18 mm:1 20 mm: 5 22 mm: 19 24 mm: 16 | 18 mm: 4 20 mm: 21 22 mm: 29 24 mm: 4 30 mm: 1 | 0.002 | |
| STJ remodelling (%) | 36 (87.8) | 59 (88.1) | 0.969 | |
| STJ remodelling size (mm) | 20 mm: 1 22 mm: 4 24 mm: 18 26 mm: 13 | 20 mm: 1 22 mm: 12 24 mm: 38 26 mm: 7 30 mm: 1 | 0.064 | |
| Use of the aPP (%) | 6 (14.6) | 15 (22.4) | 0.323 | |
| AVR operation | 132 | |||
| Bioprosthetic valve (%) | 93 (70.5) | |||
| Mechanical valve (%) | 39 (29.5) | |||
| Aortic prosthetic valve size (mm) | 19 mm: 5 21 mm: 9 23 mm: 34 25 mm: 53 27 mm: 31 | |||
| Group-PB (n = 41) | Group-PT (n = 67) | Group-R (n = 132) | |
|---|---|---|---|
| 7-day TTE | |||
| Aortic regurgitation grade (>II) (%) | 0 (0) | 0(0) | 0 (0) |
| LVDd (mm) | 54.0 (50.3–58.4) | 49.8 (47.0–55.0) | 52.0 (48.0–56.2) |
| LVDs (mm) | 41.0 (35.0–47.3) | 36.5 (32.0–43.0) | 39.0 (33.8–45.0) |
| LVEF (%) | 45.0 (35.1–58.0) | 54.0 (41.0–60.0) | 50.0 (37.0–59.0) |
| Peak PG (mmHg) | 19.1 (13.2–25.3) | 8.8 (6.2–12.2) | 13.6 (10.7–17.9) |
| Mean PG (mmHg) | 11.7 (7.6–15.0) | 5.1 (3.4–6.4) | 7.5 (6.0–10.7) |
| Vmax (m/s) | 2.2 (1.9–2.5) | 1.5 (1.4–1.9) | 1.9 (1.6–2.3) |
| AVA (cm2) | 1.7 (1.4–2.1) | 2.6 (2.1–3.1) | 1.8 (1.5–2.2) |
| 1-year TTE | |||
| Aortic regurgitation grade (>II) (%) | 1 (2.9) | 2 (3.3) | 0 (0.0) |
| LVDd (mm) | 51.0 (49.0–53.0) | 48.7 (44.5–51.5) | 48.0 (44.0–51.0) |
| LVDs (mm) | 34.4 (31.0–37.6) | 30.5 (28.0–34.0) | 31.9 (27.3–35.0) |
| LVEF (%) | 59.0 (56.5–64.0) | 63.0 (60.3–67.7) | 64.0 (58.3–69.0) |
| Peak PG (mmHg) | 20.0 (13.0–28.9) | 8.4 (6.0–12.0) | 15.6 (10.5–20.2) |
| Mean PG (mmHg) | 11.6 (7.7–15.8) | 4.4 (3.0–6.1) | 8.2 (5.7–11.3) |
| Vmax (m/s) | 2.4 (1.9–2.9) | 1.7 (1.4–1.8) | 2.1 (1.6–2.3) |
| AVA (cm2) | 2.1 (1.5–2.6) | 2.6 (2.1–3.0) | 2.0 (1.6–2.3) |
| <Group-PB vs. Group-R (Reference)> | ||||||||||
| Evaluated Time Point | Outcome Type | Outcome | Unadjusted (n = 173) | IPTW (n = 169) | ||||||
| 7-day TTE | Ordered category | Odds ratio | 95% CIs | p-value | Odds ratio | 95% CIs | p-value | |||
| Aortic regurgitation grade | 1.44 | 0.691 | 3.004 | 0.331 | 1.278 | 0.776 | 2.105 | 0.335 | ||
| Continuous | Coefficient | 95% CIs | p-value | Coefficient | 95% CIs | p-value | ||||
| Peak PG (mmHg) | 5.373 | 2.488 | 8.258 | <0.001 | 5.467 | 3.016 | 7.918 | <0.001 | ||
| Mean PG (mmHg) | 3.373 | 1.702 | 5.043 | <0.001 | 3.413 | 1.999 | 4.826 | <0.001 | ||
| Vmax (m/s) | 0.303 | 0.116 | 0.489 | 0.002 | 0.345 | 0.171 | 0.520 | <0.001 | ||
| AVA (cm2) | −0.168 | −0.397 | 0.060 | 0.147 | −0.322 | −0.534 | −0.111 | 0.003 | ||
| 1-year TTE | Ordered category | Odds ratio | 95% CIs | p-value | Odds ratio | 95% CIs | p-value | |||
| Aortic regurgitation grade | 0.356 | 0.164 | 0.770 | 0.009 | 0.476 | 0.283 | 0.799 | 0.005 | ||
| Continuous | Coefficient | 95% CIs | p-value | Coefficient | 95% CIs | p-value | ||||
| Peak PG (mmHg) | 5.402 | 2.146 | 8.658 | 0.001 | 5.647 | 2.833 | 8.462 | <0.001 | ||
| Mean PG (mmHg) | 3.250 | 1.367 | 5.133 | <0.001 | 3.504 | 1.923 | 5.084 | <0.001 | ||
| Vmax (m/s) | 0.357 | 0.048 | 0.667 | 0.025 | 0.588 | 0.298 | 0.878 | <0.001 | ||
| AVA (cm2) | 0.335 | 0.036 | 0.633 | 0.028 | 0.105 | −0.214 | 0.423 | 0.517 | ||
| <Group-PT vs. Group-R (Reference)> | ||||||||||
| Evaluated time point | Outcome type | Outcome | Unadjusted (n = 199) | IPTW (n = 192) | ||||||
| 7-day TTE | Ordered category | Odds ratio | 95% CIs | p-value | Odds ratio | 95% CIs | p-value | |||
| Aortic regurgitation grade | 0.595 | 0.325 | 1.091 | 0.094 | 0.437 | 0.29 | 0.661 | <0.001 | ||
| Continuous | Coefficient | 95% CIs | p-value | Coefficient | 95% CIs | p-value | ||||
| Peak PG (mmHg) | −4.810 | −7.111 | −2.509 | <0.001 | −4.827 | −6.887 | −2.768 | <0.001 | ||
| Mean PG (mmHg) | −2.980 | −4.261 | −1.699 | <0.001 | −2.899 | −4.014 | −1.785 | <0.001 | ||
| Vmax (m/s) | −0.282 | −0.448 | −0.116 | 0.001 | −0.261 | −0.431 | −0.091 | 0.003 | ||
| AVA (cm2) | 0.618 | 0.419 | 0.817 | <0.001 | 0.453 | 0.268 | 0.638 | <0.001 | ||
| 1-year TTE | Ordered category | Odds ratio | 95% CIs | p-value | Odds ratio | 95% CIs | p-value | |||
| Aortic regurgitation grade | 0.234 | 0.117 | 0.465 | <0.001 | 0.281 | 0.178 | 0.445 | <0.001 | ||
| Continuous | Coefficient | 95% CIs | p-value | Coefficient | 95% CIs | p-value | ||||
| Peak PG (mmHg) | −6.451 | −8.671 | −4.230 | <0.001 | −6.417 | −8.476 | −4.357 | <0.001 | ||
| Mean PG (mmHg) | −3.603 | −4.868 | −2.338 | <0.001 | −3.636 | −4.811 | −2.460 | <0.001 | ||
| Vmax (m/s) | −0.291 | −0.569 | −0.014 | 0.040 | −0.172 | −0.490 | 0.147 | 0.285 | ||
| AVA (cm2) | 0.659 | 0.449 | 0.869 | <0.001 | 0.636 | 0.434 | 0.837 | <0.001 | ||
| <Group-PB vs. Group-PT (Reference)> | ||||||||||
| Evaluated time point | Outcome type | Outcome | Unadjusted (n = 108) | IPTW (n = 52) | ||||||
| 7-day TTE | Ordered category | Odds ratio | 95% CIs | p-value | Odds ratio | 95% CIs | p-value | |||
| Aortic regurgitation grade | 2.285 | 1.022 | 5.108 | 0.044 | 0.708 | 0.308 | 1.629 | 0.417 | ||
| Continuous | Coefficient | 95% CIs | p-value | Coefficient | 95% CIs | p-value | ||||
| Peak PG (mmHg) | 10.184 | 7.004 | 13.363 | <0.001 | 5.586 | −0.106 | 11.277 | 0.054 | ||
| Mean PG (mmHg) | 6.353 | 4.475 | 8.232 | <0.001 | 3.827 | 0.663 | 6.992 | 0.019 | ||
| Vmax (m/s) | 0.585 | 0.404 | 0.765 | <0.001 | 0.337 | 0.028 | 0.647 | 0.033 | ||
| AVA (cm2) | −0.786 | −1.050 | −0.522 | <0.001 | −0.264 | −0.659 | 0.132 | 0.187 | ||
| 1-year TTE | Ordered category | Odds ratio | 95% CIs | p-value | Odds ratio | 95% CIs | p-value | |||
| Aortic regurgitation grade | 1.272 | 0.571 | 2.835 | 0.556 | 0.582 | 0.268 | 1.263 | 0.171 | ||
| Continuous | Coefficient | 95% CIs | p-value | Coefficient | 95% CIs | p-value | ||||
| Peak PG (mmHg) | 11.853 | 8.347 | 15.358 | <0.001 | 3.323 | −2.536 | 9.182 | 0.259 | ||
| Mean PG (mmHg) | 6.853 | 4.829 | 8.876 | <0.001 | 2.893 | −0.413 | 6.200 | 0.085 | ||
| Vmax (m/s) | 0.649 | 0.386 | 0.912 | <0.001 | 0.148 | −0.239 | 0.536 | 0.442 | ||
| AVA (cm2) | −0.324 | −0.713 | 0.065 | 0.101 | 0.335 | −0.256 | 0.925 | 0.259 | ||
| Univariable Linear Regression | Stepwise Multivariable Linear Regression (R2 = 0.806) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95%CIs | p Value | Coefficient | 95%CIs | p Value | Std Coefficient | VIF | |||
| Age | −0.073 | −0.389 | 0.244 | 0.638 | ||||||
| Body surface area | 18.693 | −6.562 | 43.947 | 0.139 | ||||||
| Male sex | 8.268 | −11.876 | 28.413 | 0.403 | ||||||
| Ventriculoaortic junction | −1.843 | −3.310 | −0.376 | 0.016 | ||||||
| Sinus of Valsalva | −0.352 | −0.810 | 0.106 | 0.125 | ||||||
| STJ | −0.289 | −0.928 | 0.350 | 0.356 | ||||||
| LVDd | −0.352 | −0.992 | 0.288 | 0.266 | ||||||
| LVDs | −0.362 | −0.838 | 0.113 | 0.128 | ||||||
| LVEF | 0.218 | −0.465 | 0.900 | 0.515 | ||||||
| Peak PG | 0.330 | 0.050 | 0.611 | 0.023 | ||||||
| Vmax | 6.205 | 0.219 | 12.191 | 0.043 | ||||||
| AVP or VSRR | −7.218 | −16.805 | 2.369 | 0.132 | ||||||
| Cusp plication | −14.667 | −27.919 | −1.414 | 0.032 | ||||||
| External suture annuloplasty size index | −3.426 | −6.826 | −0.027 | 0.048 | ||||||
| Geometric height | −1.805 | −3.462 | −0.149 | 0.034 | −1.315 | −2.605 | −0.025 | 0.046 | −0.303 | 1.502 |
| Free margin length | −0.896 | −1.663 | −0.130 | 0.024 | −0.806 | −1.388 | −0.225 | 0.010 | −0.415 | 1.524 |
| Use of the aPP | 27.905 | 11.795 | 44.014 | 0.002 | 25.290 | 14.689 | 35.891 | <0.001 | 0.586 | 1.024 |
| Subgroup-H (n = 20) | Subgroup-L (n = 22) | p-Value | |
|---|---|---|---|
| Peak PG at 7-day TTE (mmHg) | 25.5 (22.4–35.0) | 13.3 (10.8–18.1) | |
| Patient characteristics | |||
| Age (year) | 41.0 (22.8–49.0) | 39.0 (28.0–49.3) | 0.804 |
| Body surface area (m2) | 1.8 (1.7–2.0) | 1.8 (1.7–1.9) | 0.431 |
| Male (%) | 20 (100) | 21 (95.5) | 0.335 |
| Hypertension | 9 (45.0) | 5 (22.7) | 0.126 |
| Dyslipidaemia | 2 (10.0) | 0 | 0.129 |
| Diabetes mellitus | 0 | 0 | |
| Haemodialysis | 0 | 0 | |
| Atrial fibrillation | 0 | 0 | |
| Preoperative transthoracic echocardiography | |||
| LVDd (mm) | 65.9 (59.2–73.0) | 64.5 (59.0–73.0) | 0.791 |
| LVDs (mm) | 46.9 (40.1–51.9) | 46.0 (39.0–56.3) | 0.738 |
| LVEF (%) | 58.2 (51.1–60.7) | 57.5 (50.0–60.7) | 0.622 |
| Peak PG (mmHg) | 17.2 (12.2–23.9) | 17.5 (8.6–23.5) | 0.525 |
| Mean PG (mmHg) | 9.6 (7.5–12.4) | 7.5 (4.4–16.4) | 0.480 |
| Vmax (m/s) | 2.2 (1.8–2.7) | 2.2 (1.8–2.7) | 0.571 |
| AVA (cm2) | 3.2 (2.2–3.9) | 3.5 (3.0–4.3) | 0.092 |
| Preoperative transoesophageal echocardiography | |||
| Ventriculoaortic junction (mm) | 29.0 (28.0–30.0) | 30.0 (28.0–33.0) | 0.322 |
| Sinus of Valsalva (mm) | 34.0 (33.0–39.0) | 38.0 (35.0–40.0) | 0.019 |
| STJ (mm) | 29.0 (27.0–31.8) | 30.0 (25.0–35.5) | 0.200 |
| Intraoperative cusp measurement | |||
| Geometric height (mm) | 21.5 (20.0–24.0) | 24.5 (22.3–25.0) | 0.017 |
| Free margin length (mm) | 38.0 (35.0–40.0) | 44.5 (38.5–46.3) | 0.020 |
| Operations | |||
| AVP: Remodelling | 16: 4 | 10: 12 | 0.021 |
| Cusp plication (%) | 18 (90.0) | 21 (95.5) | 0.493 |
| External suture annuloplasty size index | 11.4 (10.9–13.0) | 12.6 (12.4–13.4) | 0.003 |
| Use of the aPP (%) | 4 (20.0) | 2 (9.1) | 0.313 |
| Decalcification and shaving (%) | 2 (10.0) | 2 (9.1) | 0.920 |
| 1-year transthoracic echocardiography | |||
| Aortic regurgitation grade (>II) (%) | 0 | 1 (4.5) | 0.324 |
| LVDd (mm) | 52.0 ± 3.4 | 50.3 ± 4.1 | 0.174 |
| Reverse remodelling rate (%) in LVDd | −20.6 ± 8.7 | −21.9 ± 8.6 | 0.684 |
| LVDs (mm) | 35.1 ± 1.0 | 34.7 ± 1.0 | 0.757 |
| Reverse remodelling rate (%) in LVDs | −24.7 ± 12.2 | −23.1 ± 10.9 | 0.699 |
| LVEF (%) | 59.7 ± 5.8 | 58.7 ± 9.8 | 0.717 |
| Peak PG (mmHg) | 25.3 ± 11.1 | 17.4 ± 8.7 | 0.029 |
| Mean PG (mmHg) | 13.8 ± 6.5 | 9.7 ± 5.1 | <0.001 |
| Vmax (m/s) | 2.5 ± 0.6 | 2.2 ± 0.5 | 0.094 |
| AVA (cm2) | 2.0 ± 0.9 | 2.7 ± 1.2 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saku, K.; Arimura, S.; Takagi, T.; Masuzawa, A.; Matsumura, Y.; Yoshitake, M.; Nagahori, R.; Murotani, K.; Kunihara, T. Haemodynamic Assessment and Outcomes of Aortic Valvuloplasty for Aortic Regurgitation in Patients with Bicuspid Aortic Valve. J. Clin. Med. 2024, 13, 7544. https://doi.org/10.3390/jcm13247544
Saku K, Arimura S, Takagi T, Masuzawa A, Matsumura Y, Yoshitake M, Nagahori R, Murotani K, Kunihara T. Haemodynamic Assessment and Outcomes of Aortic Valvuloplasty for Aortic Regurgitation in Patients with Bicuspid Aortic Valve. Journal of Clinical Medicine. 2024; 13(24):7544. https://doi.org/10.3390/jcm13247544
Chicago/Turabian StyleSaku, Kosuke, Satoshi Arimura, Tomomitsu Takagi, Akihiro Masuzawa, Yoko Matsumura, Michio Yoshitake, Ryuichi Nagahori, Kenta Murotani, and Takashi Kunihara. 2024. "Haemodynamic Assessment and Outcomes of Aortic Valvuloplasty for Aortic Regurgitation in Patients with Bicuspid Aortic Valve" Journal of Clinical Medicine 13, no. 24: 7544. https://doi.org/10.3390/jcm13247544
APA StyleSaku, K., Arimura, S., Takagi, T., Masuzawa, A., Matsumura, Y., Yoshitake, M., Nagahori, R., Murotani, K., & Kunihara, T. (2024). Haemodynamic Assessment and Outcomes of Aortic Valvuloplasty for Aortic Regurgitation in Patients with Bicuspid Aortic Valve. Journal of Clinical Medicine, 13(24), 7544. https://doi.org/10.3390/jcm13247544






