Is There a Role for Surgery in the Treatment of Metastatic Urothelial Carcinoma?
Abstract
1. Introduction
2. Materials and Methods
3. Results
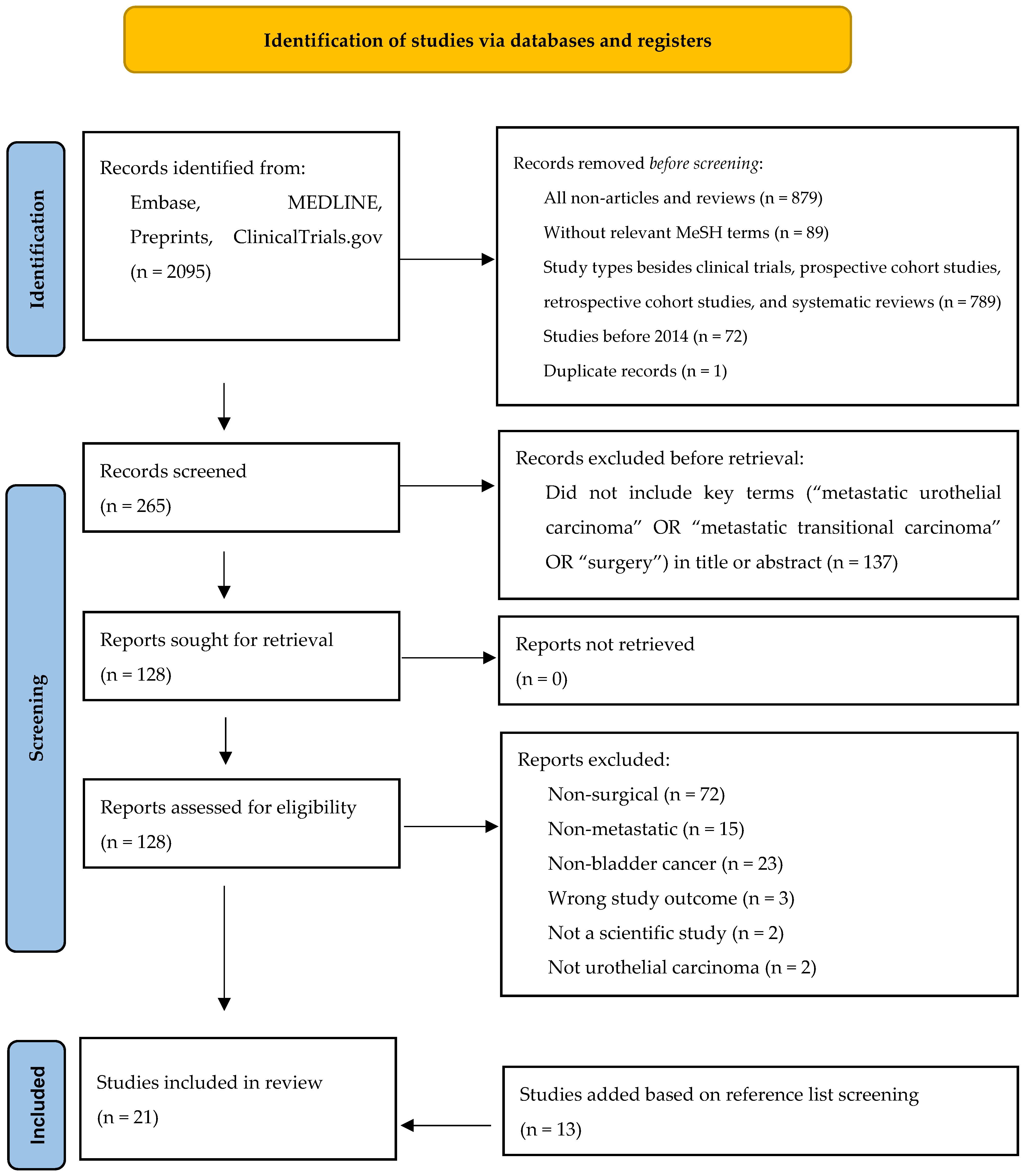
3.1. Search Results
3.2. Study Characteristics
3.3. Studies Limited to Pelvic or Retroperitoneal Lymph Node Metastases
3.4. Studies Including Supra-Regional LN Involvement and Distant Metastases
3.5. Studies That Did Not Specify the Site of Metastasis
3.6. Studies Included a Variety of Cytoreductive Surgical Methodologies
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Svatek, R.S.; Hollenbeck, B.K.; Holmäng, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Dinh, T.; Lee, J. The health economics of bladder cancer: An updated review of the published literature. Pharmacoeconomics 2014, 32, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Dursun, F.; Mackay, A.; Guzman, J.C.A.; Wenker, E.; Klaassen, Z.; O’Malley, P.; Bhindi, B.; Perez, C.O.; Xu, J.; Roh, T.; et al. Utilization and outcomes of metastasectomy for patients with metastatic urothelial cancer: An analysis of the national cancer database. Urol. Oncol. 2022, 40, 61.e21–61.e28. [Google Scholar] [CrossRef]
- Faltas, B.M.; Gennarelli, R.L.; Elkin, E.; Nguyen, D.P.; Hu, J.; Tagawa, S.T. Metastasectomy in older adults with urothelial carcinoma: Population-based analysis of use and outcomes. Urol. Oncol. 2018, 36, 9.e11–9.e17. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J., Sr.; Einhorn, L.H.; Elson, P.J.; Crawford, E.D.; Kuebler, P.; Tannock, I.; Raghavan, D.; Stuart-Harris, R.; Sarosdy, M.F.; Lowe, B.A. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J. Clin. Oncol. 1992, 10, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- Logothetis, C.J.; Dexeus, F.H.; Finn, L.; Sella, A.; Amato, R.J.; Ayala, A.G.; Kilbourn, R.G. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J. Clin. Oncol. 1990, 8, 1050–1055. [Google Scholar] [CrossRef]
- Ho, P.L.; Willis, D.L.; Patil, J.; Xiao, L.; Williams, S.B.; Melquist, J.J.; Tart, K.; Parikh, S.; Shah, J.B.; Delacroix, S.E.; et al. Outcome of patients with clinically node-positive bladder cancer undergoing consolidative surgery after preoperative chemotherapy: The, M.D. Anderson Cancer Center Experience. Urol. Oncol. 2016, 34, 59.e1–59.e598. [Google Scholar] [CrossRef]
- Iwamoto, H.; Izumi, K.; Shimura, Y.; Natsagdorj, A.; Maolake, A.; Takezawa, Y.; Nohara, T.; Shigehara, K.; Kadono, Y.; Mizpkami, A. Metastasectomy Improves Survival in Patients with Metastatic Urothelial Carcinoma. Anticancer Res. 2016, 36, 5557–5561. [Google Scholar] [CrossRef]
- Mir, M.C.; Matin, S.F.; Bex, A.; Spiess, P.E.; Thompson, R.H.; Grob, B.; Poppel, H.V. The role of surgery in the management of metastatic kidney cancer: An evidence-based collaborative review. Minerva Urol. Nefrol. 2018, 70, 109–125. [Google Scholar] [CrossRef]
- Penna, C.; Nordlinger, B. Colorectal metastasis (liver and lung). Surg. Clin. N. Am. 2002, 82, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.W.; Murray, K.S.; Donat, S.M.; Herr, H.W.; Bochner, B.H.; Dalbagni, G. The Outcome of Post-Chemotherapy Retroperitoneal Lymph Node Dissection in Patients with Metastatic Bladder Cancer in the Retroperitoneum. Bladder Cancer 2019, 5, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Mariani, L.; Lo Vullo, S.; Yu, E.Y.; Woods, M.E.; Wong, Y.; Harshman, L.C.; Alva, A.; Sternberg, C.N.; Bamias, A.; et al. Lack of Effectiveness of Postchemotherapy Lymphadenectomy in Bladder Cancer Patients with Clinical Evidence of Metastatic Pelvic or Retroperitoneal Lymph Nodes Only: A Propensity Score-based Analysis. Eur. Urol. Focus 2019, 5, 242–249. [Google Scholar] [CrossRef]
- Sweeney, P.; Millikan, R.; Donat, M.; Wood, C.G.; Radtke, A.S.; Pettaway, C.A.; Grossman, H.B.; Dinney, C.P.; Swanson, D.A.; Pisters, L.L. Is there a therapeutic role for post-chemotherapy retroperitoneal lymph node dissection in metastatic transitional cell carcinoma of the bladder? J. Urol. 2003, 169, 2113–2117. [Google Scholar] [CrossRef]
- Zargar-Shoshtari, K.; Zargar, H.; Lotan, Y.; Shah, J.B.; Van Rhijn, B.W.; Daneshmand, S.; Spiess, P.E.; Black, P.C. A Multi-Institutional Analysis of Outcomes of Patients with Clinically Node Positive Urothelial Bladder Cancer Treated with Induction Chemotherapy and Radical Cystectomy. J. Urol. 2016, 195, 53–59. [Google Scholar] [CrossRef]
- Abe, T.; Shinohara, N.; Harabayashi, T.; Sazawa, A.; Maruyama, S.; Suzuki, S.; Nonomura, K. Impact of multimodal treatment on survival in patients with metastatic urothelial cancer. Eur. Urol. 2007, 52, 1106–1113. [Google Scholar] [CrossRef]
- de Vries, R.R.; Nieuwenhuijzen, J.A.; Meinhardt, W.; Bais, E.M.; Horenblas, S. Long-term survival after combined modality treatment in metastatic bladder cancer patients presenting with supra-regional tumor positive lymph nodes only. Eur. J. Surg. Oncol. 2009, 35, 352–355. [Google Scholar] [CrossRef]
- Dodd, P.M.; McCaffrey, J.A.; Herr, H.; Mazumdar, M.; Bacik, J.; Higgins, G.; Boyle, M.G.; Scher, H.I.; Bajorin, D.F. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J. Clin. Oncol. 1999, 17, 2546–2552. [Google Scholar] [CrossRef]
- Lehmann, J.; Suttmann, H.; Albers, P.; Volkmer, B.; Gschwend, J.E.; Fencher, G.; Spahn, M.; Heidenreich, A.; Odenthal, A.; Seif, C.; et al. Surgery for metastatic urothelial carcinoma with curative intent: The German experience (AUO AB 30/05). Eur. Urol. 2009, 55, 1293–1299. [Google Scholar] [CrossRef]
- Li, R.; Kukreja, J.E.B.; Seif, M.A.; Petros, F.G.; Campbell, M.T.; Nguyen, J.V.; Gonzalez, G.M.N.; Kamat, A.M.; Pisters, L.L.; Dinnery, C.P.; et al. The role of metastatic burden in cytoreductive/consolidative radical cystectomy. World J. Urol. 2019, 37, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Preisser, F.; Nazzani, S.; Tian, Z.; Fossati, N.; Gandaglia, G.; Gallina, A.; Soulieres, D.; Tilki, D.; Montosorsi, F.; et al. More Extensive Lymph Node Dissection Improves Survival Benefit of Radical Cystectomy in Metastatic Urothelial Carcinoma of the Bladder. Clin. Genitourin. Cancer 2019, 17, 105–113.e2. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Taguchi, S.; Kanatani, A.; Kawai, T.; Ikeda, M.; Urakami, S.; Matsumoto, A.; Komemushi, Y.; Miyakawa, J.; Yamada, D.; et al. Oncologic Outcome of Metastasectomy for Urothelial Carcinoma: Who Is the Best Candidate? Ann. Surg. Oncol. 2017, 24, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Krege, S.; Suhr, J.; Rübben, H. Impact of surgical resection of bladder cancer metastases refractory to systemic therapy on performance score: A phase II trial. Urology 2001, 57, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Collazo Lorduy, A.; Stern, A.; Fahmy, O.; Pinotti, R.; Galsky, M.D.; Gakis, G. Survival after Metastasectomy for Metastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Bladder Cancer 2017, 3, 121–132. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Walsh, G.L.; Pisters, L.L.; Shen, Y.; Swanson, D.A.; Logothetis, C.J.; Millikan, R.E. Is there a role for surgery in the management of metastatic urothelial cancer? The, M.D. Anderson experience. J. Urol. 2004, 171, 145–148. [Google Scholar] [CrossRef]
- Steven, K.; Poulsen, A.L. Radical cystectomy and extended pelvic lymphadenectomy: Survival of patients with lymph node metastasis above the bifurcation of the common iliac vessels treated with surgery only. J. Urol. 2007, 178 Pt 1, 1218–1224. [Google Scholar] [CrossRef]
- Moschini, M.; Xylinas, E.; Zamboni, S.; Mattei, A.; Niegisch, G.; Yu, E.Y.; Bamias, A.; Agarwal, N.; Sridhar, S.S.; Sternberg, C.N.; et al. Efficacy of Surgery in the Primary Tumor Site for Metastatic Urothelial Cancer: Analysis of an International, Multicenter, Multidisciplinary Database. Eur. Urol. Oncol. 2020, 3, 94–101. [Google Scholar] [CrossRef]
- Xing, Q.; Ji, C.; Wang, Y.; Wang, X.; Zhu, Z. Metastasectomy could not improve the survival of metastatic urothelial carcinoma: Evidence from a meta-analysis. Transl. Cancer Res. 2020, 9, 1567–1576. [Google Scholar] [CrossRef]
- Cowles, R.S.; Johnson, D.E.; McMurtrey, M.J. Long-term results following thoracotomy for metastatic bladder cancer. Urology 1982, 20, 390–392. [Google Scholar] [CrossRef]
- Herr, H.W.; Donat, S.M. Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. J. Urol. 2001, 165, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Siefker-Radtke, A.O.; Millikan, R.E.; Tu, S.M.; Moore, D.F., Jr.; Smith, T.L.; Williams, D.; Logothetis, C.J. Phase III trial of fluorouracil, interferon alpha-2b, and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in metastatic or unresectable urothelial cancer. J. Clin. Oncol. 2002, 20, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Donat, S.M.; Bajorin, D.F. Post-chemotherapy surgery in patients with unresectable or regionally metastatic bladder cancer. J. Urol. 2001, 165, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.P.; Mertens, L.S.; van Rhijn, B.W.; Bex, A.; van der Poel, H.G.; Meinhardt, W.; Kerst, J.M.; Bergman, A.M.; Fioole-Bruining, A.; van Werkhoven, E.; et al. Induction chemotherapy followed by surgery in node positive bladder cancer. Urology 2014, 83, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Sonpavde, G.P.; Powles, T.; Claps, M.; Burotto, M.; Schenker, M.; Sade, J.P.; BAmias, A.; Beuzeboc, P.; Bedke, J.; et al. Characterization of complete responders to nivolumab + gemcitabine-cisplatin vs gemcitabine-cisplatin alone and patients with lymph node–only metastatic urothelial carcinoma from the CheckMate 901 trial. J. Clin. Oncol. 2024, 42 (Suppl. 16), 4509. [Google Scholar] [CrossRef]
- Erdi, Y.E. Limits of Tumor Detectability in Nuclear Medicine and PET. Mol. Imaging Radionucl. Ther. 2012, 21, 23–28. [Google Scholar] [CrossRef]
- Alevizakos, M.; Bellmunt, J. Adjuvant immunotherapy for muscle-invasive urothelial carcinoma of the bladder. Expert Rev. Anticancer Ther. 2022, 22, 259–267. [Google Scholar] [CrossRef]
- Bhalla, S.; Passarelli, R.; Biswas, A.; De, S.; Ghodoussipour, S. Plasma-Derived Cell-Free DNA as a Biomarker for Early Detection, Prognostication, and Personalized Treatment of Urothelial Carcinoma. J. Clin. Med. 2024, 13, 2057. [Google Scholar] [CrossRef]
- Pfail, J.; Capellan, J.; Passarelli, R.; Kaldany, A.; Chua, K.; Lichtbroun, B.; Srivastava, A.; Golombos, D.; Jang, T.L.; Pitt, H.A.; et al. National Surgical Quality Improvement Program audit of contemporary perioperative care for radical cystectomy. BJU Int. 2024. [Google Scholar] [CrossRef]
- Chua, K.J.; Patel, H.V.; Srivastava, A.; Doppalapudi, S.K.; Lichtbroun, B.; Patel, N.; Elsamra, S.E.; Singer, E.A.; Jang, T.L.; Ghodoussipour, S.B. Annual trends of cystectomy complications: A contemporary analysis of the NSQIP database. Urol. Oncol. 2023, 41, 390.e19–390.e26. [Google Scholar] [CrossRef]
- Maisch, P.; Lunger, L.; Düwel, C.; Schmid, S.C.; Horn, T.; Gschwend, J.E.; Sauter, A.; Heck, M.M. Outcomes of palliative cystectomy in patients with locally advanced pT4 bladder cancer. Urol. Oncol. 2021, 39, 368.e11–368.e17. [Google Scholar] [CrossRef] [PubMed]
| Study | Sample Size | Site(s) of Metastasis | Criteria for Intervention | Surgical Intervention | Survival | Conclusion(s) | Limitation(s)/Quality |
|---|---|---|---|---|---|---|---|
| Studies limited to pelvic and retroperitoneal lymph node involvement | |||||||
| Ho et al., 2016 [8] (Single-institution retrospective) | 55 | Pelvic LN and RPLN | Neoadjuvant chemotherapy | RC + metastasectomy | 5-year OS: -Overall: 40.4% -pN0: 66% -cN1–3: 17.6% -cM1: 0% | Extirpative surgery can be curative in some populations after chemotherapy | -Limited sample size -No control group -NOS: Poor |
| Liu et al., 2019 [13] (Single-institution retrospective) | 13 | RPLN | Good response to neoadjuvant chemotherapy | Metastasectomy +/− primary surgery (12 RC) | -Median PFS: 14 months -Median CSS: 21 months -2-year DSS 50% and 34% in patients with and without complete chemotherapy response | -Metastasectomy may improve survival in select populations -Incomplete response to chemotherapy is a poor prognosticator | -Limited sample size -No control group -NOS: Poor |
| Necchi et al., 2019 [14] (Multi-institution retrospective) | 242 | Pelvic LN and RPLN | Neoadjuvant chemotherapy | RC with pelvic or RPLND | 36-month OS: 51.7% vs. 41.1% in patients with and without LND | No significant OS benefit | -No histological confirmation of LN involvement -NOS: Poor |
| Sweeney et al., 2003 [15] (Single-institution phase II prospective study) | 11 | RPLN | -Biopsy-proven mUC to RPLN -Good response to chemotherapy | RC with PLND and complete bilateral RPLND | -4-year DSS: 36% -Median DSS: 14 months -Median RFS: 7 months | -RPLND has curative potential in mUC -DSS and RFS significantly increased if tumor in <3 nodes | -Limited sample size -NOS: Poor |
| Zargar-Shoshtari et al., 2016 [16] (Multi-institution retrospective) | 304 | Pelvic LN | Neoadjuvant chemotherapy | RC | Median OS: 22 months | Improved OS associated with pN0, negative margins, and excision of >15 nodes | -No baseline patient data -No standardization of chemotherapy -No control group -NOS: Poor |
| Studies including supra-regional lymph node involvement and sites of distant metastases | |||||||
| Abe et al., 2007 [17] (Single-institution retrospective) | 12 | Bone, liver, LN, local recurrence, lung | -Good performance status -Response to chemotherapy -Single site of metastasis | Metastasectomy | -Median OS with intervention: 42 months -Median OS for observation: 10 months | Metastasectomy may contribute to long-term disease control in select population | -Limited sample size -No prospective selection criteria -NOS: Poor |
| De Vries et al., 2008 [18] (Single-institution retrospective) | 14 | Supra-regional LN | -Neoadjuvant chemotherapy -Good performance status | RC + metastasectomy | -Median OS: 10.1 months -3 year DSS: 36% -5 year DSS: 24% | -Metastasectomy can improve survival in select patients -Neoadjuvant chemotherapy response may influence survival | -Limited sample size -No control group -NOS: Poor |
| Dodd et al., 1999 [19] (Single-institution retrospective) | 30 | Bone, liver, LN, lung | -Neoadjuvant chemotherapy -Single site of metastasis | RC + metastasectomy | 5-year survival: 20% | Metastasectomy may improve 5-year survival in select patients | -Limited sample size -No prospective selection criteria -No control group -NOS: Poor |
| Faltas et al., 2018 [4] (SEER Medicare study) | 497 | Bone, brain, liver, LN, lung | Unknown | Metastasectomy +/− primary surgery (99 RC, 54 NephU) | -Mean OS: 19 months -3-year survival: 38% | -Metastasectomy may improve survival in select patients -Safety profile of metastasectomy is comparable to primary surgery | -Population limited to age >65 -Limited patient information -No control group -NOS: Poor |
| Iwamoto et al., 2016 [9] (Single-institution retrospective) | 7 | Lymph nodes, visceral | -Good performance status -Single site of metastasis | Metastasectomy +/− primary surgery (3 RC, 1 NephU, 1 Partial Ureterectomy) | Metastasectomy and CRP < 1 mg/dL are predictors of improved PFS and OS | Patients with CRP < 1 mg/dL may experience survival benefit with metastasectomy | -Limited sample size -No prospective selection criteria -NOS: Poor |
| Lehmann et al., 2009 [20] (Multi-institution retrospective) | 44 | Adrenal gland, bone, brain, LN, lung, skin, small intestine | -Limited metastatic foci -Good neoadjuvant chemotherapy response | RC + metastasectomy | -OS: 35 months -PFS: 19 months -5-year survival: 28% | Metastasectomy may improve survival in select patients | -Limited sample size -No prospective selection criteria -Heterogeneous treatment algorithms -NOS: Poor |
| Li et al., 2019 [21] (Single-institution retrospective) | 43 | Bone, LN, lung | Unknown | RC | -5-yr CSS: 19.9% -Single-metastasis CSS: 26 months -Multiple-metastasis CSS: 7.9 months | -Improved CSS for site of metastasis vs. multiple metastases -No OS difference by metastatic site -Minimal survival benefit for multiple metastases | -Limited sample size -No prospective selection criteria -NOS: Poor |
| Mazzone et al., 2018 [22] (SEER database study) | 319 | Lymph nodes, other | Unknown | RC, RC with LND | -Median OS 14 vs. 8 months for RC and non-RC patients with mUC -CSM 13 vs. 10 months in RC vs. RC + LND | -Survival benefit for RC in mUC -More extensive LND lowered OM (>13 LN) | -Limited patient information -No prospective selection criteria -NOS: Poor |
| Nakagawa et al., 2017 [23] (Multi-institutional retrospective) | 37 | Local recurrence, LN, lung | -Lesion resectability, -Patient health status | RC + metastasectomy with curative intent | -Median OS: 34.3 months -5-year CSS: 39.7% | Metastasectomy may improve survival in select patients | -Limited sample size -No prospective selection criteria -No control group -NOS: Poor |
| Otto et al., 2001 [24] (Single-institution phase II prospective study) | 70 | Bone, LN, lung, peritoneum, skin | -Disease progression after chemotherapy -Resectability -Age > 21 -ASA score < 4 -NYHA score < 4 | RC + metastasectomy | -Median survival: 7 months -1 yr OS: 30% -2 yr OS: 19% | -No OS benefit with metastasectomy -Metastasectomy enhanced quality of life in patients with symptomatic disease | -Participation limited to patients with poor prognosis -NOS: Poor |
| Patel et al., 2017 [25] (Meta-analysis) | 412 | Bone, brain, LN, lung, Skin | Varied by study | Metastasectomy | Improved OS with metastasectomy in meta-analysis of 5 of 17 included studies | Lack of uniform reporting and prospective trials limit the formulation of general recommendations | -Only 3 of 17 studies were RCTs -Variable reporting of treatment and outcomes -A single study contributed 90% of OS data -NOS: Poor |
| Siefker-Radtke et al., 2004 [26] (Single-institution retrospective) | 31 | Brain, LN, lung, skin | -Single site of metastasis -Response to chemotherapy -Resectability -No evidence of rapid progression | Primary surgery + metastasectomy | -Median OS: 31 months -Medial DFS: 7 months -5 yr OS: 33% | Metastasectomy may improve survival in select patients | -Limited sample size -NOS: Poor |
| Steven et al., 2007 [27] (Single-institution retrospective) | 22 | Supra-aortic LN | No chemotherapy | RC + metastasectomy | -5 yr OS: 37% -Improved OS in patients with < 6 involved LN | -Extended LND provides accurate staging and improves survival -RC should include extensive LND | -Limited sample size -NOS: Poor |
| Studies that did not specify site of metastasis | |||||||
| Dursun et al., 2021 [3] (NCDB Study) | 556 | Unknown | Unknown | Metastasectomy +/− RC or CMT | No difference in 2-year and 5-year survival compared to matched cohort | No OS benefit for metastasectomy in mUC | -Limited patient information -NOS: Poor |
| Moschini et al., 2020 [28] (Multi-institution retrospective) | 47 | Unknown | Unknown | RC | 36-month CSS and OS improved in patients with a single site of metastasis | Metastasectomy may improve survival in patients with a single site of metastasis | -Limited sample size -Limited patient information -No prospective selection criteria |
| Xing et al., 2020 [29] (Meta-analysis) | 8 studies | Unknown | Unknown | Metastasectomy | No OS benefit with metastasectomy | No OS benefit for metastasectomy in mUC | -Limited patient information -Variable reporting of treatment and outcomes -NOS: Poor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhalla, S.; Pfail, J.; Ghodoussipour, S. Is There a Role for Surgery in the Treatment of Metastatic Urothelial Carcinoma? J. Clin. Med. 2024, 13, 7498. https://doi.org/10.3390/jcm13247498
Bhalla S, Pfail J, Ghodoussipour S. Is There a Role for Surgery in the Treatment of Metastatic Urothelial Carcinoma? Journal of Clinical Medicine. 2024; 13(24):7498. https://doi.org/10.3390/jcm13247498
Chicago/Turabian StyleBhalla, Sophia, John Pfail, and Saum Ghodoussipour. 2024. "Is There a Role for Surgery in the Treatment of Metastatic Urothelial Carcinoma?" Journal of Clinical Medicine 13, no. 24: 7498. https://doi.org/10.3390/jcm13247498
APA StyleBhalla, S., Pfail, J., & Ghodoussipour, S. (2024). Is There a Role for Surgery in the Treatment of Metastatic Urothelial Carcinoma? Journal of Clinical Medicine, 13(24), 7498. https://doi.org/10.3390/jcm13247498






