Differential Cardiotoxicity of Ibrutinib Versus Chemoimmunotherapy in Chronic Lymphocytic Leukemia: A Population-Based Study
Abstract
1. Introduction
2. Method
2.1. TriNetX Platform
2.2. Patient Selection
2.3. Propensity Score Matching (PSM)
2.4. Outcome Measurement
2.5. Statistical Analyses
3. Results
4. Primary and Secondary Outcomes
5. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scarfo, L.; Ferreri, A.J.; Ghia, P. Chronic lymphocytic leukaemia. Crit. Rev. Oncol. Hematol. 2016, 104, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.; Fink, A.M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Fürstenau, M.; Eichhorst, B. Novel Agents in Chronic Lymphocytic Leukemia: New Combination Therapies and Strategies to Overcome Resistance. Cancers 2021, 13, 1336. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Mulder, T.A.; Osterborg, A. BTK Inhibitors in Chronic Lymphocytic Leukemia: Biological Activity and Immune Effects. Front. Immunol. 2021, 12, 686768. [Google Scholar] [CrossRef]
- Zelenetz, A.D.; Gordon, L.I.; Wierda, W.G.; Abramson, J.S.; Advani, R.H.; Andreadis, C.B.; Bartlett, N.; Byrd, J.C.; Czuczman, M.S.; Fayad, L.E.; et al. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 1.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 326–362. [Google Scholar] [CrossRef]
- Chanan-Khan, A.; Cramer, P.; Demirkan, F.; Fraser, G.; Silva, R.S.; Grosicki, S.; Pristupa, A.; Janssens, A.; Mayer, J.; Bartlett, N.L.; et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): A randomised, double-blind, phase 3 study. Lancet Oncol. 2016, 17, 200–211. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef]
- Quartermaine, C.; Ghazi, S.M.; Yasin, A.; Awan, F.T.; Fradley, M.; Wiczer, T.; Kalathoor, S.; Ferdousi, M.; Krishan, S.; Habib, A.; et al. Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2023, 5, 570–590. [Google Scholar] [CrossRef]
- Mattsson, A.; Sylvan, S.E.; Asklid, A.; Wiggh, J.; Winqvist, M.; Lundin, J.; Mansouri, L.; Rosenquist, R.; Johansson, H.; Österborg, A.; et al. Risk-adapted bendamustine + rituximab is a tolerable treatment alternative for elderly patients with chronic lymphocytic leukaemia: A regional real-world report on 141 consecutive Swedish patients. Br. J. Haematol. 2020, 191, 426–432. [Google Scholar] [CrossRef]
- Chiattone, C.S.; Gabus, R.; Pavlovsky, M.A.; Akinola, N.O.; Varghese, A.M.; Arrais-Rodrigues, C. Management of Chronic Lymphocytic Leukemia in Less-Resourced Countries. Cancer J. 2021, 27, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jain, N.; Ayer, T.; Wierda, W.G.; Flowers, C.R.; O’Brien, S.M.; Keating, M.J.; Kantarjian, H.M.; Chhatwal, J. Economic Burden of Chronic Lymphocytic Leukemia in the Era of Oral Targeted Therapies in the United States. J. Clin. Oncol. 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Varghese, A.M.; Sood, N.; Daniel, S.M. Current pricing model for cancer drugs—Is it ‘Justum Pretium’ for the developing world? Taking management of chronic lymphocytic leukaemia in India into consideration. Br. J. Haematol. 2020, 190, e292–e294. [Google Scholar] [CrossRef]
- Langan, S.M.; Schmidt, S.A.; Wing, K.; Ehrenstein, V.; Nicholls, S.G.; Filion, K.B.; Klungel, O.; Petersen, I.; Sorensen, H.T.; Dixon, W.G.; et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ 2018, 363, k3532. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nhu, N.T.; Tran, V.K.; Nguyen, T.T.H.; Lin, C.F. Efficacy and Safety of Bruton Tyrosine Kinase Inhibitor Monotherapy Compared with Combination Therapy for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1996. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Sabrie, N.; Leong, D.; Pang, A.; Austin, P.C.; Prica, A.; Nanthakumar, K.; Calvillo-Argüelles, O.; Lee, D.S.; Thavendiranathan, P. Cardiovascular Risk Associated With Ibrutinib Use in Chronic Lymphocytic Leukemia: A Population-Based Cohort Study. J. Clin. Oncol. 2021, 39, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.; Tang, B.; Azmi, S.; Yang, K.; Han, Y.; Zhang, X.; Roeker, L.; Wallis, N.; Stern, J.C.; Hedrick, E.; et al. A real-world study to assess the association of cardiovascular adverse events (CVAEs) with ibrutinib as first-line (1L) treatment for patients with chronic lymphocytic leukaemia (CLL) in the United States. eJHaem 2023, 4, 135–144. [Google Scholar] [CrossRef]
- St-Pierre, F.; Ma, S. Use of BTK Inhibitors in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): A Practical Guidance. Blood Lymphat. Cancer Targets Ther. 2022, 12, 81–98. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illes, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.-P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Sanz, R.G.; et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kazmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, D.; Feder, A.L.; Trum, M.; Kreitmeier, K.G.; Stengel, L.; Maier, L.S.; Sag, C.M. Ibrutinib impairs IGF-1-dependent activation of intracellular Ca handling in isolated mouse ventricular myocytes. Front. Cardiovasc. Med. 2023, 10, 1190099. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kinoshita, T.; Sukbuntherng, J.; Chang, B.Y.; Elias, L. Ibrutinib Inhibits ERBB Receptor Tyrosine Kinases and HER2-Amplified Breast Cancer Cell Growth. Mol. Cancer Ther. 2016, 15, 2835–2844. [Google Scholar] [CrossRef]
- Chen, S.T.; Azali, L.; Rosen, L.; Zhao, Q.; Wiczer, T.; Palettas, M.; Gambril, J.; Kola-Kehinde, O.; Ruz, P.; Kalathoor, S.; et al. Hypertension and incident cardiovascular events after next-generation BTKi therapy initiation. J. Hematol. Oncol. 2022, 15, 92. [Google Scholar] [CrossRef]
- Xiao, L.; Salem, J.E.; Clauss, S.; Hanley, A.; Bapat, A.; Hulsmans, M.; Iwamoto, Y.; Wojtkiewicz, G.; Cetinbas, M.; Schloss, M.J.; et al. Ibrutinib-Mediated Atrial Fibrillation Attributable to Inhibition of C-Terminal Src Kinase. Circulation 2020, 142, 2443–2455. [Google Scholar] [CrossRef]
- Leszek, P.; Klotzka, A.; Bartus, S.; Burchardt, P.; Czarnecka, A.M.; Dlugosz-Danecka, M.; Gierlotka, M.; Kosela-Paterczyk, H.; Krawczyk-Ozog, A.; Kubiatowski, T.; et al. A practical approach to the 2022 ESC cardio-oncology guidelines: Comments by a team of experts—Cardiologists and oncologists. Kardiol. Pol. 2023, 81, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Bensken, W.P.; Vu, L.; Dong, W.; Koroukian, S.M.; Caimi, P. Ibrutinib Is Associated With Increased Cardiovascular Events and Major Bleeding in Older CLL Patients. JACC CardioOncol. 2023, 5, 233–243. [Google Scholar] [CrossRef]
- Dickerson, T.; Wiczer, T.; Waller, A.; Philippon, J.; Porter, K.; Haddad, D.; Guha, A.; Rogers, K.A.; Bhat, S.; Byrd, J.C.; et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019, 134, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018, Centers for Disease Control and Prevention. Available online: https://stacks.cdc.gov/view/cdc/87559 (accessed on 25 September 2024).
- Reda, G.; Fattizzo, B.; Cassin, R.; Mattiello, V.; Tonella, T.; Giannarelli, D.; Massari, F.; Cortelezzi, A. Predictors of atrial fibrillation in ibrutinib-treated CLL patients: A prospective study. J. Hematol. Oncol. 2018, 11, 79. [Google Scholar] [CrossRef]
- Natarajan, G.; Terrazas, C.; Oghumu, S.; Varikuti, S.; Dubovsky, J.A.; Byrd, J.C.; Satoskar, A.R. Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells. Oncoimmunology 2016, 5, e1057385. [Google Scholar] [CrossRef]
- Pretorius, L.; Du, X.J.; Woodcock, E.A.; Kiriazis, H.; Lin, R.C.; Marasco, S.; Medcalf, R.L.; Ming, Z.; Head, G.A.; Tan, J.W.; et al. Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am. J. Pathol. 2009, 175, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2020, 43, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol. 2019, 1, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Wendtner, C.M. Bendamustine plus rituximab in chronic lymphocytic leukemia: Is there life in the old dog yet? Haematologica 2018, 103, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; de Lima Lopes, G.; Yusof, M.M.; Rubagumya, F.; Rutkowski, P.; Sengar, M. Barriers in access to oncology drugs—A global crisis. Nat. Rev. Clin. Oncol. 2023, 20, 7–15. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Bretagne, M.; Lebrun-Vignes, B.; Groarke, J.D.; Johnson, D.B.; Yang, T.; Reddy, N.M.; Funck-Brentano, C.; Brown, J.R.; et al. Cardiovascular Toxicities Associated With Ibrutinib. J. Am. Coll. Cardiol. 2019, 74, 1667–1678. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Guha, A.; Derbala, M.H.; Zhao, Q.; Wiczer, T.E.; Woyach, J.A.; Byrd, J.C.; Awan, F.T.; Addison, D. Ventricular Arrhythmias Following Ibrutinib Initiation for Lymphoid Malignancies. J. Am. Coll. Cardiol. 2018, 72, 697–698. [Google Scholar] [CrossRef]
- Mulay, S.; Boruchov, A. Recurrent and partially reversible cardiomyopathy occurring during treatment with bendamustine and rituximab. Leuk. Lymphoma 2015, 56, 805–807. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Kopecky, S.L.; Specks, U.; Bharucha, K.; Fervenza, F.C. Non-ischemic cardiomyopathy after rituximab treatment for membranous nephropathy. J. Renal Inj. Prev. 2017, 6, 18–25. [Google Scholar] [CrossRef]
- Patil, V.; Lunge, S.; Doshi, B. Cardiac side effect of rituximab. Indian J. Drugs Dermatol. 2020, 6, 49. [Google Scholar] [CrossRef]
- Poterucha, J.T.; Westberg, M.; Nerheim, P.; Lovell, J.P. Rituximab-induced polymorphic ventricular tachycardia. Tex. Heart Inst. J. 2010, 37, 218–220. [Google Scholar]
- Quek, L.S.; Bolen, J.; Watson, S.P. A role for Bruton’s tyrosine kinase (Btk) in platelet activation by collagen. Curr. Biol. 1998, 8, S1. [Google Scholar] [CrossRef]
- Elefante, A.; Czuczman, M.S. Bendamustine for the treatment of indolent non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Am. J. Health Syst. Pharm. 2010, 67, 713–723. [Google Scholar] [CrossRef]
| Characteristic | Before PSM Ibrutinib (n = 2704) | Before PSM B+ Anti CD20 (n = 1075) | Standardised Difference | After PSM Ibrutinib (n = 977) | After PSM B+ Anti CD20 (n = 977) | Standardised Difference |
|---|---|---|---|---|---|---|
| Age at Index (Mean ± SD) | 67.5 ± 10.8 | 65.5 ± 10.7 | 0.183 | 66.0 ± 10.6 | 65.7 ± 10.4 | 0.027 |
| Male | 1729 (63.9%) | 657 (61.1%) | 0.017 | 609 (62.3%) | 601 (61.5%) | 0.017 |
| Female | 975 (36.1%) | 418 (38.9%) | 0.058 | 368 (37.7%) | 376 (38.5%) | 0.017 |
| White | 2117 (78.3%) | 778 (72.4%) | 0.138 | 716 (73.3%) | 720 (73.7%) | 0.009 |
| Black or African American | 132 (4.9%) | 41 (3.8%) | 0.052 | 39 (4%) | 39 (4%) | <0.001 |
| Hepatomegaly and splenomegaly | 331 (12.2%) | 238 (22.1%) | 0.265 | 188 (19.2%) | 196 (20.1%) | 0.021 |
| Abnormal coagulation profile | 10 (0.4%) | 11 (1%) | 0.079 | 10 (1%) | 10 (1%) | <0.001 |
| Personal history of antineoplastic chemotherapy | 133 (4.9%) | 72 (6.7%) | 0.076 | 51 (5.2%) | 55 (5.6%) | 0.018 |
| Heart failure | 0 (0%) | 10 (0.9%) | 0.137 | 0 (0%) | 0 (0%) | -- |
| Diabetes mellitus | 180 (6.7%) | 79 (7.3%) | 0.027 | 59 (6%) | 70 (7.2%) | 0.045 |
| Hypertensive diseases | 0 (0%) | 41 (3.8%) | 0.282 | 0 (0%) | 0 (0%) | -- |
| AF/flutter | 0 (0%) | 10 (0.9%) | 0.137 | 0 (0%) | 0 (0%) | -- |
| Overweight, obesity, and other hyperalimentation | 137 (5.1%) | 58 (5.4%) | 0.015 | 49 (5%) | 51 (5.2%) | 0.009 |
| Disorders of lipoprotein metabolism | 497 (18.4%) | 210 (19.5%) | 0.029 | 180 (18.4%) | 186 (19%) | 0.016 |
| Chronic kidney disease (CKD) | 101 (3.7%) | 58 (5.4%) | 0.080 | 37 (3.8%) | 45 (4.6%) | 0.041 |
| Ischemic heart diseases | 149 (5.5%) | 74 (6.9%) | 0.057 | 55 (5.6%) | 59 (6%) | 0.017 |
| Cerebral infarction | 19 (0.7%) | 17 (1.6%) | 0.083 | 10 (1%) | 11 (1.1%) | 0.010 |
| Pulmonary embolism | 29 (1.1%) | 23 (2.1%) | 0.085 | 17 (1.7%) | 19 (1.9%) | 0.015 |
| Surgical procedures on the cardiovascular system | 1348 (49.9%) | 696 (64.7%) | 0.305 | 615 (62.9%) | 618 (63.3%) | 0.006 |
| Alpha-blockers/related | 242 (8.9%) | 92 (8.6%) | 0.014 | 90 (9.2%) | 87 (8.9%) | 0.011 |
| Ace inhibitors | 143 (5.3%) | 53 (4.9%) | 0.016 | 52 (5.3%) | 48 (4.9%) | 0.019 |
| Beta blockers/related | 319 (11.8%) | 150 (14%) | 0.064 | 122 (12.5%) | 119 (12.2%) | 0.009 |
| Calcium channel blockers | 134 (5%) | 67 (6.2%) | 0.056 | 36 (3.7%) | 44 (4.5%) | 0.041 |
| Diuretics | 331 (12.2%) | 194 (18%) | 0.162 | 141 (14.4%) | 151 (15.5%) | 0.029 |
| Antiarrhythmics | 696 (25.7%) | 477 (44.4%) | 0.398 | 397 (40.6%) | 404 (41.4%) | 0.015 |
| Antilipemic agents | 552 (20.4%) | 183 (17%) | 0.087 | 139 (14.2%) | 161 (16.5%) | 0.062 |
| Angiotensin ii inhibitor | 109 (4%) | 48 (4.5%) | 0.022 | 27 (2.8%) | 30 (3.1%) | 0.018 |
| Anticoagulants | 617 (22.8%) | 436 (40.6%) | 0.388 | 373 (38.2%) | 360 (36.8%) | 0.027 |
| Platelet aggregation inhibitors | 415 (15.3%) | 196 (18.2%) | 0.077 | 168 (17.2%) | 169 (17.3%) | 0.003 |
| Outcome | Cohort | Patients in Cohort | Patients with Outcome (%) | HR (95% CI) |
|---|---|---|---|---|
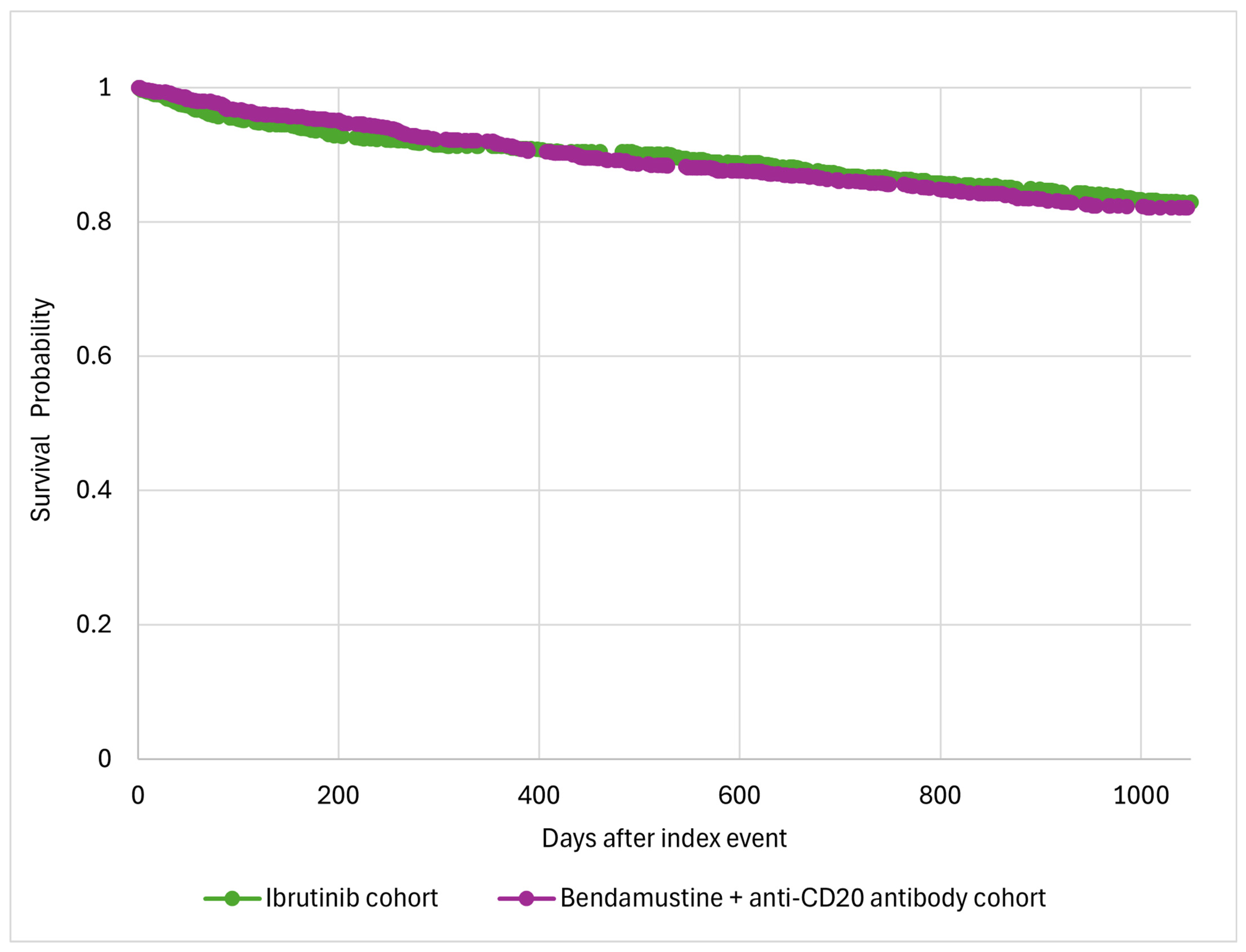
All-cause death | Ibrutinib | 977 | 151 (15.5%) | 0.94 (0.75–1.17) |
| B+ anti-CD20 antibody | 977 | 159 (16.3%) | ||
AF/Flutter | Ibrutinib | 977 | 101 (10.3%) | 1.88 (1.35–2.62) |
| B+ anti-CD20 antibody | 977 | 55 (5.6%) | ||
Hypertension | Ibrutinib | 977 | 230 (23.5%) | 1.21 (1.00–1.47) |
| B+ anti-CD20 antibody | 977 | 194 (19.9%) | ||
Heart failure | Ibrutinib | 977 | 17 (1.7%) | 0.94 (0.48–1.83) |
| B+ anti-CD20 antibody | 977 | 18 (1.8%) | ||
Ventricular arrhythmias | Ibrutinib | 977 | 18 (1.5%) | 2.15 (0.87–5.29) |
| B+ anti-CD20 antibody | 977 | 10 (1.0%) | ||
Bleeding | Ibrutinib | 977 | 83 (8.5%) | 1.14 (0.83–1.56) |
| B+ anti-CD20 antibody | 977 | 73 (7.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majrashi, A.; Gue, Y.X.; Shantsila, A.; Williams, S.; Lip, G.Y.H.; Pettitt, A.R. Differential Cardiotoxicity of Ibrutinib Versus Chemoimmunotherapy in Chronic Lymphocytic Leukemia: A Population-Based Study. J. Clin. Med. 2024, 13, 7492. https://doi.org/10.3390/jcm13237492
Majrashi A, Gue YX, Shantsila A, Williams S, Lip GYH, Pettitt AR. Differential Cardiotoxicity of Ibrutinib Versus Chemoimmunotherapy in Chronic Lymphocytic Leukemia: A Population-Based Study. Journal of Clinical Medicine. 2024; 13(23):7492. https://doi.org/10.3390/jcm13237492
Chicago/Turabian StyleMajrashi, Abdulrahman, Ying X. Gue, Alena Shantsila, Stella Williams, Gregory Y. H. Lip, and Andrew R. Pettitt. 2024. "Differential Cardiotoxicity of Ibrutinib Versus Chemoimmunotherapy in Chronic Lymphocytic Leukemia: A Population-Based Study" Journal of Clinical Medicine 13, no. 23: 7492. https://doi.org/10.3390/jcm13237492
APA StyleMajrashi, A., Gue, Y. X., Shantsila, A., Williams, S., Lip, G. Y. H., & Pettitt, A. R. (2024). Differential Cardiotoxicity of Ibrutinib Versus Chemoimmunotherapy in Chronic Lymphocytic Leukemia: A Population-Based Study. Journal of Clinical Medicine, 13(23), 7492. https://doi.org/10.3390/jcm13237492








