The Burden of ABO-Incompatible Kidney Transplantation: Readmission Rates and Complications, a Twenty-Year Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Institutional Review Board Approval
2.2.1. Protocol for ABO-Incompatible Kidney Transplantation
2.2.2. Readmission Rate
2.2.3. Complications
2.2.4. Donor-Specific Antibodies
2.2.5. Graft Function
2.2.6. Rejection
2.2.7. Data Collection
2.2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Readmissions
3.3. Perioperative Hospitalization Duration
3.4. Kidney Function
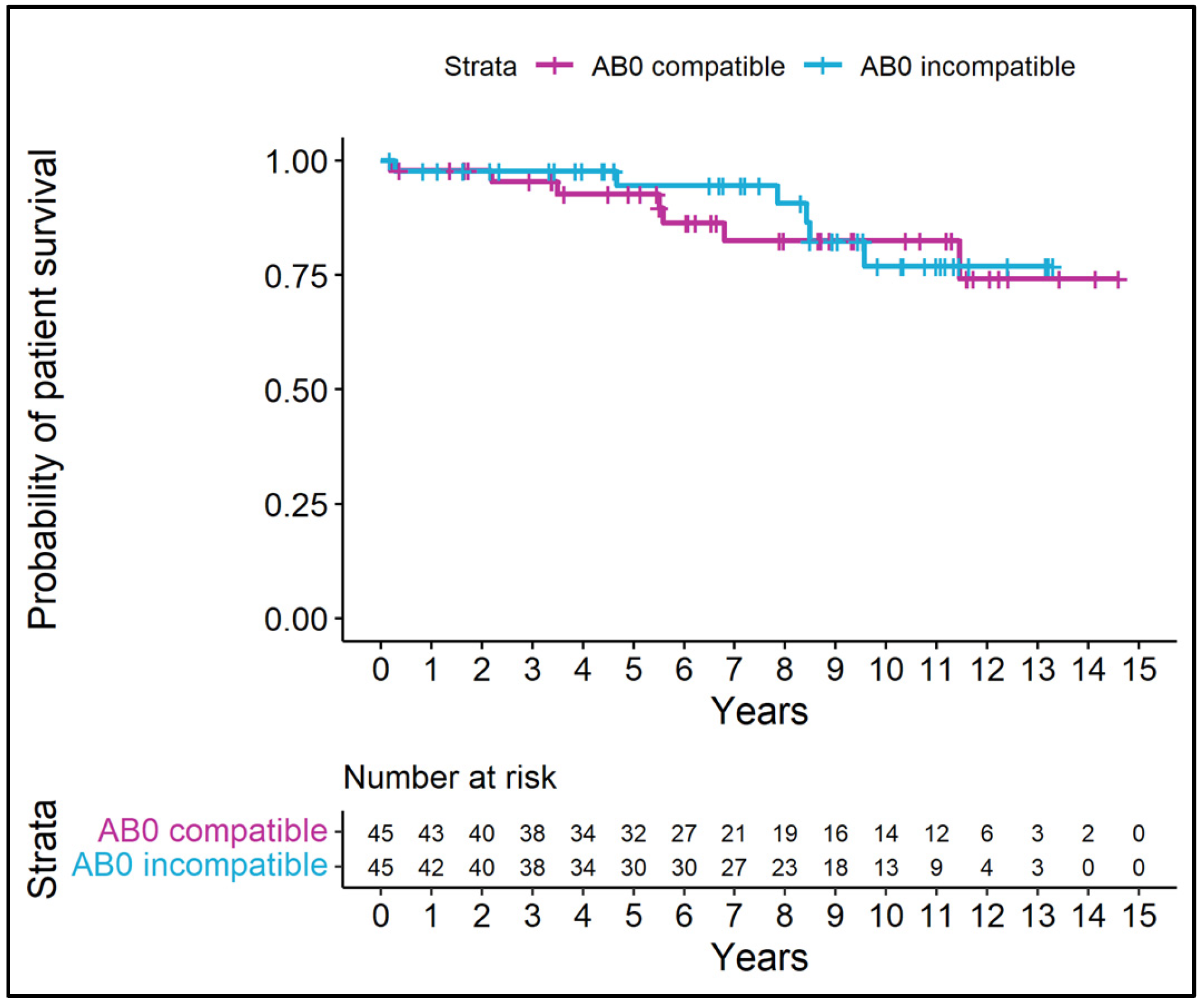
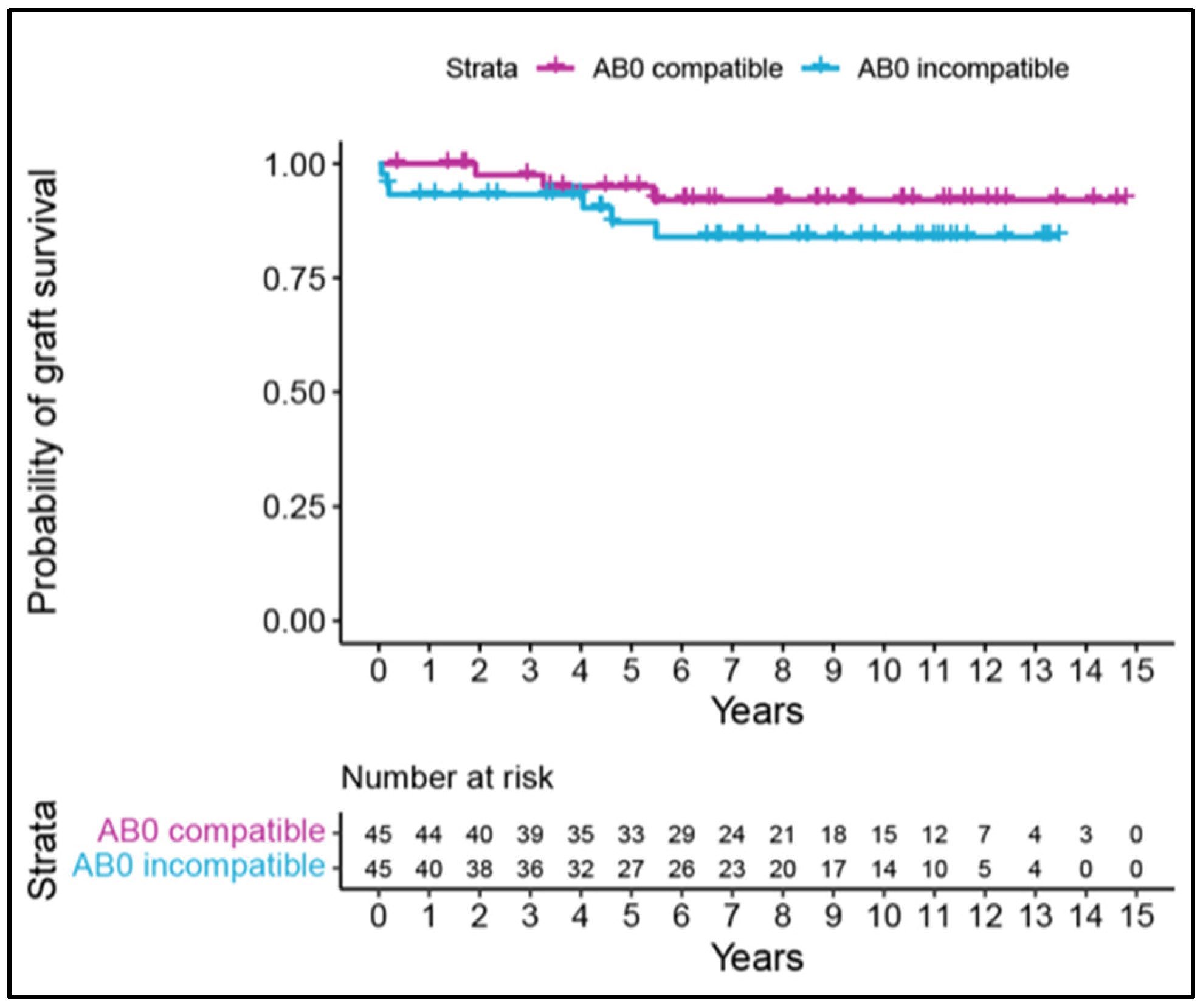
3.5. Patient and Graft Survival
3.6. Postoperative Complications
3.6.1. CCI
3.6.2. Major Complications (Clavien–Dindo Classification ≥ 3a)
3.7. Infection
3.8. Rejection
3.9. Tumor Incidence
3.10. NODAT
3.11. Cardiovascular Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABOi | ABO incompatible |
| ABOc | ABO compatible |
| ABOi-LDKT | ABO-incompatible live-donor kidney transplantation |
| ABOc-LDKT | ABO-compatible live-donor kidney transplantation |
| ABOi-LDKTr | ABO-incompatible live-donor kidney transplant recipient |
| ABOc-LDKTr | ABO-compatible live-donor kidney transplant recipient |
| ACS | acute coronary syndrome |
| CCI | comprehensive complication index |
| CKD-epi | Chronic Kidney Disease Epidemiology Collaboration |
| DDKT | deceased donor kidney transplantation |
| DGF | delayed graft function |
| DSA | donor-specific antibodies |
| eGFR | estimated glomerular filtration rate |
| ESRD | end-stage renal disease |
| KPD | kidney paired donation |
| KT | kidney transplantation |
| LDKT | living donor kidney transplantation |
| MFI | mean fluorescent index |
| NODAT | new-onset diabetes after transplantation |
References
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, P.I.; Cecka, J.M.; Gjertson, D.W.; Takemoto, S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 1995, 333, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.; Shin, M.; Kim, E.; Moon, J.; Jung, G.; Choi, G.; Kwon, C.; Joh, J.; Kim, S. Comparison of outcomes of living and deceased donor kidney grafts surviving longer than 5 years in Korea. Transplant. Proc. 2010, 42, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Zeier, M.; Döhler, B.; Opelz, G.; Süsal, C. ABO-incompatible kidney transplantation. Front. Immunol. 2017, 8, 234. [Google Scholar] [CrossRef]
- Lentine, K.L.; Axelrod, D.; Klein, C.; Simpkins, C.; Xiao, H.; Schnitzler, M.A.; Tuttle-Newhall, J.E.; Dharnidharka, V.R.; Brennan, D.C.; Segev, D.L. Early clinical complications after ABO incompatible live donor kidney transplantation: A national study of Medicare-insured recipients. Transplantation 2014, 98, 54. [Google Scholar] [CrossRef]
- de Weerd, A.E.; van Agteren, M.; Leebeek, F.W.; Ijzermans, J.N.M.; Weimar, W.; Betjes, M.G.H. ABO-incompatible kidney transplant recipients have a higher bleeding risk after antigen-specific immunoadsorption. Transpl. Int. 2015, 28, 25–33. [Google Scholar] [CrossRef]
- Hall, E.C.; Engels, E.A.; Montgomery, R.A.; Segev, D.L. Cancer risk following ABO incompatible living donor kidney transplantation. Transplantation 2013, 96, 476. [Google Scholar] [CrossRef]
- Haugen, C.E.; King, E.A.; Bae, S.; Bowring, M.G.; Holscher, C.M.; Garonzik-Wang, J.; McAdams-DeMarco, M.; Segev, D.L. Early hospital readmission in older and younger kidney transplant recipients. Am. J. Nephrol. 2018, 48, 235–241. [Google Scholar] [CrossRef]
- Tavares, M.G.; Junior, H.T.-S.; Pestana, J.O.M. Early Hospital Readmission (EHR) in kidney transplantation: A review article. Braz. J. Nephrol. 2020, 42, 231–237. [Google Scholar] [CrossRef]
- Uchida, J.; Kosoku, A.; Naganuma, T.; Tanaka, T.; Nakatani, T. Latest insights on ABO-incompatible living-donor renal transplantation. Int. J. Urol. 2020, 27, 30–38. [Google Scholar] [CrossRef]
- Goldfarb-Rumyantzev, A.; Hurdle, J.F.; Scandling, J.; Wang, Z.; Baird, B.; Barenbaum, L.; Cheung, A.K. Duration of end-stage renal disease and kidney transplant outcome. Nephrol. Dial. Transplant. 2005, 20, 167–175. [Google Scholar] [CrossRef][Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Slankamenac, K.; Nederlof, N.; Pessaux, P.; De Jonge, J.; Wijnhoven, B.P.; Breitenstein, S.; Oberkofler, C.E.; Graf, R.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann. Surg. 2014, 260, 757–763. [Google Scholar] [CrossRef]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.-A. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef]
- Clavien, P.A.; Vetter, D.; Staiger, R.D.; Slankamenac, K.; Mehra, T.; Graf, R.; Puhan, M.A. The Comprehensive Complication Index (CCI®): Added value and clinical perspectives 3 years ‘down the line’. Ann. Surg. 2017, 265, 1045–1050. [Google Scholar] [CrossRef]
- Inker, L.A.; Titan, S. Measurement and estimation of GFR for use in clinical practice: Core curriculum 2021. Am. J. Kidney Dis. 2021, 78, 736–749. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Coresh, J. GFR estimation: From physiology to public health. Am. J. Kidney Dis. 2014, 63, 820–834. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.G.; Kim, B.S.; Kim, M.S.; Kim, S.I.; Kim, Y.S.; Huh, K.H. Early hospital readmissions after ABO-or HLA-incompatible living donor kidney transplantation. Sci. Rep. 2019, 9, 3246. [Google Scholar] [CrossRef]
- Zschiedrich, S.; Jänigen, B.; Dimova, D.; Neumann, A.; Seidl, M.; Hils, S.; Geyer, M.; Emmerich, F.; Kirste, G.; Drognitz, O.; et al. One hundred ABO-incompatible kidney transplantations between 2004 and 2014: A single-centre experience. Nephrol. Dial. Transplant. 2016, 31, 663–671. [Google Scholar] [CrossRef]
- Speer, C.; Kälble, F.; Nusshag, C.; da Silva, L.P.; Schaier, M.; Becker, L.E.; Klein, K.; Sommerer, C.; Beimler, J.; Leo, A.; et al. Outcomes and complications following ABO-incompatible kidney transplantation performed after desensitization by semi-selective immunoadsorption-a retrospective study. Transpl. Int. 2019, 32, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Kang, S.S.; Park, S.B.; Park, U.J.; Kim, H.T.; Cho, W.H.; Han, S. Comparison of clinical outcomes between ABO-compatible and ABO-incompatible spousal donor kidney transplantation. Kidney Res. Clin. Pract. 2016, 35, 50–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thukral, S.; Kumar, D.; Ray, D.S. Comparative analysis of ABO-incompatible kidney transplantation with ABO-compatible transplantation: A single-center experience from Eastern India. Saudi J. Kidney Dis. Transplant. 2019, 30, 97. [Google Scholar]
- Opelz, G.; Morath, C.; Süsal, C.; Tran, T.H.; Zeier, M.; Döhler, B. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: Results from 101 centers. Transplantation 2015, 99, 400–404. [Google Scholar] [CrossRef]
- Massie, A.B.; Orandi, B.J.; Waldram, M.M.; Luo, X.; Nguyen, A.Q.; Montgomery, R.A.; Lentine, K.L.; Segev, D.L. Impact of ABO-incompatible living donor kidney transplantation on patient survival. Am. J. Kidney Dis. 2020, 76, 616–623. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, J.Y.; Kim, S.-H.; Kim, D.H.; Lim, S.J.; Shin, S.; Kim, Y.H.; Jung, J.H.; Park, S.-K.; Kwon, H.; et al. Acute rejection and infectious complications in ABO-and HLA-incompatible kidney transplantations. Ann. Transplant. 2020, 25, e927420-1. [Google Scholar] [CrossRef]
- de Weerd, A.E.; van den Brand, J.A.; Bouwsma, H.; de Vries, A.P.; Dooper, I.P.M.; Sanders, J.S.F.; Christiaans, M.H.; van Reekum, F.E.; van Zuilen, A.D.; Bemelman, F.J.; et al. ABO-incompatible kidney transplantation in perspective of deceased donor transplantation and induction strategies: A propensity-matched analysis. Transpl. Int. 2021, 34, 2706–2719. [Google Scholar] [CrossRef]
- Eskandary, F.; Böhmig, G.A. ABO-Incompatibility: Time to Challenge the Paradigm of Equivalence in Live-Donor Kidney Transplantation? Transpl. Int. 2022, 35, 10281. [Google Scholar] [CrossRef]
- Montgomery, J.R.; Berger, J.C.; Warren, D.S.; James, N.; Montgomery, R.A.; Segev, D.L. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation 2012, 93, 603. [Google Scholar] [CrossRef]
- Ponticelli, C.; Cucchiari, D.; Bencini, P. Skin cancer in kidney transplant recipients. J. Nephrol. 2014, 27, 385–394. [Google Scholar] [CrossRef]
- Stoff, B.; Salisbury, C.; Parker, D.; Zwald, F.O. Dermatopathology of skin cancer in solid organ transplant recipients. Transplant. Rev. 2010, 24, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Segev, D.L.; Kucirka, L.M.; Gentry, S.E.; Montgomery, R.A. Utilization and outcomes of kidney paired donation in the United States. Transplantation 2008, 86, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Basu, G.; Daniel, D.; Rajagopal, A.; Neelakantan, N.; John, G.T. A Model for Human Leukocyte Antigen–Matched Donor-Swap Transplantation in India. Transplantation 2008, 85, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Nikzad, A.; Akbarpour, M.; Rees, M.A.; Roth, A.E. Global kidney chains. Proc. Natl. Acad. Sci. USA 2021, 118, e2106652118. [Google Scholar] [CrossRef]
- Biró, P.; Haase-Kromwijk, B.; Andersson, T.; Ásgeirsson, E.I.; Baltesová, T.; Boletis, I.; Bolotinha, C.; Bond, G.; Böhmig, G.; Burnapp, L.; et al. Building kidney exchange programmes in Europe—An overview of exchange practice and activities. Transplantation 2019, 103, 1514. [Google Scholar] [CrossRef]
| Matching Variables | ABO Compatible | ABO Incompatible |
|---|---|---|
| n | 45 | 45 |
| Male n (%) | 29 (64.4) | 29 (64.4) |
| Female n (%) | 16 (35.6) | 16 (35.6) |
| Age at KT (mean (SD)) | 49.5 (10.8) | 50.1 (11.3) |
| Recurrent disease n (%) | 12 (26.7) | 12 (26.7) |
| Year of KT (mean (SD)) | 2011.4 (3.9) | 2011.8 (3.9) |
| 0 to <7 months dialysis n (%) | 17 (37.8) | 17 (37.8) |
| 7 to 24 months dialysis n (%) | 15 (33.3) | 15 (33.3) |
| >24 months dialysis n (%) | 13 (28.9) | 13 (28.9) |
| Variable | ABO Compatible | ABO Incompatible | p-Value |
|---|---|---|---|
| eGFR at 1 year | 47.5 [41.2, 55.0] | 59.0 [47.0, 68.5] | 0.004 |
| eGFR at 5 years | 50.0 [39.0, 63.0] | 60.0 [46.2, 70.0] | 0.136 |
| eGFR at 10 years | 44.0 [32.5, 50.5] | 62.0 [43.5, 71.5] | 0.236 |
| Variable | ABO Compatible | ABO Incompatible | p-Value |
|---|---|---|---|
| CCI < 3 months | 33.7 [28.2, 37.5] | 31.4 [20.9, 35.2] | 0.438 |
| CCI < 6 months | 33.7 [29.9, 35.6] | 33.7 [33.7, 39.6] | 0.875 |
| CCI < 12 months | 33.1 [23.7, 38.2] | 20.9 [20.9, 31.7] | 0.25 |
| CCI > 12 months | 31.9 [24.7, 39.1] | 27.1 [23.2, 34.3] | 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berchtold, C.; Huebel, K.; Roessler, F.; Graf, N.; Dutkowski, P.; Lehmann, K.; Mueller, T.; de Rougemont, O. The Burden of ABO-Incompatible Kidney Transplantation: Readmission Rates and Complications, a Twenty-Year Analysis. J. Clin. Med. 2024, 13, 7477. https://doi.org/10.3390/jcm13237477
Berchtold C, Huebel K, Roessler F, Graf N, Dutkowski P, Lehmann K, Mueller T, de Rougemont O. The Burden of ABO-Incompatible Kidney Transplantation: Readmission Rates and Complications, a Twenty-Year Analysis. Journal of Clinical Medicine. 2024; 13(23):7477. https://doi.org/10.3390/jcm13237477
Chicago/Turabian StyleBerchtold, Caroline, Kerstin Huebel, Fabian Roessler, Nicole Graf, Philipp Dutkowski, Kuno Lehmann, Thomas Mueller, and Olivier de Rougemont. 2024. "The Burden of ABO-Incompatible Kidney Transplantation: Readmission Rates and Complications, a Twenty-Year Analysis" Journal of Clinical Medicine 13, no. 23: 7477. https://doi.org/10.3390/jcm13237477
APA StyleBerchtold, C., Huebel, K., Roessler, F., Graf, N., Dutkowski, P., Lehmann, K., Mueller, T., & de Rougemont, O. (2024). The Burden of ABO-Incompatible Kidney Transplantation: Readmission Rates and Complications, a Twenty-Year Analysis. Journal of Clinical Medicine, 13(23), 7477. https://doi.org/10.3390/jcm13237477






