The Impact of the Coexistence of Frailty Syndrome and Cognitive Impairment on Early and Midterm Complications in Older Patients with Acute Coronary Syndromes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Procedure
- (a)
- TIMI major bleeding;
- (b)
- Ventricular arrhythmia;
- (c)
- Cardiac conduction disturbances requiring cardiac stimulation;
- (d)
- Cardiac arrest;
- (e)
- Early stent thrombosis;
- (f)
- Acute heart failure (Killip–Kimball class III/IV);
- (g)
- Stroke;
- (h)
- Prolonged hospital stay (>8 days);
- (i)
- In-hospital death.
- (a)
- Recurrent ACS;
- (b)
- Recurrent revascularisation;
- (c)
- Stroke;
- (d)
- Death.
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
3.2. Early Complications
3.3. Major Adverse Cardiovascular and Cerebrovascular Events (MACCEs) at 6 Months
4. Discussion
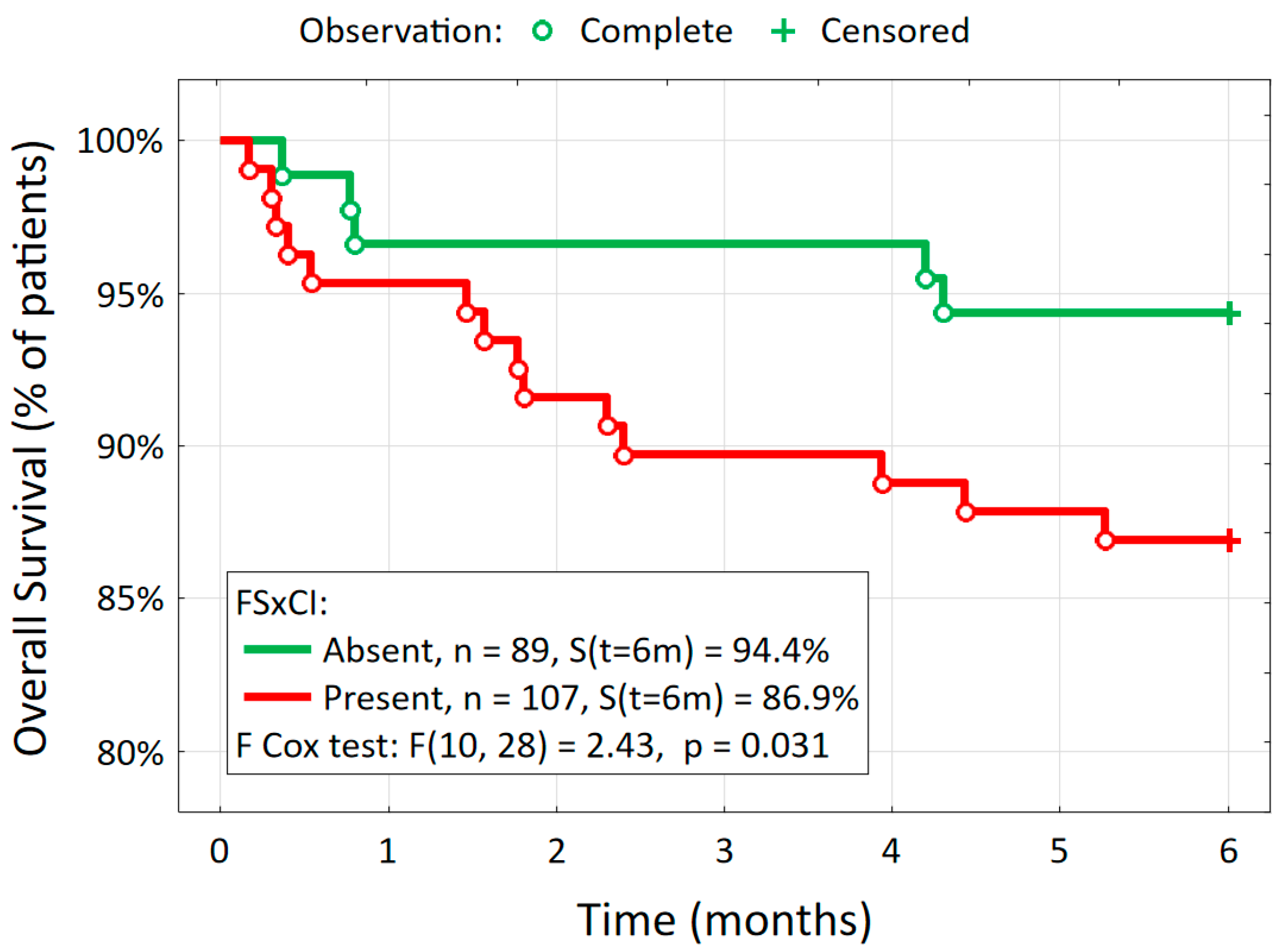
The Major Adverse Cardiovascular and Cerebrovascular Events (MACCEs) at 6 Months
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Guralnik, J.M.; Studenski, S.; Fried, L.P.; Cutler, G.B., Jr.; Walston, J.D.; The Interventions on Frailty Working Group. Designing Randomized, Controlled Trials Aimed at Preventing or Delaying Functional Decline and Disability in Frail, Older Persons: A Consensus Report. J. Am. Geriatr. Soc. 2004, 52, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive Frailty: Rational and Definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Ekerstad, N.; Swahn, E.; Janzon, M.; Alfredsson, J.; Löfmark, R.; Lindenberger, M.; Carlsson, P. Frailty Is Independently Associated with Short-Term Outcomes for Elderly Patients with Non–ST-Segment Elevation Myocardial Infarction. Circulation 2011, 124, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Westerhout, C.M.; Alexander, K.P.; Roe, M.T.; Winters, K.J.; Cyr, D.D.; Fox, K.A.; Prabhakaran, D.; Hochman, J.S.; Armstrong, P.W.; et al. Frailty Is Associated with Worse Outcomes in Non-ST-Segment Elevation Acute Coronary Syndromes: Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Prasitlumkum, N.; Doyle, K.S.; Ding, K.R.; Natarajan, B.; Mukherjee, A.; Varadarajan, P.; Pai, R.G. The Impact of Cognitive Impairment in Patients with Acute Coronary Syndrome Undergoing Percutaneous Revascularization: A Systematic Review and Meta-Analysis. Coron. Artery Dis. 2022, 33, e59–e66. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Bonanad, C.; García-Blas, S.; Ruiz, V.; Fernández-Cisnal, A.; Sastre, C.; Ruescas, A.; Valero, E.; González, J.; Mollar, A.; et al. Long-Term Prognostic Value of Cognitive Impairment on Top of Frailty in Older Adults after Acute Coronary Syndrome. J. Clin. Med. 2021, 10, 444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ijaz, N.; Jamil, Y.; Brown, C.H.; Krishnaswami, A.; Orkaby, A.; Stimmel, M.B.; Gerstenblith, G.; Nanna, M.G.; Damluji, A.A. Role of Cognitive Frailty in Older Adults With Cardiovascular Disease. J. Am. Heart Assoc. 2024, 13, e033594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tokuda, K.; Tanaka, A.; Uemura, Y.; Shibata, N.; Iwama, M.; Sakaguchi, T.; Yoshida, R.; Negishi, Y.; Tashiro, H.; Tanaka, M.; et al. Long-Term Clinical Outcomes Following Percutaneous Coronary Intervention in Patients Aged 90 Years and Older. J. Cardiol. 2024, 84, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.J.; van Assen, M.A.L.M.; Luijkx, K.G.; Schols, J.M.G.A. The Predictive Validity of the Tilburg Frailty Indicator: Disability, Health Care Utilization, and Quality of Life in a Population at Risk. Gerontologist 2012, 52, 619–631. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Uchmanowicz, I.; Jankowska-Polańska, B.; Uchmanowicz, B.; Kowalczuk, K.; Gobbens, R. Validity and Reliability of the Polish Version of the Tilburg Frailty Indicator (TFI). J. Frailty Aging 2016, 5, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Stańczak, J. MMSE: Polska Normalizacja [Eng. MMSE: Polish Normalization]; Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego [Eng. Psychological Testing Laboratory of the Polish Psychological Association]: Warszawa, Poland, 2010; ISBN 9788360733707. [Google Scholar]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Voshaar, R.C.O. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Lowres, N.; Woolaston, A.; Naismith, S.L.; Gallagher, R. Prevalence and Patterns of Cognitive Impairment in Acute Coronary Syndrome Patients: A Systematic Review. Eur. J. Prev. Cardiol. 2020, 27, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, D.; Kaczmarek-Majer, K.; Rzeźniczak, J.; Klamecka-Pohl, K.; Ganowicz-Kaatz, T.; Słomczyński, M.; Budzianowski, J.; Pieszko, K.; Hiczkiewicz, J.; Tykarski, A.; et al. Cognitive Impairment in Cardiovascular Patients after Myocardial Infarction: Prospective Clinical Study. J. Clin. Med. 2023, 12, 4954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borges, M.K.; Canevelli, M.; Cesari, M.; Aprahamian, I. Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis. Front. Med. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avila-Funes, J.A.; Carcaillon, L.; Helmer, C.; Carrière, I.; Ritchie, K.; Rouaud, O.; Tzourio, C.; Dartigues, J.; Amieva, H. Is Frailty a Prodromal Stage of Vascular Dementia? Results from the Three-City Study. J. Am. Geriatr. Soc. 2012, 60, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Scafato, E.; Frisardi, V.; Seripa, D.; Logroscino, G.; Maggi, S.; Imbimbo, B.P.; Galluzzo, L.; Baldereschi, M.; Gandin, C.; et al. Frailty Syndrome and the Risk of Vascular Dementia: The Italian Longitudinal Study on Aging. Alzheimer’s Dement. 2013, 9, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Scafato, E.; Seripa, D.; Lozupone, M.; Imbimbo, B.P.; D’Amato, A.; Tortelli, R.; Schilardi, A.; Galluzzo, L.; Gandin, C.; et al. Reversible Cognitive Frailty, Dementia, and All-Cause Mortality. Ital. Longitud. Study Aging 2017, 18, 89.e1–89.e8. [Google Scholar] [CrossRef] [PubMed]
- Fogg, C.; Meredith, P.; Culliford, D.; Bridges, J.; Spice, C.; Griffiths, P. Cognitive Impairment Is Independently Associated with Mortality, Extended Hospital Stays and Early Readmission of Older People with Emergency Hospital Admissions: A Retrospective Cohort Study. Int. J. Nurs. Stud. 2019, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Bonanni, M.; Bueti, F.M.; Matteucci, A.; Cammalleri, L.; Stifano, G.; Muscoli, S.; Romeo, F. Multidimensional Prognostic Index (MPI) in Elderly Patients with Acute Myocardial Infarction. Aging Clin. Exp. Res. 2021, 33, 1875–1883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bagai, A.; Chen, A.Y.; Udell, J.A.; Dodson, J.A.; McManus, D.D.; Maurer, M.S.; Enriquez, J.R.; Hochman, J.; Goyal, A.; Henry, T.D.; et al. Association of Cognitive Impairment With Treatment and Outcomes in Older Myocardial Infarction Patients: A Report From the NCDR Chest Pain–MI Registry. J. Am. Heart Assoc. 2019, 8, e012929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hajduk, A.M.; Saczynski, J.S.; Tsang, S.; Geda, M.E.; Dodson, J.A.; Ouellet, G.M.; Goldberg, R.J.; Chaudhry, S.I. Presentation, Treatment, and Outcomes of Older Adults Hospitalized for Acute Myocardial Infarction According to Cognitive Status: The SILVER-AMI Study. Am. J. Med. 2021, 134, 910–917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levine, D.A.; Langa, K.M.; Galecki, A.; Kabeto, M.; Morgenstern, L.B.; Zahuranec, D.B.; Giordani, B.; Lisabeth, L.D.; Nallamothu, B.K. Mild Cognitive Impairment and Receipt of Treatments for Acute Myocardial Infarction in Older Adults. J. Gen. Intern. Med. 2020, 35, 28–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- A Byrne, R.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826, Erratum in Eur. Heart J. 2024, 45, 1145. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, K.; Pyrpyris, N.; Iliakis, P.; Kanatas, P.; Theofilis, P.; Sakalidis, A.; Apostolos, A.; Tsioufis, P.; Papanikolaou, A.; Aznaouridis, K.; et al. Optimal Management of High Bleeding Risk Patients Undergoing Percutaneous Coronary Interventions: Where Do We Stand? J. Cardiol. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]

| Coexistence of Frailty Syndrome and Cognitive Impairment (FSxCI) | p | OR [95% CI] | ||
|---|---|---|---|---|
| Present n = 107 | Absent n = 89 | |||
| Age, years, mean (SD) | 77.9 (8.1) | 70.8 (5.8) | <0.001 | × |
| Age ≥ 74 years | 76 (71.0%) | 23 (25.8%) | <0.001 | 7.04 [3.74; 13.2] |
| Gender | ||||
| Female, n (%) | 45 (42.1%) | 32 (36.0%) | 0.463 | 1.29 [0.72; 2.31] |
| Risk factors for CHD | ||||
| Nicotine dependence, n (%) | 15 (14.0%) | 26 (29.2%) | 0.013 | 0.40 [0.19; 0.80] |
| Hypertension, n (%) | 87 (81.3%) | 55 (61.8%) | 0.004 | 2.69 [1.41; 5.14] |
| Diabetes, n (%) | 32 (29.9%) | 27 (30.3%) | 1.000 | 0.98 [0.53; 1.81] |
| Renal failure, n (%) | 23 (21.5%) | 19 (21.4%) | 1.000 | 1.01 [0.51; 2.00] |
| COPD, n (%) | 12 (11.2%) | 6 (6.7%) | 0.328 | 1.75 [0.63; 4.86] |
| Type of ACS: STEMI | 53 (49.5%) | 46 (51.7%) | 0.776 | 0.92 [0.52; 1.61] |
| Management | ||||
| Conservative, n (%) | 12 (11.2%) | 6 (6.7%) | 0.328 | 1.75 [0.63; 4.86] |
| Invasive, n (%) | 95 (88.8%) | 83 (93.3%) | ||
| PCI, n (%) | 85 (79.4%) | 75 (84.3%) | 0.460 | 0.72 [0.34; 1.51] |
| CABG, n (%) | 10 (9.3%) | 8 (9.0%) | 1.000 | 1.04 [0.39; 2.77] |
| Effectiveness of PCI: optimal result (TIMI = 3) | 75 (88.2%) | 61 (82.8%) | 0.877 | 1.75 [0.63; 4.86] |
| Time from pain onset to re-opening of artery for patients with STEMI (hours) | n = 53 | n = 46 | 0.951 | |
| <6 h, n (%) | 38 (71.7%) | 33 (71.7%) | 1.00 (ref) | |
| 6–12 h, n (%) | 12 (22.6%) | 11 (23.9%) | 0.95 [0.37; 2.43] | |
| >12 h, n (%) | 3 (5.7%) | 2 (4.4%) | 1.30 [0.21; 8.28] | |
| Pharmacological treatment | ||||
| Beta-blocker | 93 (86.9%) | 83 (93.3%) | 0.162 | 0.48 [0.18; 1.31] |
| ACEI/sartan | 85 (79.4%) | 76 (85.4%) | 0.350 | 0.66 [0.31; 1.40] |
| OAC/NOAC | 14 (13.1%) | 7 (7.9%) | 0.258 | 1.76 [0.68; 4.58] |
| 11 (10.3%) | 14 (15.7%) | 0.287 | 0.61 [0.26; 1.43] | |
| Antiplatelet drugs ASA + P2Y12 inhibitor | 106 (99.1%) | 87 (97.8%) | 0.592 | 2.44 [0.22; 27.3] |
| Statin | 103 (96.3%) | 84 (94.4%) | 0.734 | 1.53 [0.40; 5.89] |
| LVEF (%), mean (SD) | 44.0 (10.1) | 46.5 (9.7) | 0.081 | × |
| Univariate Logistic Regression | Mulivariate Logistic Regression | ||||
|---|---|---|---|---|---|
| b | p | beta | p | OR [95% CI] | |
| Age (years) | 0.135 | <0.001 | 0.128 | <0.001 | 1.14 [1.08; 1.19] |
| Age ≥ 74 years | 1.951 | <0.001 | - | - | - |
| Nicotine dependence | −2.169 | <0.001 | - | - | - |
| Hypertension | 0.977 | 0.004 | 0.783 | 0.036 | 2.18 [1.06; 4.54] |
| Coexistence of Frailty Syndrome and Cognitive Impairment (FSxCI) | p | OR [95% CI] | ||
|---|---|---|---|---|
| Present n = 107 | Absent n = 89 | |||
| Early complications, n (%) | ||||
| Total early complications | 66 (61.7) | 33 (37.1) | <0.001 | 2.73 [1.53–4.88] |
| Bleeding | 3 (2.8%) | 3 (3.4%) | 1.000 | 0.83 [0.16–4.20] |
| Ventricular arrhytmia | 6 (5.6%) | 2 (2.2%) | 0.296 | 2.58 [0.51–13.1 |
| Conduction disturbances requiring cardiac stimulation | 5 (4.7%) | 1 (1.1%) | 0.224 | 4.31 [0.49–37.6] |
| Cardiac arrest | 4 (3.7%) | 3 (3.4%) | 1.000 | 1.11 [0.24–5.11] |
| Early stent thrombosis | 2 (1.9%) | 2 (2.2%) | 1.000 | 0.83 [0.11–6.00] |
| Acute heart failure | 10 (9.3%) | 4 (4.5%) | 0.267 | 2.19 [0.66–7.24] |
| Stroke | 3 (2.8%) | 4 (4.5%) | 0.704 | 0.61 [0.13–2.81] |
| Prolonged hospital stay (>8 days) | 56 (52.3%) | 28 (31.5%) | 0.004 | 2.39 [1.33–4.30] |
| In-hospital death | 4 (3.7%) | 2 (2.2%) | 0.691 | 1.69 [0.30–9.45] |
| Major adverse cardiovascular and cerebrovascular events (MACCEs) at 6 months, n (%) | ||||
| Total MACCE | 18 (16.8) | 6 (6.7) | 0.047 | 2.80 [1.06–7.39] |
| MI | 2 (1.9%) | 1 (1.1%) | 1.000 | 1.68 [0.15–18.8] |
| Stroke | 2 (1.9%) | 0 (0.0%) | 0.502 | 4.29 [0.20–89.5] |
| Recurrent revascularization | 1 (0.9%) | 1 (1.1%) | 1.000 | 0.83 [0.05–13.5] |
| Death | 14 (13.1%) | 5 (5.6%) | 0.093 | 2.53 [0.87–7.32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wontor, R.; Lisiak, M.; Łoboz-Rudnicka, M.; Ołpińska, B.; Wyderka, R.; Dudek, K.; Łoboz-Grudzień, K.; Jaroch, J. The Impact of the Coexistence of Frailty Syndrome and Cognitive Impairment on Early and Midterm Complications in Older Patients with Acute Coronary Syndromes. J. Clin. Med. 2024, 13, 7408. https://doi.org/10.3390/jcm13237408
Wontor R, Lisiak M, Łoboz-Rudnicka M, Ołpińska B, Wyderka R, Dudek K, Łoboz-Grudzień K, Jaroch J. The Impact of the Coexistence of Frailty Syndrome and Cognitive Impairment on Early and Midterm Complications in Older Patients with Acute Coronary Syndromes. Journal of Clinical Medicine. 2024; 13(23):7408. https://doi.org/10.3390/jcm13237408
Chicago/Turabian StyleWontor, Radosław, Magdalena Lisiak, Maria Łoboz-Rudnicka, Bogusława Ołpińska, Rafał Wyderka, Krzysztof Dudek, Krystyna Łoboz-Grudzień, and Joanna Jaroch. 2024. "The Impact of the Coexistence of Frailty Syndrome and Cognitive Impairment on Early and Midterm Complications in Older Patients with Acute Coronary Syndromes" Journal of Clinical Medicine 13, no. 23: 7408. https://doi.org/10.3390/jcm13237408
APA StyleWontor, R., Lisiak, M., Łoboz-Rudnicka, M., Ołpińska, B., Wyderka, R., Dudek, K., Łoboz-Grudzień, K., & Jaroch, J. (2024). The Impact of the Coexistence of Frailty Syndrome and Cognitive Impairment on Early and Midterm Complications in Older Patients with Acute Coronary Syndromes. Journal of Clinical Medicine, 13(23), 7408. https://doi.org/10.3390/jcm13237408





